Abstract
Background
Wood identification is extremely important in the modern forest industry. Teak (Tectona grandis L. family verbanaceae), Black Rosewood (Dalbergia latifolia L. family Fabaceae), and Ben Teak (Lagerstroemia lanceolata L. family Lytheraceae) are significant economic timber species in tropical countries. T. grandis is one of the most expensive hardwoods. It occurs naturally in India, Myanmar, Thailand, Java, and Laos in South Asia. L. lanceolata a strong associate of T. grandis occurs in deciduous forest-adjoining states of Karnataka such as Andhra Pradesh and Telangana state in India. D. latifolia distributed in India, Indonesia, Myanmar, Nepal, Philippines, Sri Lanka, and Vietnam. The selected valuable timbers threatened illegal logging and deforestation. It is challenging for non-taxonomist to accurately recognize and identify the timber species based on the conventional methods. DNA barcoding technologies and large-scale screening of DNA variation in trees can be done routinely and much faster with higher taxonomic resolution than morphological determination methods. In this study, we selected two plastid-specific ribulose bisphosphate carboxylase (rbcL), maturase K (matK), and one nuclear-specific trnH-ITS (intergenic spacer region)-conserved barcoding marker to compare species inter- and intradetailed discrimination. The aim of this study was to develop an accurate species and their provenance identification to support conservation efforts of three selected species viz T. grandis, D. latifolia, and L. lanceolata in Karnataka, India.
Results
Among the three selected markers, only two markers (rbcL and trnH-ITS) were having potential to amplify and discriminate the intra- and interspecific regions of selected timbers and would have been used to get barcodes for each species. Additionally, rbcL (90–96%) has a higher discrimination dominance over trnH-ITS (80–86%). From the barcoding results, it was found that the T. grandis and D. latifolia samples that belong to the Institute of Wood Science and Technology Bangalore are genetically more related to Dharwad than Hassan region in Karnataka.
Conclusion
The present study provided a database in which DNA sequences from unknown samples could be matched as well as required sequences from which other timbers or plant species could be identified. The use of inbuilt unique properties of DNA database within a timber would serve as an important molecular element in forensic forestry to support the determination of identifying the species and their adulterants.
Similar content being viewed by others
Introduction
Black Rosewood (Dalbergia latifolia f: Fabaceae), Ben Teak (Lagerstroemia lanceolata f: Lytheraceae), and Teak (Tectona grandis f: Verbenaceae) are incredibly significant economic timber species. T. grandis is one of the most expensive hardwoods in the world (Kollert and Walotek 2015). It is indigenous to peninsular and central India, Myanmar, Indonesia, Thailand, and Laos in South Asia and has also emerged as a major hardwood plantation species in a number of other countries in Africa, West Indies, and Sri Lanka (Mohanan et al. 1997) (Hansen et al. 2017). L. lanceolata is found in the deciduous forest-adjoining state of Karnataka such as Andhra Pradesh and Telangana states, and it is a strong associate of T. grandis in the forest of Karnataka, India. D. latifolia mostly distributed in India, Indonesia, Myanmar, Nepal, Philippines, Sri Lanka, and Vietnam (Orwa et al. 2009). These three valuable timber species are threatened by illegal logging and deforestation. In an effort to reduce and ultimately halt illegal logging and trade in, a challenge for the molecular biologists is the identification of plants at an intraspecific and interspecific level using barcode sequences that differentiate among closely related species or intervarietal is the most significant (Han et al. 2016). DNA barcoding is a widely used molecular marker technology, which uses a standardized and conserved but diversed DNA sequence to identify species and the origins of timber and to track logs along supply chains (Kang et al. 2017). This technique could serve as another tool to help identify and trace valuable timbers (Finkeldey et al. 2009). A significant assessment of DNA variation can be done faster and with higher taxonomic resolution than morphological determination methods (Lowe 2007). The development of DNA marker for species identification method would greatly contribute to identify wood species and wood products through region and source verification to trace legally from illegally harvested logs or timber products (Lowe and Cross 2011). The major influence of DNA barcoding is that once a solid reference database has been established, the method does not require expert taxonomic knowledge in order to identify specific samples (Hartvig et al. 2015). This technique has facilitated marketing and sale of poor-quality wood of other species in place of the novel timber species. Although DNA barcoding has been successfully used to discriminating plants or crop species, applying this technique for discriminating forest tree species is difficult. Plant plastid and mitochondrial genomes exhibit a low rate of mutation and nucleotide substitution (Palmer 1985). DNA barcoding studies in the plants have been proposed a few plastid-coding regions (rbcL and matK) and non-coding region (ITS-trnH) as potential barcodes (Pettengill and Neel 2010). The study was mainly focused on the sequence analysis, calculation of genetic distance, variable sites, molecular weight of bases, amplified polymorphism, barcode development of multiple sequence alignment, use of a neighbor-joining algorithm, and tree construction analysis. In the present study, the main focus was to develop an accurate species identification method for the three selected T. grandis, D. latifolia, and L. lanceolata species and this was employed by developing potential DNA barcodes to support conservation efforts of the genus.
Materials and methods
Sampling sites
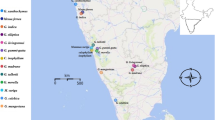
The samples of selected species were collected from three broad natural-growing population regions along Haliyal, Barchi range Dharwad under Western Ghats, Hassan (Alur taluka) and Bangalore, Karnataka, India (Fig. 1). Details of the sample location were described in (Table 1). Superior phenotypes were chosen for this research study based on height, girth, and disease-free tree.
Sample collection
For the development of barcodes from the selected three species, three samples (three leaves and three cores) of each species were selected and core and leaf sampling was carried out. Core sample was collected at 1.3 m from the ground level through using the conventional increment borer (Mora coretax original, Sweden; 100 mm) (core diameter 5.15 mm). The hole in the tree was properly sealed with paraffin wax to prevent infection. Core and leaf samples were put into a plastic cover and immediately placed into dried silica gel. After the arrival to the laboratory, the samples that had been placed in the silica gel were cleaned and kept for drying at least 3 to 4 days with the change of silica gel which absorb all moisture content of the samples and then transferred the dried samples to − 20 °C cryogenic freezer to maintain the quality for further analysis.
Genomic DNA isolation
Total genomic DNA was isolated from silica-dried leaves and wood samples by using a modified CTAB protocol (Fatima et al. 2018a, 2018b).
DNA quantification
The yield of extracted DNA was quantified by nanodrop at 260 and 280 nm wavelength. The purity of DNA shows a ration between 1.80 to 2.0 (Sambrook et al. 1989). DNA suitability for sequencing and barcoding was checked by running the samples on 1% (w/v) agarose gel (Jiao et al. 2013) stained with 4 μL of 0.3% ethidium bromide.
PCR amplification through universal barcode primers (RbcL, matK, and trnH-ITS)
Three candidate barcoding loci rbcL, matK, and ITS-trnH (CBOL Plant Working Group 2009) were selected and synthesized by Integrated DNA Technologies Pvt. Ltd. (Table 2). DNA amplification was carried out in a 13-μL reaction volume containing 1.5 μL genomic DNA (30 ng/ μL), 2 μL primers (10 mM both forward and reverse), 1.5 μL PCR buffer, 1.5 μL dNTPs (10 mM), 1.5 μL MgCl2, 0.3 μL Taq polymerase (3 U/ μL) (Bangalore genie), and 4.2 μL double distilled water. Amplification cycle consists of an initial 3-min denaturation at 94 °C, 30 cycles for 1 min at 60–61 °C, 1 min at 72 °C, and final extension step for 10 min at 72 °C.
Separation of amplified PCR products and purification
The amplified product loaded with 5 μL loading buffer was size fractionated by electrophoresis on a 2% agarose gel (Saiki et al. 2017) with 0.3% ethidium bromide. The bands are visualized on UV transilluminator (Syngen G:Box) to test out the bands. PCR amplified products were purified using EZ-10 spin column PCR purification kit (Life Technologies, California USA) following the manufacturer’s standard instructions.
DNA sequencing and primer development
Sanger sequencing of the purified PCR products was carried out in Eurofins Genomics India Pvt. Ltd. Database search was done in the nucleotide database of the US National Center for the Biotechnology Information (NCBI, www.ncbi.nlm.nih.gov) or the Barcode of Life Database (BOLD, http://www.boldsystems.org/) to identify homologs of the related genes. Gene sequences from ITS-trnH and rbcL were submitted to NCBI sequence data submission bank (http://www.ncbi.nlm.nih.gov/genbank./) to obtain accession numbers. The provenance-specific barcode primers from the sequenced data of rbcL and ITS-trnH were designed by Primer3 version 0.4.0 software online (http://bioinfo.ut.ee/primer3-0.4.0) (Untergasser et al. 2012).
Multiple sequence alignment and barcode generation
Multiple sequence alignment comparison brought out the genus identification in between the provenances. Nucleotide base FASTA files of all the species alighned through online European Bioinformatics Institute (EMBL-EMBI) (Clustal Omega; http://www.ebi.ac.uk/Tools/msa/clustalo/) program. The nucleotide sequences of ITS-trnH and rbcL were aligned separately and analyzed. Barcode construction for rbcL and ITS-trnH region of each selected species was done by Eurofins Genomics India Pvt. Ltd.
Tree construction and phylogenetic analysis
Tree construction is a method that uses the MEGA software to construct a neighbor-joining (NJ) tree for each marker and with the different combinations of barcode markers under the Kimura-2 parameter substitution model (Hall 2013). The phylogenetic trees were constructed by using algorithm programs in molecular evolutionary genetic analysis (MEGA7) software (Kumar et al. 2007). The bootstrap support of the NJ tree was assessed using 1500 replicates. The selected species were effectively differentiated if all the individuals of the correspondinding species formed a group in phylogenetic tree (Hollingsworth et al. 2009).
Results and discussion
The total genomic DNA that has been extracted from the selected species concentration was estimated by UV spectrophotometer using the standard formula 50 × OD260 × dilution factor/100, and the graphs were recorded (Fig. 2).
Barcode primer amplification
Three barcode primer PCR products were randomly chosen from the wood samples of selected species to verify the sequencing and barcode development of the selected species (Kang et al. 2017). A total of seven individuals representing all the three species were successfully amplified and sequenced using two DNA barcodes (rbcL and ITS-trnH). The selected barcode regions were amplified with the barcode primers, and the eluted product gave a good read frame length in the forward direction (Table 3). However, there was a difficulty in the amplification and alignment sequencing with matK primer (Fig. 3a–c). From the above gel pictures, it was clearly depicting that only rbcL and ITS-trnH were amplified in all the tree species. Further analysis revealed the species-wise difference in D. latifolia, T. grandis, and L. lanceolata, but it did not discriminate the provenances of the samples (Fig. 3d). The success rate of PCR amplification sequencing was the higher for rbcL compared to ITS-trnH in the selected tree species (Fig. 4).
PCR amplification product generated by using plastid DNA. a rbcL for (i) T. grandis, (ii) D. latifolia, and (iii) L. lanceolata. b matK for (i) T.grandis, (ii) D. latifolia, and (iii) L. lanceolata. c ITS-trnH for (i) T.grandis, (ii) D. latifolia, and (iii) L. lanceolata. d Selected primer rbcL amplified and showing clear bands in all three timbers: (i) T. grandis, (ii) D. latifolia, and (iii) L. lanceolata
Nucleotide variation and sequencing success rate
The average sequence characteristics of the studied barcodes have been tabulated (Table 4). The total sequences obtained from the rbcL and ITS-trnH region of all the selected species were aligned based on their provenances by using (MSA Clustal W1.2).
The final aligned partial sequences of rbcL in T. grandis had a length of 619 bp for Dharwad, 528 bp for Hassan, and 653 bp for IWST Bangalore with the variable sites, and for D. latifolia, 502 bp for Dharwad, 514 bp for Hassan, and 608 bp for IWST Bangalore which revealed the location specific base differences in the selected species. In L. lanceolata, it was 620 bp length sequence. For T. grandis, the average bp sequences obtained by using rbcL marker was 600 bp with the 75 bp variable sites. In case of D. latifolia, the average bp sequence length by using rbcL primer is 541 bp with the variable sites of 85 bp. In L. lanceolata sequences, the bp average was found to be 620 bp with the 56 variable sites. The total number of SNPs (single nucleotide polymorphisms) was found more in rbcL than in ITS-trnH marker sequences. The total number of SNPs produced by using rbcL in T. grandis was 97 followed by 68 in D. latifolia and 91 in L. lanceolata. CpG% was more in rbcL sequences compared to ITS-trnH sequences. In T. grandis, CpG% was found 97% by using rbcL marker followed by 85% in D. latifolia and 91% in L. lanceolata (Table 4).
For ITS-trnH region, the final aligned partial sequences of T. grandis had a length of 477 bp for Dharwad, 455 bp for Hassan, and 314 bp for IWST Bangalore, and for D. latifolia, 741 bp for Dharwad, 761 bp for Hassan, and 775 bp for IWST Bangalore which revealed the location specific base differences in the selected species. For L. lanceolata, it was 305 bp length sequence. For T. grandis, the average of bp sequences obtained by using ITS-trnH marker was 415 bp with 93 bp variable sites. In case of D. latifolia, the average bp sequence length by using ITS-trnH primer was 759 bp with the variable sites of 115 bp. In L. lanceolata sequences, the bp average by using ITS-trnH primer was found to be 305 bp with the 68 variable sites. The total number of SNPs produced by using ITS-trnH in T. grandis was 16 followed by 13 in D. latifolia and 11 in L. lanceolata. In T. grandis, CpG% was found 85% by using ITS-trnH marker followed by 59% in D. latifolia and 73% in L. lanceolata (Table 4).
The numbers of polymorphic sites among the species with different region samples were ranged from 78 for D. latifolia, 85 for L. lanceolata, and 267 for T. grandis, and the number of variable sites of the rbcL regions increased when the sequence length varies (Table 4).
Nucleotide discrimination-based approach for species identification
ITS-trnH and rbcL could assign the characteristic nucleotide positions, and differences also showed for all the species with 100% correct division. Nucleotide sequence analysis was done using rbcL and ITS-trnH loci for all the species with 100% correct classification. In case of ITS-trnH in D. latifolia, A and T nucleotides showed low variable whereas C and G were highly variable. Adenine ranged from 160 (Dharwad) to 174 (IWSTB) with the average of 66. Thymine base was varied in between 108 (Dharwad) and 125 (Hassan) with the mean value 114 followed by cytosine which ranged from 193 (Hassan) to 237 (IWSTB) with the average 219 base and by guanine which ranges between 192 (Dharwad) and 232 (IWSTB) with the mean value of 218 base. Similarly, in case of rbcL, G nucleotide was a low variation whereas A, T, and C were showing high variation in D. latifolia. Adenine variation ranged from 122 (IWSTB) to 160 (Hassan) with the average value of 143. Thymine base was ranged from 146 (IWSTB) to 168 (Hassan) with the mean value 155 followed by cytosine which ranged from 91 (IWSTB) to 151 (Hassan) with the average of 117 and by guanine nucleotide variation which ranged from 108 (IWSTB) to 127 (Hassan) with the average value of 116, respectively (Fig. 5; Table 5).
In T. grandis, adenine nucleotide was showing low variation while thymine, cytosine, and guanine were found to be extremely variable which corresponds to the provenances. In case of ITS-trnH sequences of T. grandis, A and T nucleotides showed low variable whereas C and G were highly variable. Adenine ranged from 40 (IWSTB) to 97 (Dharwad) with the average of 78 nucleotides. Thymine base was diverse in between 49 (IWSTB) and 85 (Dharwad) with the mean value of 114 followed by cytosine which ranged from 114 (IWSTB) to 160 (Dharwad) with the average of 135 nucleotides and by guanine which ranges between 99 (IWSTB) and 144 (Dharwad and Hassan) with the mean value of 129 base. Similarly, in case of rbcL, adenine nucleotide was showing low variation whereas cytosine, guanine, and thymine were showing high variation in T. grandis. Adenine variation ranged from 147 (Hassan) to 176 (Dharwad) with the average value of 166 nucleotides. Thymine base was ranged from 151 (Hassan) to 170 (IWSTB) with the mean value of 159 followed by cytosine which ranged from 97 (Dharwad) to 129 (IWSTB) with the average of 113 and by guanine nucleotide variation which ranged from 118 (Hassan)) to 146 (IWSTB) with the mean of 129 nucleotides (Fig. 6; Table 5).
Multiple sequence alignment and discrimination of species
We analyzed three individuals of the selected species from different regions of Karnataka, and the sequences of the two examined regions of plastid DNA (rbcL) and nuclear DNA (ITS-trnH) were examined. Multiple sequence alignment of the selected three species sequences was carried out by using the online software ClustalW (v 1.2.4). We manually calculated the sequence alignments for SNPs and found unique SNPs in all the selected species which were observed with rbcL and ITS-trnH barcoding markers.
The total number of sequences of D. latifolia by using ITS-trnH markers was found to be 742 bp in IWST, 742 bp in Dharwad, and 762 bp in Hassan, Karnataka. Total rbcL sequences found in D. latifolia were 609 bp in IWSTB, 503 bp in Dharwad, and 515 bp in Hassan, Karnataka. We found 28 unique nucleotide sites in D. latifolia by using ITS-trnH. These were the 116th and 762th sites of the ITS-trnH sequences at which A and T respectively were present in D. latifolia of IWSTB and in Dharwad while G was present in D. latifolia Hassan. In case of D. latifolia, a total of 38 unique sites were found by using rbcL markers. These were the rbcL sequences at which A and T were present in Dharwad and Hassan while C and G were found in Hassan, Karnataka (Fig. 7).
The total number of sequences of T. grandis which was obtained by using ITS-trnH markers was 315 bp in IWSTB, 478 bp in Dharwad, and 456 bp in Hassan. The sequence number of rbcL markers found in IWSTB was 654 bp, in Dharwad 620 bp, and in Hassan region 529 bp. In T. grandis, 25 unique sites were found by ITS-trnH sequences. These were the 133th to 478th sites of the ITS-trnH at which C and G were present in T. grandis of Dharwad and IWSTB, respectively, while A and T were present in T. grandis of Hassan, Karnataka. A total of 53 unique sites were found in T. grandis sequences by using rbcL markers in which 62th to 529th A, G, and T was present in Dharwad and Hassan while C and G were found in IWSTB Karnataka, respectively (Fig. 8).
Bioinformatics analyses of sequences and barcode generation
The rbcL and ITS-trnH sequences from T. grandis, D. latifolia, and L. lanceolata were completely identical with all other sequences of the selected species. BLAST search in the NCBI nucleotide database was done using the sequences of all three species deposited in the GenBank showing 100% homology with the nuclear DNA regions. Comparison of the detected sequences with entries in the database finally provided the plant species identification. The nucleotide sequences obtained were submitted to DNA databank (accession number MG920556, MG920558, MG920557, MG920565, MG920313).
The developed barcodes for the D. latifolia by using ITS-trnH and rbcL are showed in Fig. 9 with their respective provenances ((i) Dharwad, (ii) Hassan, and (iii) IWST Bangalore, Karnataka) respectively. The developed barcodes for the T. grandis by using ITS-trnH and rbcL are showed in Fig. 10 with their respective provenances ((i) Dharwad, (ii) Hassan, and (iii) IWST Bangalore, Karnataka), followed by L. lanceolata in which the developed barcodes by using ITS-trnH and rbcL which showed with their respective provenances ((i) Hassan, Karnataka) are showed in Fig. 11.
Phylogenetic analysis
Total aligned molecular sequences from the selected three species by rbcL and ITS-trnH were used to generate a dendrogram which represented the genetic relationship among the selected species based on their provenances. The numbers of base substitutions per site in between sequences are shown in the dendrogram. The standard error estimates are shown above the diagonal site. Analyses were conducted using the Kimura-2 parameter model (Kimura 1980). The analysis involved three nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + non-coding. Gaps and missing data were eliminated from all the positions. There were a total of 312 positions in the final dataset. Evolutionary analyses were performed by MEGA7 (Kimura 1980 and Kumar et al. 2007).
According to evolutionary divergence by using the rbcL and ITS-trnH sequences, it was found that intraspecific variations in D. latifolia from Dharwad and IWST Bangalore samples were closely related (0.023–1.040) than the samples of Hassan. The case of Hassan and IWST Bangalore was quite distant (0.115–1.071), whereas Hassan and Dharwad were more closely related than IWST Bangalore (0.008–0.122) (Fig. 12a).
T. grandis belongs to Dharwad and IWST Bangalore region which were more related to each other (0.004–0.048) than Hassan samples. Hassan and IWST Bangalore were found to be quite distantly related (0.045–0.008), whereas Hassan and Dharwad were more related than IWST Bangalore (0.002–0.008) (Fig. 12b).
On the other hand, L. lanceolata samples from Hassan and Dharwad were diverged at the rate of 0.04634–0.3290 (Table 6). From the above results, it was concluded that the geographical effect and genetic effect were both high in Bangalore and Hassan populations rather than in Dharwad populations. Both factors were acted upon the selected tree species and gene low variability based on all the climatic as well as genetic conditions. Conserved gene variability was slow in Dharwad, IWST Bangalore, and Hassan populations and resulted less variability.
Primer development
In this study, we developed region-specific (IWSTB, Dharwad, and Hassan, Karnataka) barcode primers for each selected species (D. latifolia, T. grandis, and L. lanceolata) by using rbcL- and ITS-trnH-generated marker sequences Table 7.
Discussion
This study aims to develop a DNA marker to identify the species of T. grandis, D. latifolia and L. lanceolata by a multilocus DNA barcoding approach. The chloroplast DNA region rbcL and nuclear region ITS could be amplified successfully through the fresh wood samples and confirmed that fresh wood is a good source of DNA for wood tissue barcoding in Aquilaria sinensis (Jiao et al. 2013). In this study, the rbcL and ITS-trnH regions of chloroplast and nuclear DNA were amplified by using the wood tissues of respective timber samples (Fig. 3a–c). Lee et al. (2016) developed a DNA barcode to identify seven Aquilaria species using eight barcode candidate loci (matK, rbcL, rpoB, rpoC1, psbA-trnH, trnL-trnE, ITS, and ITS2) and the results revealed that all single barcodes amplified and sequenced with the selected primers. The results revealed that all single barcodes could be easily amplified and sequenced with the selected primers. In the present study, we found that rbcL marker is individually abled to discriminate species of the selected timbers (Fig. 3d). Previous studies revealed that matK barcode individually or in combination with other chloroplast DNA barcodes yielded a low success rate for species discrimination, but it has the ability to cluster studied Pterocarpus species according to their broad geographic origins (Jiao et al. 2018). Amplification and sequencing of the matK barcoding region is problematic due to high sequence variability in the primer binding sites (Hollingsworth et al. 2011). In the present study, the overall lowest rate of amplification and sequencing was reported with matK barcoding marker (Fig. 1b). Hassold et al. (2016) characterized diagnostic polymorphisms in the three DNA barcoding markers (matK, rbcL, and trnL) that allowed rapid discrimination between the Dalbergia species of Madagascar and its distribution range. Li et al. (2011) suggested that trn-F would be a good alternative locus due to high variation within trn-F marker and recommended the adoption of rbcL + matK for fern studies. The ITS barcoding markers which perform as an influential marker at the species level show high levels of interspecific divergence (Alvarez and Wendel 2003). In this study, we used three barcode markers in which rbcL and ITS-trnH performed 97% of the amplification and sequencing Table 3. Kang et al. (2017) reported a success rate of amplification and sequencing of matK fragment (50–57%). In the present study, the success rate of amplification and sequencing of the selected markers is 96 to 94% for rbcL, 91 to 80% for ITS-trnH, and 43 to 30% for matK with the selected species. Nuclear ITS-trnH fragment represents a more promising DNA barcode marker than the maternally inherited chloroplast barcodes in Aceraceae (Han et al. 2016), while in this study, both rbcL and ITS barcoding markers were amplified and generate good results with all the selected species Figs. 9, 10, and 11. DNA barcoding with ITS marker alone revealed unknown species and proved useful in identifying species listed in endangered species appendixes (Meullner et al. 2011).
Nithaniyal et al. (2014) studied that rbcL and matK markers correctly identified 136 species out of 143 tropical dry evergreen species. The success rate of amplification and sequencing of ITS marker could be variable for tropical forest plant in different regions (Kang et al. 2017). In this study, the selected species belongs to tropical forest, and amplification followed by sequencing was found highly successful by using ITS markers. D. olivery species were accurately identified by rbcL, matK, and ITS barcode markers (Hartvig et al. 2015). Bhagwat et al. (2015) reported matK and matK + rbcL as the most suitable barcodes to differentiate Dalbergia species. In this present study, we found that not only combinations of the barcodes but also alone maker could identify the species. ITS sequences ranged from 663 to 666 bp with 94 variable sites and 80 informative sites. The alignment length was 69 bp with 19 insertion and deletion of 1–3 bp within the aligned regions in the genus Decalepis (Apocynaceae) (Mishra et al. 2017a, 2017b). In our study, ITS-trnH ranged from with variable sites 68 to 115 (Table 4). Tsumura et al. (2011) developed a dataset and found nucleotide substitutions between the four wood plant groups, which provide convenient sites for developing markers. The highest nucleotide diversity was found in White Meranti species out of four species (Yellow, Valau, and Red Meranti). The two unique nucleotides found in S. albida were not present in any other Shorea species which were the 11th and 1225th sites of the trnH-psbA-trnK sequence, at which G and C respectively were present in S. albida, while A and G were present in all other Shorea species. In this study, we found 28 unique nucleotide sites in D. latifolia by using ITS-trnH and 38 unique sites was found by using rbcL markers (Fig. 7; Table 5). In T. grandis, 25 unique sites were found by using ITS-trnH sequences and 53 unique sites were found by using rbcL markers which indicated that rbcL sequences having more bp variation in their unique sequences than ITS-trnH unique sequences. The above rbcL and ITS-trnH sequencing and barcoding results of T. grandis, D. latifolia and L. lanceolata trees samples, revealed a considerable discrimination in intra-species and inter-species level in the selected regions of Karnataka (Table 1). (Hassan, IWST Bangalore, and Dharwad. Genetic distance base neighbor-joining analysis revealed the character-based identification of the traded species in Terminalia. Hassold et al. (2016) tested matK, rbcL, and trnL barcoding markers to differentiate Dalbergia species between Madagascar and from other areas of its distributional range. Phylogenetic analysis revealed that the Dalbergia studies from monophyletic groups contain two subgroups, one of which corresponds to a single species. Phylogenetic study of Terminalia species revealed the average intraspecific variations with the combination of 0.0188 to 0.0019 were less than the distance to the nearest neighbor (0.106 ± 0.009) with the matK and ITS (Mishra et al. 2017a, 2017b). In the present study, we calculated divergence among the individuals of the species (intraspecific divergence) with the combination of rbcL and ITS-trnH phylogenetic analysis revealed in T. grandis (0.004–0.048), D. latifolia (0.008–0.122), and L. lanceolata (0.046–0.329) (Table 6). The genetic distance between sequence pair after multiple sequence alignment was helpful in determining the genetic relatedness between the species of selected timbers at the molecular level. Li et al. 2011 performed the DNA barcoding technique to generate barcode sequences for Aquilaria species identification and applied the barcodes to identify the source species of agarwood found in the market. DNA barcoding of eight Dalbergia species from Vietnam recommended ITS locus as a potential barcode based on UPGMA analysis and nucleotide diversity (Phong et al. 2014), but in this study, we found that rbcL marker was a strong barcode marker for D. latifolia and T. grandis and based on genetic distance UPGMA study. Phylogenetic analysis of Santalum album with its adulterants Osyris wightiana and Erythroxylem monogynum formed the most genetically similar cluster by using rbcL, matK, and trnH-psbA barcoding markers (Dev et al. 2014). In the present study, genetic similarity with low intraspecific variation was reported in T. grandis, D. latifolia and L. lanceolata trees samples by using the rbcL and ITS-trnH markers. Through the barcoding sequence results and phylogenetic analysis, D. latifolia samples of IWST Bangalore are genetically more related to Dharwad than Hassan, Karnataka (Fig. 12a). Phylogenetic analysis of T. grandis IWST Bangalore samples was genetically more related to Dharwad than Hassan, Karnataka (Fig. 12b). Out of three universal primers, rbcL and ITS-trnH detected suitable and distinguishable gene sequences for the selected timber species, and that could have been used to get barcodes for forest timer species.
Conclusions
DNA-based barcoding is an invaluable tool for detecting errors in identifications of the tree species. The ratio of successfully identified species to all three sampled species was calculated as the proportion of species that were discriminated. From the above sequences, barcodes and pairwise distance matrix indicated their closeness during evolution and help to draw their phylogenetic tree. The present study provided a database to which the selected DNA sequences from unknown samples could be matched as well as necessary sequences from which other timbers or plant species could be identified and used for species identification. This study was able to identify wood samples, which represent that DNA barcoding has the potential to be an identification tool in the selected timbers.
Abbreviations
- A:
-
Adenine
- C:
-
Cytosine
- CTAB:
-
Cetyl trimethyl ammonium bromide
- G:
-
Guanine
- PCR:
-
Polymerase chain reaction
- SSR:
-
Simple sequence repeats
- T:
-
Thymine
- TAE:
-
Tris-acetate-EDTA
References
Alvarez I, Wendel JF (2003) Ribosomal ITS sequences and plant phylogenetic inference. Mol Phylogenet Evol 29:417–434
Bhagwat RM, Dholakia BB, Kadoo NY, Balasundaran M, Gupta VS (2015) Two new potential barcodes to discriminate Dalbergia species. PLoS One 10(11):e0142965
CBOL Plant Working Group (2009) A DNA barcode for land plants. Proc Natl Acad Sci U S A 106(31):12794–12797
Dev SA, Muralidharan EM, Sujanpal P, Balasundaran M (2014) Identification of market adulterants in east Indian sandalwood using DNA barcoding. Ann For Sci 71:517–522
Fatima T, Srivastava A, Hanur VS, Rao SM (2018a) An effective wood DNA extraction protocol for three economic important timber species of India. Am J Plant Sci 9:139–149
Fatima T, Srivastava A, Somashekar PV, Hanur VS (2018b) Optimization of genomic DNA extraction routine from ROSEWOOD (Dalbergia latifolia) and BEN TEAK (Lagerstroemia lanceolata). Int J Gen 6:446–451
Finkeldey R, Leinemann L, Gailing O (2009) Molecular genetic tools to infer the origin of forest plants and wood. Appl Microbiol Biotechnol 5:1251–1258
Hall BG (2013) Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol 30:1229–1235
Han WY, Duan D, Ma XF, Jia Y, Liu ZL, Zhao GF, Li ZH (2016) Efficient identification of the forest tree species in Aceraceae using DNA barcodes. Front Plant Sci (7):1707
Hansen OK, Changtragoon S, Ponoy B, Lopez J, Richard J, Kjaer ED (2017) Worldwide translocation of Teak- origin of landraces and present genetic base. Tree Genet Genomes 13:87
Hartvig I, Czako M, Kjaer ED, Nielson LR, Theilade I (2015) The use of DNA barcoding in identification and conservation of Rosewood (Dalbergia spps). PLoS One 9(1371):1–24
Hassold S, Lowry PP, Bauert MR, Razafintsalama A, Ramamonjisoa L, Widmer A (2016) DNA barcoding of Malagasy rosewoods: towards a molecular identification of CITES-listed Dalbergia species. PLoS One 10(1371):1–17
Hollingsworth ML, Clark AA, Forrest LL, Richardson J, Pennington RT, Long DG (2009) Selecting barcoding loci for plants: evaluation of seven candidate loci with species-level sampling in three divergent groups of land plants. Mol Ecol Resour 9:439–457
Hollingsworth PM, Graham SW, Little DP (2011) Choosing and using a plant DNA barcode. PLoS One 6:e19254
Jiao L, Yin Y, Cheng Y, Jiang X (2013) DNA barcoding for identification of the endangered species Aquilaria sinensis: comparison of data from heated or aged wood samples. J Holzforschung 4:487–494
Jiao L, Yu M, Wiedenhoeft AC, He T, Li J, Liu B, Jiang X, Yin Y (2018) DNA barcode authentication and library development for the wood of six commercial Pterocarpus species: the critical role of Xylarium specimens. Sci Rep 28:1945
Kang Y, Deng Z, Zang R, Long W (2017) DNA barcoding analysis and phylogenetic relationships of tree species in tropical cloud forest. Sci Rep 7:12564. 1–12564. 9
Kimura MA (1980) Simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kollert W, Walotek J (2015) Global Teak trade in the aftermath of Myanmar’s log export ban. Food and agriculture organization of the united nations. http://www.fao.org/3/a-i5023e.pdf.
Kumar S, Stecher G, Tamura K (2007) MEGA.7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Lee SY, Ng WL, Mahat MN, Nazre M, Mohamed R (2016) DNA barcoding of the endangered Aquilaria (Thymelaeaceae) and its application in species authentication of agarwood products traded in the market. PLoS One 11(4):e0154631. https://doi.org/10.1371/journal.pone.0154631
Li F-W, Kuo L-Y, Rothfels CJ, Ebihara A, Chiou W-L, Windham MD, Pryer KM (2011) rbcL and matK earn two thumbs up as the core DNA barcode for ferns. PLoS One 6(10):e26597
Lowe AJ (2007) Can we use DNA to identify the geographic origin of tropical timber. Proceedings of the international workshop “Fingerprinting methods for the identification of timber origins”, Bonn
Lowe AJ, Cross HB (2011) The application of DNA methods to tree tracking and origin verification. J IAWA 2:251–262
Meullner AN, Schaefer H, Lahaye R (2011) Evaluation of candidate DNA barcoding loci for economically important tree species of the mahogany family (Meliaceae). Mol Ecol Resour 11:450–460
Mishra P, Kumar A, Nagireddy A, Shukla AK, Sundaresan V (2017a) Evaluation of single and multilocus DNA barcodes towards species delineation in complex tree genus Terminalia. PLoS One 12(8):e0182836
Mishra P, Kumar A, Sivaraman G, Shukla AK, Kaliyamoorthy RK, Slater A, Velusamy S (2017b) Character-based DNA barcoding for authentication and conservation of IUCN red listed threatened species of genus Decalepis (Apocynaceae). Sci Rep (7):14910
Mohanan C, Sharma JK, Florence EJM (1997) Nursery diseases of teak in India. Proceeding of the International Teak Symposium, Thiruvananthapuram, pp 107–112
Nithaniyal S, Newmaster SG, Ragupathy S, Krishnamoorthy D, Vassou SL, Parani M (2014) DNA barcode authentication of wood samples of threatened and commercial timber trees within the tropical dry Evergreen Forest of India. PLoS One 9(9):e107669
Orwa CA, Mutua R, Jamnadass RS, Anthony S (2009) Agroforestry tree database: a tree reference and selection guide version 4.0. World Agroforestry Centre, Kenya
Palmer JD (1985) Evolution of chloroplast and mitochondrial DNA in plants and algae. In: MACINTY RJ, ILl MI (eds) Monographs in evolutionary biology: mole evol genet, pp 131–240
Pettengill JB, Neel MC (2010) An evaluation of candidate plant DNA barcodes and assignment methods in diagnosing 29 species in the genus Agalinis (Orobanchaceae). Am J Bot 97(8):1391–406
Phong DT, Tang DV, Hien VTT, Ton ND, Van HN (2014) Nucleotide diversity of a nuclear and four chloroplast DNA regions in rare tropical wood species of Dalbergia in Vietnam: a DNA barcode identifying utility. Asian J Appl Sci 2:116–125
Saiki FA, Bernardi AP, Reis MS, Faoro H, Souza EM, Pedrosa FO, Mantovani A, Guidolin AF (2017) Development and validation of the forest SSR markers for Mimosa scabrella Benth. Genet Mol Res 16;16(1). https://doi.org/10.4238/gmr16019571
Sambrook J, Fritesh EDF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold spring Harbor Laboratory Press, NY, p 2344
Tsumura Y, Kado Y, Yoshida K, Abe H, Ohtani M, Taguchi Y, Fukue Y, Tani N, Ueno S, Yoshimura K, Kamiya K, Harada K, Takeuchi Y, Diway B, Finkeldey R, Naiem M, Indrioko S, Ng KKS, Muhammad N, Lee SL (2011) Molecular database for classifying Shorea species (Dipterocarpaceae) and technique for checking the legitimacy of timber and wood products. J Plant Res 124:35–48
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40:15
Acknowledgements
The authors are thankful to the Director, IWST, Group Coordinator Research, Head Genetics and Tree Improvement Division, molecular biology lab members in the Institute of Wood Science and Technology, the Additional Principal Chief Conservator of Forest (research), and the Karnataka Forest Department for financial support. The authors also wish to thank Hassan DFO, Dharwad DFO, and Barchi Haliyal forest range officers for their assistance on field trips, tree identification, and sample collection.
Funding
The authors declare that this work was funded by the Karnataka Forest Department in Karnataka, India (Project ID-KFD/DNA 2014-2016).
Availability of data and materials
We declare that all data generated or analyzed during this study are included in this manuscript.
Author information
Authors and Affiliations
Contributions
TF completed the sample collection in the field and laboratory experiment work, the data analysis, and the writing of the draft of the manuscript. AS was involved in designing the experiments and selecting the species and the study location. SPV carried out the sample identification and collection in the field. VSH was involved in the result interpretations and reviewed the manuscript. MSR was involved in reviewing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fatima, T., Srivastava, A., Somashekar, P.V. et al. Development of DNA-based species identification and barcoding of three important timbers. Bull Natl Res Cent 43, 76 (2019). https://doi.org/10.1186/s42269-019-0116-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-019-0116-8