Abstract
Background
Purified cannabidiol (CBD), a non-psychoactive phytocannabinoid, has gained regulatory approval to treat intractable childhood epilepsies. Despite this, artisanal and commercial CBD-dominant hemp-based products continue to be used by epilepsy patients. Notably, the CBD doses used in these latter products are much lower than that found to be effective in reducing seizures in clinical trials with purified CBD. This might be because these CBD-dominant hemp products contain other bioactive compounds, including phytocannabinoids and terpenes, which may exert unique effects on epilepsy-relevant drug targets. Voltage-gated sodium (NaV) channels are vital for initiation of neuronal action potential propagation and genetic mutations in these channels result in epilepsy phenotypes. Recent studies suggest that NaV channels are inhibited by purified CBD. However, the effect of cannabis-based products on the function of NaV channels is unknown.
Methods
Using automated-planar patch-clamp technology, we profile a hemp-derived nutraceutical product (NP) against human NaV1.1–NaV1.8 expressed in mammalian cells to examine effects on the biophysical properties of channel conductance, steady-state fast inactivation and recovery from fast inactivation.
Results
NP modifies peak current amplitude of the NaV1.1–NaV1.7 subtypes and has variable effects on the biophysical properties for all channel subtypes tested. NP potently inhibits NaV channels revealing half-maximal inhibitory concentration (IC50) values of between 1.6 and 4.2 μg NP/mL. Purified CBD inhibits NaV1.1, NaV1.2, NaV1.6 and NaV1.7 to reveal IC50 values in the micromolar range. The CBD content of the product equates to IC50 values (93–245 nM), which are at least an order of magnitude lower than purified CBD. Unlike NP, hemp seed oil vehicle alone did not inhibit NaV channels, suggesting that the inhibitory effects of NP are independent of hemp seed oil.
Conclusions
This CBD-dominant NP potently inhibits NaV channels. Future study of the individual elements of NP, including phytocannabinoids and terpenes, may reveal a potent individual component or that its components interact to modulate NaV channels.
Similar content being viewed by others
Background
Nearly two-thirds of epilepsies are classified as being genetic in origin (Berkovic et al. 2006; Miller et al. 1998; Speed et al. 2014). Advances in genome sequencing have enabled the systematic screening of patient genomes to identify mutations in single genes that are responsible for epilepsy (Perucca and Perucca 2019). These monogenic epilepsies all report mutations in molecular components of neuronal signalling, with mutations in NaV channels being the most prevalent (Parrini et al. 2017). Functional characterization of the biophysical properties of these disease-associated mutant channels has provided insight on the molecular mechanisms by which NaV channel dysfunction contributes to epileptogenesis. NaV channel mutations that have been identified exhibit altered biophysical properties representative of both gain-of-function (GOF) and loss-of-function (LOF) phenotypes (Berecki et al. 2019; Berecki et al. 2018; Blanchard et al. 2015; de Kovel et al. 2014).
Mutations in SCN1A, the gene that encodes voltage-gated sodium channel NaV1.1, account for the greatest number of mutations identified and range in severity across various epilepsy types (Depienne et al. 2009; Djemie et al. 2016). De novo LOF mutations in SCN1A are present in over 80% of patients with Dravet syndrome (DS), a severe form of childhood-onset epilepsy. As NaV1.1 channels are expressed predominantly in GABAergic interneurons, LOF mutations result in reduced interneuron activity, thereby causing an imbalance between excitatory and inhibitory neurotransmission (Brunklaus and Zuberi 2014). Mutations in SCN2A and SCN8A, the genes that encode NaV1.2 and NaV1.6, respectively, have been implicated in Lennox-Gastaut syndrome (LGS), another severe childhood epilepsy (Epi4K. 2013). NaV1.2 and NaV1.6 are the primary NaV channels expressed in excitatory pyramidal neurons (Hu et al. 2009). Functional studies indicate that the majority of SCN2A and SCN8A mutations are GOF, resulting in neuronal hyperexcitability (Epi4K. 2013; Estacion et al. 2014; Veeramah et al. 2012).
Many epilepsy syndromes, including DS and LGS, are resistant to current treatments creating an urgent need for novel therapies with improved side effect profiles. Cannabis-based products are increasingly being used in intractable epilepsies (Suraev et al. 2018; Suraev et al. 2017), following numerous media stories featuring significant improvements in treatment-resistant childhood epilepsy patients using medicinal cannabis. These artisanal and commercially manufactured products contain hundreds of potentially bioactive compounds, including phytocannabinoids and terpenoids. Both phytocannabinoids and terpenes modulate a range of membrane proteins, including ion channels, and have exhibited anticonvulsant properties in preclinical seizure models (Anderson et al. 2019a, b; Gray and Whalley 2020). A purified preparation of the non-psychoactive phytocannabinoid CBD, Epidiolex™, was approved by drug regulatory agencies in the USA, Europe and Australia for the treatment of DS, LGS and tuberous sclerosis complex. While the anticonvulsant mode of action of CBD is unclear, cellular studies have shown that CBD affects several ion channels, such as 5-HT1AR, TRP channels, T-type calcium channels, GABAA receptors and NaV channels (Ghovanloo et al. 2018; Watkins 2019).
Recent studies suggest that NaV channel currents (NaV1.1-NaV1.7) are attenuated by purified CBD (Duan et al. 2008; Ghovanloo et al. 2018; Okada et al. 2005). However, the effect of cannabis-based products on the function of NaV channels is unknown. Here we use automated-planar patch-clamp technology to characterize the effects of a nutraceutical product (NP) against the human voltage-gated sodium channel family (NaV1.1-NaV1.8). We examined the effects of NP on the biophysical properties of channel conductance, steady-state fast inactivation and recovery from fast inactivation. Additionally, on the same biophysical parameters, we characterized the effects of a hemp seed oil preparation that contained the natural terpenes but was devoid of phytocannabinoids.
Methods
Tissue culture and transfection
HEK293T cells stably expressing SCN1A, SCN2A, SCN3A, SCN5A or SCN9A and CHO cells stably expressing SCN4A or SCN8A were maintained as previously described (Richards et al. 2018). CHO cells were transiently co-transfected with the pcDNA3.1-SCN10A construct as previously described (Knapp et al. 2012) and a GFP construct, using Lipofectamine 3000, according to the manufacturer’s instructions (Thermofisher).
Planar patch-clamp electrophysiology
Patch-clamp recordings were made using a Patchliner® (Nanion Technologies, Munich, Germany) in the whole-cell configuration as previously described (Richards et al. 2018). Briefly, cells were prepared in suspension at a density of 1×106–5x107 cells/mL. The external recording solution comprised (in mM): 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 5 D-glucose, 10 HEPES, pH 7.4 with NaOH, ~295 mOsm. The internal recording solution comprised (in mM): 50 CsCl, 60 CsF, 10 NaCl, 20 EGTA, 10 HEPES, pH 7.2 with CsOH, ~285 mOsm. NP was diluted in DMSO. Medium single-hole planar -16 chips with an average resistance of ~2.5 MΩ were used. Chip and whole-cell capacitance were fully compensated, and 50% series resistance compensation applied. Recordings were acquired at 50 kHz with the low pass filter set to 10 kHz in PATCHMASTER (HEKA Instruments, NY, USA) and performed at 27°C. Offline analysis was performed using Microsoft Excel, MatLab R2019a (MathWorks) and GraphPad Prism 8 (Molecular Devices). Biophysical analysis was performed using MatLab scripts, incorporating the curve fitting equations described below, tailored to enable analysis of multiple-cell data output acquired using automated-planar patch-clamp technology.
Voltage clamp protocols
Voltage protocols were used, as previously described (Richards et al. 2018). Briefly, to study the voltage-dependence of activation, cells were held at −120 mV and depolarized to test potentials, in 5 mV increments, between −120 and +50 mV for 100 ms. To study steady-state fast inactivation, cells were held at conditioning pre-pulse potentials ranging from −120 to +30 mV in 5 mV increments from a holding potential of −120 mV and a test pulse at 5 mV for 20 ms. Recovery from fast inactivation was studied by pre-pulsing the cells to 0 mV from a holding potential of −120 mV for 50 ms, to fully inactivate channels. The voltage was then stepped back to the holding potential for variable interpulse intervals (ipi from 0 to 39 ms in 3 ms increments). To test channel availability, the voltage was stepped to 0 mV for 50 ms. To determine IC50 values for NP, hemp seed oil and purified CBD, cells were held at −80 mV, stepped to −120 mV for 200 ms followed by 50 ms test depolarization to 0 mV every 2 s for 30 s in the presence of vehicle control (DMSO). The cells were then exposed to NP (0.03–9 μg NP/mL), hemp seed oil (0.03–9 μg hemp seed oil/mL) or purified CBD (0.1–100 μM) sequentially for 5 min. Currents for individual cells were averaged over 24-s periods directly before application and following a 5-min exposure of NP, hemp seed oil or purified CBD. Leak subtraction was applied before normalization of current amplitude. Normalized mean data were fit to the Hill equation.
Curve fitting and data analysis
To examine the voltage-dependence of activation, normalized current-voltage (I-V) relationships were converted to conductance (G) using the following equation: G=I/(V-Vr) where Vr is the reversal potential for Na+. The voltage-dependence of conductance and availability were normalized and fitted to a Boltzmann equation: G=1/(1+exp[(V-V0.5)/a]), where a is the slope of the half-maximum, V is the potential of the given pulse and V0.5 is the potential for the half-maximal activation/inactivation. To measure recovery from inactivation, normalized currents were plotted against ipi and data fitted with the equation I/Imax=1−exp/(rc+x), where Imax is maximal current; rc recovery rate constant; x is time. The time course of inactivation was fitted to a single exponential function I/Imax=I0+A*exp(-t-t0/τ)+C, where I0 is the non-inactivating component, Imax is the peak current, t is time and A is the component for the time constant τ. Time constants were plotted against voltage and the data fitted with a decaying exponential equation Y=span*exp(-K*x)+plateau, where span is the starting point of the curve, K is the decay factor, plateau is the value the curve decays to and x is time.
Nutraceutical product
The nutraceutical product (NP), Ananda Hemp 600 and hemp seed oil were provided for free by Ananda Professional Pty Ltd. NP was produced by ethanol extraction of hemp and stored at 4°C. Experiments were performed over a 4-month period, which complies with the 12-month shelf life, and comparable bioactive effects were observed throughout the series of experiments. The cold-pressed hemp seed oil was devoid of phytocannabinoids and terpenoids. The NP contained the following phytocannabinoids (mg/g product): cannabidiolic acid (CBDA) 1.4, cannabidiol (CBD) 18.3, cannabinol (CBN) 0.3 and Δ9-tetrahydrocannabinol (Δ9-THC) 3 (Additional file 1). Further characterization by LC-MS/MS (Suraev et al. 2018) showed cannabidivarinic acid (CBDVA), cannabidivarin (CBDV), cannabigerolic acid (CBGA), cannabigerol (CBG), Δ9-tetrahydrocannabinolic acid (THCA), Δ9-tetrahydrocannabidvarinic acid (THCVA), Δ9-tetrahydrocannabidvarin (THCV) and cannabichromene (CBC) were not present. Analysis by GC-MS (Suraev et al. 2018) showed the NP had the following terpene content (mg/g hemp seed oil): α-pinene 1.6, D-limonene 0.6, β-linalool 2.1 and β-caryophyllene 3.1. The highest concentration tested (9 μg NP/mL), equates to molar exposures of (nM): CBDA 35, CBD 520, CBN 9, Δ9-THC 86, α-pinene 106, D-limonene 40, β-linalool 60 and β-caryophyllene 137. Final drug concentrations contained 0.1% DMSO.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 8 (Molecular Devices) software, with a p value <0.05 considered statistically significant. Data values are expressed as mean±SEM. Comparisons were made between the vehicle and extract datasets using paired Student’s t tests. Comparisons were also made between IC50 values for pure CBD and the equivalent molar IC50 of CBD found in NP using unpaired Student’s t tests.
Results
Previous studies show that CBD is able to modulate the function of human NaV channels; however, data showing how commercially available phytocannabinoid preparations affect these ion channels is lacking (Duan et al. 2008; Ghovanloo et al. 2018; Okada et al. 2005). Here, we examined the impact of an orally administered phytocannabinoid-containing nutraceutical product (NP), on eight human voltage-dependent sodium channel subtypes, NaV1.1-NaV1.8. We examined the impact of NP on the biophysical properties of each channel and determined potency.
NP inhibits peak sodium currents
Whole-cell current recordings were recorded from cells expressing a single NaV isoform using automated-planar patch-clamp technology. NP (3 μg NP/mL) significantly inhibited the peak amplitude of sodium currents elicited by six of the NaV channel subtypes NaV1.1 (peak current (nA): DMSO −1.8±0.4, NP −1.3±0.3, p=0.01), NaV1.2 (peak current (nA): DMSO −1.0±0.2, NP −0.8±0.2, p=0.006), NaV1.4 (peak current (nA): DMSO −4.4±1.1, NP −3.0±1.1, p=0.002), NaV1.5 (peak current (nA): DMSO −8.2±1.0, NP −5.9±1.0, p=0.0004), NaV1.6 (peak current (nA): DMSO −1.5±0.3, NP −1.0±0.3, p=0.03) and NaV1.7 (peak current (nA): DMSO −1.9±0.3, NP −1.2±0.2, p=0.03) (Fig. 1a, b). NaV1.8 currents were not affected by NP (Fig. 1a, b). Interestingly, at the same concentration, NP significantly potentiated NaV1.3 peak current amplitude (peak current (nA): DMSO −1.2±0.2, NP −1.4±0.2, p=0.04) (Fig. 1a, b). Sodium channel currents were recorded in the presence of 3 μg hemp seed oil/mL. Hemp seed oil had no effect on the peak current amplitude elicited by NaV1.1, NaV1.2, NaV1.4, NaV1.5, NaV1.6, NaV1.7 or NaV1.8 (Fig. 1b and Additional file 2). However, similar to what was seen with NP, hemp seed oil potentiated NaV1.3 peak current amplitude (peak current (nA): DMSO −0.9±0.2, Hemp −1.2±0.2, p=0.04) (Fig. 1b and Additional file 2).
NP inhibits peak current amplitude of NaV channels. a Representative current traces, evoked by the indicated voltage protocol (inset), in the presence of DMSO vehicle (
 ) or NP (3 μg NP/mL) for NaV1.1 (
) or NP (3 μg NP/mL) for NaV1.1 (
 ), NaV1.2 (
), NaV1.2 (
 ), NaV1.3 (
), NaV1.3 (
 ), NaV1.4 (
), NaV1.4 (
 ), NaV1.5 (
), NaV1.5 (
 ), NaV1.6 (
), NaV1.6 (
 ), NaV1.7 (
), NaV1.7 (
 ) and NaV1.8 (
) and NaV1.8 (
 ) expressed in HEK 293 or CHO cells. Horizontal scale bars (2 ms) apply to all traces. b Mean normalized peak current amplitude (I/Imax) in the presence of DMSO vehicle (
) expressed in HEK 293 or CHO cells. Horizontal scale bars (2 ms) apply to all traces. b Mean normalized peak current amplitude (I/Imax) in the presence of DMSO vehicle (
 ) or NP (3 μg NP/mL) for NaV1.1 (
) or NP (3 μg NP/mL) for NaV1.1 (
 ; n=10), NaV1.2 (
; n=10), NaV1.2 (
 ; n=8), NaV1.3 (
; n=8), NaV1.3 (
 ; n=10), NaV1.4 (
; n=10), NaV1.4 (
 ; n=6), NaV1.5 (
; n=6), NaV1.5 (
 ; n=10), NaV1.6 (
; n=10), NaV1.6 (
 ; n=11), NaV1.7 (
; n=11), NaV1.7 (
 ; n=7), NaV1.8 (
; n=7), NaV1.8 (
 ; n=7) and DMSO vehicle (
; n=7) and DMSO vehicle (
 or, hemp seed oil (3 μg hemp seed oil/mL) for NaV1.1 (
or, hemp seed oil (3 μg hemp seed oil/mL) for NaV1.1 (
 ; n=9), NaV1.2 (
; n=9), NaV1.2 (
 ; n=5), NaV1.3 (
; n=5), NaV1.3 (
 ; n=5), NaV1.4 (
; n=5), NaV1.4 (
 ; n=9), NaV1.5 (
; n=9), NaV1.5 (
 ; n=5), NaV1.6 (
; n=5), NaV1.6 (
 ; n=5), NaV1.7 (
; n=5), NaV1.7 (
 ; n=11) and NaV1.8 (
; n=11) and NaV1.8 (
 ; n=7). Data points are mean±SEM. Peak current amplitudes in the presence of NP were compared to DMSO vehicle (
; n=7). Data points are mean±SEM. Peak current amplitudes in the presence of NP were compared to DMSO vehicle (
 ) currents. Peak current amplitudes in the presence of hemp seed oil were compared to DMSO vehicle (
) currents. Peak current amplitudes in the presence of hemp seed oil were compared to DMSO vehicle (
 ) currents. Statistical significance is marked as *p<0.05; **p<0.01
) currents. Statistical significance is marked as *p<0.05; **p<0.01
Divergent effects of NP on biophysical properties of NaV channels
Next, we sought to determine whether NP affects the biophysical properties of channel activation and steady-state fast inactivation. Conductance curves were fitted with a Boltzmann equation, and the voltage-dependence of half-activation (V0.5) and slope values were obtained. No changes in the voltage-dependence of activation were observed for NaV1.1, NaV1.2, NaV1.4, NaV1.6 or NaV1.7 channel subtypes (Fig. 2a, b). Although there is no effect on the voltage-dependence of activation for NaV1.1, a small, but significant change in the slope of the conductance curve was observed (slope factor: DMSO 6.1±0.4, NP 7.6±0.6 p=0.002). This suggests that NP has a GOF effect on NaV1.1 since a larger slope factor indicates greater activation of the channel at voltages negative to V0.5. NP (3 μg NP/mL) treatment resulted in significant hyperpolarizing shifts in the voltage-dependence of activation for NaV1.3 (V0.5 act (mV): DMSO −7.3±1.7, NP −12.6±1.5, p=0.003) and NaV1.8 (V0.5 act (mV): DMSO −0.1±2.1, NP −7.0±1.3, p=0.02), consistent with an increase in channel availability (Fig. 2a, b). In contrast, a significant depolarizing shift in the voltage-dependence of activation was observed for NaV1.5 (V0.5 act (mV): DMSO −46.8±2.4, NP −43.8±2.6, p=0.02) following treatment with NP, which is consistent with a decrease in channel availability (Fig. 2a, b). Again, we examined the effect of hemp seed oil alone on voltage-dependence of activation for each NaV isoform. Hemp seed oil caused small but significant hyperpolarizing shifts in the voltage-dependence of activation for NaV1.1 (V0.5 act (mV): DMSO −11.0±1.7, Hemp −15.4±1.5, p=0.002) and NaV1.2 (V0.5 act (mV): DMSO −12.6±2.1, Hemp −14.5±2.6, p=0.04) (Fig. 2d and Additional file 3). However, hemp seed oil did not affect the voltage-dependence of activation of the other sodium channel subtypes (Fig. 2d and Additional file 3). All NaV isoforms, except NaV1.2, exhibited a negative shift in the voltage-dependence of steady-state fast inactivation (V0.5 inact (mV): NaV1.1 DMSO −42.6±1.5, NP −48.3±1.8, p=0.0001; NaV1.3 DMSO −59.7±1.2, NP −64.1±0.9, p=0.002; NaV1.4 DMSO −58.0±3.5, NP −65.1±3.8, p=0.006; NaV1.5 DMSO −79.3±1.7, NP −88.4±1.8, p=0.001; NaV1.6 DMSO −52.2±2.4, NP −58.7±3.1, p=0.0004; NaV1.7 DMSO −59.7±2.3, NP −63.5±2.1, p=0.03; NaV1.8 DMSO −32.4±1.8, NP −39.6±1.3, p=0.006) in the presence of NP (Fig. 2a, c). A hyperpolarizing shift in fast inactivation is indicative of reduced channel availability. Hemp seed oil also resulted in a negative shift in the voltage-dependence of fast inactivation for all isoforms (V0.5 inact (mV): NaV1.1 DMSO −48.7±1.4, Hemp −52.9±1.2, p=0.003; NaV1.3 DMSO −55.8±1.3, Hemp −60.1±1.3, p=0.002; NaV1.4 DMSO −56.7±0.7, Hemp −64.4±1.4, p=0.005; NaV1.5 DMSO −77.9±2.4, Hemp −80.6±2.4, p=0.003; NaV1.8 DMSO −41.1±1.8, Hemp −45.7±2.4, p=0.03) except NaV1.2, NaV1.6 and NaV1.7 (Fig. 2e and Additional file 3). Despite the effects on the voltage-dependence of inactivation, neither NP nor hemp seed oil affected the time constants of fast inactivation (Additional files 4 and 5).
The effects of NP on activation and steady-state fast inactivation (SSFI). a Voltage-dependence of normalized peak conductance (G/Gmax) and SSFI (I/Imax), evoked by the indicated voltage protocols (insets), in the presence of vehicle (
 ) or NP (3 μg NP/mL) for NaV1.1 (
) or NP (3 μg NP/mL) for NaV1.1 (
 ; n=10; mean diff slope act 1.5±0.3, p=0.002), NaV1.2 (
; n=10; mean diff slope act 1.5±0.3, p=0.002), NaV1.2 (
 ; n=8), NaV1.3 (
; n=8), NaV1.3 (
 ; n=10), NaV1.4 (
; n=10), NaV1.4 (
 ; n=6; mean diff slope act 1.6±0.6, p=0.04; mean diff slope inact 0.8±0.3, p=0.03), NaV1.5 (
; n=6; mean diff slope act 1.6±0.6, p=0.04; mean diff slope inact 0.8±0.3, p=0.03), NaV1.5 (
 ; n=10; mean diff slope act 2.1±0.5, p=0.003; mean diff slope inact 0.8±0.3, p=0.003), NaV1.6 (
; n=10; mean diff slope act 2.1±0.5, p=0.003; mean diff slope inact 0.8±0.3, p=0.003), NaV1.6 (
 ; n=11; mean diff slope act 1.3±0.4, p=0.01), NaV1.7 (
; n=11; mean diff slope act 1.3±0.4, p=0.01), NaV1.7 (
 ; n=7) and NaV1.8 (
; n=7) and NaV1.8 (
 ; n=7). Boltzmann curves were fitted to pooled averages of peak conductance. b Average change in the voltage of half (V0.5) activation and c SSFI initiated by NP (3 μg NP/mL) for NaV1.1 (
; n=7). Boltzmann curves were fitted to pooled averages of peak conductance. b Average change in the voltage of half (V0.5) activation and c SSFI initiated by NP (3 μg NP/mL) for NaV1.1 (
 ; n=10), NaV1.2 (
; n=10), NaV1.2 (
 ; n=8), NaV1.3 (
; n=8), NaV1.3 (
 ; n=10), NaV1.4 (
; n=10), NaV1.4 (
 ; n=6), NaV1.5 (
; n=6), NaV1.5 (
 ; n=10), NaV1.6 (
; n=10), NaV1.6 (
 ; n=11), NaV1.7 (
; n=11), NaV1.7 (
 ; n=7) and NaV1.8 (
; n=7) and NaV1.8 (
 ; n=7). d Average change in the voltage of half V0.5 activation and e SSFI caused by hemp seed oil (3 μg hemp seed oil/mL) for NaV1.1 (
; n=7). d Average change in the voltage of half V0.5 activation and e SSFI caused by hemp seed oil (3 μg hemp seed oil/mL) for NaV1.1 (
 ; n=11), NaV1.2 (
; n=11), NaV1.2 (
 ; n=5), NaV1.3 (
; n=5), NaV1.3 (
 ; n=5), NaV1.4 (
; n=5), NaV1.4 (
 ; n=9), NaV1.5 (
; n=9), NaV1.5 (
 ; n=6), NaV1.6 (
; n=6), NaV1.6 (
 ; n=6), NaV1.7 (
; n=6), NaV1.7 (
 ; n=10) and NaV1.8 (
; n=10) and NaV1.8 (
 ; n=5). Data points are mean±SEM. Statistical significance is marked as *p<0.05; **p<0.01; ***p<0.001
; n=5). Data points are mean±SEM. Statistical significance is marked as *p<0.05; **p<0.01; ***p<0.001
However, recovery from steady-state fast inactivation was significantly slower in the presence of NP for NaV1.1 (rc: DMSO 1.0±0.1, NP 2.7±0.3, p=0.0002), NaV1.2 (rc: DMSO 1.0±0.2, NP 2.0±0.3, p=0.01), NaV1.4 (rc: DMSO 1.4±0.4, NP 5.2±1.1, p=0.01), NaV1.5 (rc: DMSO 3.4±0.3, NP 16.3±1.9, p=0.0001), NaV1.6 (rc: DMSO 1.1±0.2, NP 1.6±0.1, p=0.01) and NaV1.7 (rc: DMSO 2.7±0.2, NP 3.8±0.5, p=0.03) (Fig. 3). A slower recovery from steady-state fast inactivation suggests reduced channel availability and is consistent with a decrease in channel activity. NP did not affect the recovery from steady-state fast inactivation for either NaV1.3 or NaV1.8 (Fig. 3). Hemp seed oil only slowed the recovery from fast inactivation of NaV1.5 (rc: DMSO 4.5±0.6, Hemp 7.0±1.2, p=0.005) and had no effect on the other channels (Additional file 6).
The effect of NP on sodium channel recovery from fast inactivation. Recovery of channel availability from fast inactivation as a function of time, evoked by the indicated voltage protocol (inset), in the presence of DMSO vehicle (
 ) or NP (3 μg NP/mL) for NaV1.1 (
) or NP (3 μg NP/mL) for NaV1.1 (
 ; n=10), NaV1.2 (
; n=10), NaV1.2 (
 ; n=8), NaV1.3 (
; n=8), NaV1.3 (
 ; n=10), NaV1.4 (
; n=10), NaV1.4 (
 ; n=6), NaV1.5 (
; n=6), NaV1.5 (
 ; n=10), NaV1.6 (
; n=10), NaV1.6 (
 ; n=11), NaV1.7 (
; n=11), NaV1.7 (
 ; n=7) and NaV1.8 (
; n=7) and NaV1.8 (
 ; n=7). A hyperbola was fitted to pooled averages. Insets show the mean rc values in the presence of DMSO vehicle (black bars) or NP (coloured bars as described above). Data points are mean±SEM. Statistical significance is marked as *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001
; n=7). A hyperbola was fitted to pooled averages. Insets show the mean rc values in the presence of DMSO vehicle (black bars) or NP (coloured bars as described above). Data points are mean±SEM. Statistical significance is marked as *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001
NP potently inhibits voltage-gated sodium channels
Lastly, we determined the potency of NP on inhibition of peak current amplitude across all eight NaV channel isoforms. We constructed concentration-response curves in cells sequentially exposed to NP (0.03–9 μg NP/mL) to calculate IC50 values (Fig. 4). NP potently inhibits sodium currents through all sodium channel subtypes, except NaV1.8, with IC50 values ranging from 1.6 to 4.2 μg NP/mL (Table 1). No concentration-dependent inhibition of peak current amplitude was observed with hemp seed oil for any of the channel isoforms (Additional file 7). As CBD was the most abundant cannabinoid in the NP, we determined the IC50 values of purified CBD at a subset of the sodium channels NaV1.1, NaV1.2, NaV1.6 and NaV1.7 (Fig. 5). The results revealed IC50 values ranging from 11.9 to 18.5 μM (Table 2). Using the IC50 values for NP, we derived the equivalent molar IC50 of CBD found in NP for individual cells and compared them with purified CBD IC50 values for the sodium channels NaV1.1 (IC50 (μM): NP 0.169±0.02, CBD 18.5± 2.2, p=0.0002), NaV1.2 (IC50 (μM): NP 0.245±0.03, CBD 18.4±2.6, p=0.0001), NaV1.6 (IC50 (μM): NP 0.099±0.02, CBD 16.6±1.8, p=0.001) and NaV1.7 (IC50 (μM): NP 0.095±0.001, CBD 11.9±2.2, p=0.0001).
Discussion
Preclinical and clinical studies show CBD is anticonvulsant; however, its mechanism of action is unclear and likely involves numerous epilepsy drug targets (Devinsky et al. 2016; Pisanti et al. 2017). Many clinically effective anticonvulsants inhibit NaV channels, and purified CBD non-selectively inhibits these channels (Ghovanloo et al. 2018). However, as cannabis contains 500 phytochemicals including phytocannabinoids and terpenes, many cannabis-based products being used to treat epilepsy contain constituents beyond CBD (Nuutinen 2018). Our study shows that a CBD-dominant nutraceutical product that contains a mixture of phytocannabinoids and terpenes potently modulated various functional properties of the sodium channel subtypes NaV1.1–NaV1.8.
The IC50 values of the hemp-derived NP across NaV1.1-NaV1.7 ranged from 1.6 to 4.2 μg NP/mL, which equates to CBD IC50 values of 93–245 nM. Our data show that purified CBD inhibited a subset of NaV channels with IC50 values in the micromolar range. Thus, NP is an order of magnitude more potent than purified CBD at these NaV channels, implying that either another component of the NP potently inhibits the channels or that the various components within the NP work cooperatively to inhibit channel function. Whether the other phytocannabinoid or terpene constituents of NP alone directly affect NaV channel subtypes is unknown, although there is evidence that Δ9-THC inhibits sodium channels at micromolar concentrations (Turkanis et al. 1991) and linalool inhibits NaV channels at high micromolar concentrations (Leal-Cardoso et al. 2010). Mounting evidence supports synergistic interactions between the many molecular constituents of cannabis-derived products, consistent with the notion of an “entourage effect” (Russo 2019; Russo 2011). Future studies could explore whether an “entourage effect” or synergism between components within the NP contributes to its inhibition of NaV channel function.
NP (3 μg NP/mL) inhibited peak current amplitude across this superfamily of human sodium channels, with the exception NaV1.8, where no change was seen, and NaV1.3 where peak current amplitude was enhanced. However, hemp seed oil vehicle (3 μg hemp seed oil/mL) also enhanced peak current amplitude of NaV1.3 suggesting the effect was not specific to the NP. While postnatal expression of NaV1.3 is very low in rodent brain tissue, human tissue distribution studies have shown that NaV1.3 is retained in the adult brain (Cheah et al. 2013; Gazina et al. 2010). In adult brain, NaV1.3 expression is present in both excitatory neurons and inhibitory interneurons (Whitaker et al. 2001). Further, de novo SCN3A that are both GOF and LOF have been implicated in childhood epilepsies (Chen et al. 2015; Holland et al. 2008; Lamar et al. 2017; Vanoye et al. 2014). NP and/or hemp seed oil could provide a potential therapeutic benefit in patients with LOF NaV1.3 mutations. NP had no effect on peak current amplitude of NaV1.8, which is in contrast to previous work that shows both tonic and use-dependent inhibition of NaV1.8 channels in murine primary sensory neurons by 2 μM CBD (Zhang and Bean 2021). Since the highest concentration of CBD examined in the NP was only 175 nM, it may explain why no effect was observed. Future studies could also determine whether interactions between NP and sodium channels exhibit state-dependence.
NP had variable effects on the biophysical properties of activation, steady-state fast inactivation and recovery from fast inactivation for the channel isoforms. NP did not affect the activation properties of NaV1.1, NaV1.2, NaV1.4 or NaV1.7; however, it did cause hyperpolarizing shifts in the midpoint of activation for NaV1.3 and NaV1.8, indicative of enhanced channel availability. A depolarizing shift in the conductance curve for NaV1.5 was observed with NP, thus impeding channel function. The overall inhibitory effects of NP on NaV1.5, the cardiac sodium channel, provides some cause for concern. However, purified CBD is well tolerated in humans with no clinically significant ECG changes even at peak plasma concentrations >2 μM, consistent with purified CBD only modestly inhibiting NaV1.5 (IC50 of 3.8 μM) (Ghovanloo et al. 2018). While there is scant data available evaluating plasma exposure levels in patients using nutraceutical hemp extracts, a recent clinical trial reported that a CBD-dominant cannabis herbal extract attained a plasma concentration of ~200 nM in childhood epilepsy patients when dosed up to 10–12 mg/kg CBD per day (Huntsman et al. 2019). No serious adverse events were reported in this study; however, it would be prudent for future safety studies administering CBD-dominant cannabis based-products to incorporate ECG measures. However, it is unlikely that this product would affect sodium channels when administered as a nutraceutical product where a common dosage is 15 mg CBD twice daily, amounting to a ~0.5 mg/kg per day CBD dose (Capano et al. 2020). NP treatment resulted in hyperpolarizing shifts in the midpoint of inactivation for all channel subtypes, except NaV1.2. Hyperpolarizing shifts of channel inactivation mean that the channels move into an inactivated state more readily, indicative of the channels being functionally inhibited.
Recovery from steady-state fast inactivation, for NaV1.1, NaV1.2, NaV1.4, NaV1.5, NaV1.6 and NaV1.7, was significantly slower in the presence of NP and is also consistent with functional inhibition of these channels. Notably, these effects appear to be independent of the NP as the hemp seed oil vehicle produced the same pattern of results on channel inactivation, suggesting other components of the hemp seed oil, which is devoid of phytocannabinoids and terpenes, exert these effects.
Our results suggest that NP might be further explored to treat epilepsies which are sensitive to inhibition of voltage-gated sodium channels, including LGS where GOF mutations have been identified in SCN2A (NaV1.2) and SCN8A (NaV1.6) (Epi4K. 2013). However, it is difficult to reconcile our observation that NP inhibits NaV1.1 channel function with research suggesting that CBD-dominant hemp extracts have anticonvulsant effects in DS patients who in 80% of cases have a LOF SCN1A mutation (NaV1.1) (Depienne et al. 2009). Use of sodium channel inhibitors is contraindicated in DS because inhibiting NaV1.1 would exacerbate LOF SCN1A mutations by reducing the neuronal inhibition exerted by GABAergic interneurons (Ogiwara et al. 2007; Stein et al. 2019). Interestingly, in DS patient-derived pluripotent stem cells, 50 nM CBD attenuated the excitability of excitatory neurons and potentiated the excitability of inhibitory neurons, opposing effects resulting in reestablishment of normal network activity (Sun and Dolmetsch 2018). Furthermore, therapies that potentiate NaV1.1 channel function abolish seizures and reduce mortality in a mouse model of DS (Richards et al. 2018). It remains possible that the promiscuous target activity of the cannabinoids and terpenes in NP affects other epilepsy-relevant drug targets such as GABAA receptors (Anderson et al. 2019a; Bakas et al. 2017), G-protein coupled receptor 55 (GPR55) (Kaplan et al. 2017) and TRPV1 channels (Gray et al. 2020) which overshadow any deleterious effects of NaV1.1 channel inhibition. Alternatively, the brain concentrations of the NP might be insufficient to inhibit Nav1.1 in vivo.
Conclusion
Our present findings show that a CBD-dominant hemp-derived product potently inhibited NaV channels. NP appeared to more potently inhibit NaV channels based on its CBD content than purified CBD, although this could engender both increased therapeutic efficacy and side-effects in epilepsy patients. Future functional characterization of the components of NP, including phytocannabinoids and terpenes, may reveal a potent constituent or interactions between components to modulate sodium channels.
Availability of data and materials
The data used for this study are available from the corresponding author on reasonable request.
Abbreviations
- CBC:
-
Cannabichromene
- CBD:
-
Cannabidiol
- CBDA:
-
Cannabidiolic acid
- CBDV:
-
Cannabidivarin
- CBDVA:
-
Cannabidivarinic acid
- CBG:
-
Cannabigerol
- CBGA:
-
Cannabigerolic acid
- CBN:
-
Cannabinol
- DS:
-
Dravet syndrome
- GOF:
-
Gain-of-function
- IC50 :
-
Half-maximal inhibitory concentration
- ipi:
-
Interpulse intervals
- LGS:
-
Lennox-Gastaut syndrome
- LOF:
-
Loss-of-function
- NaV :
-
Voltage-gated sodium
- NP:
-
Nutraceutical product
- SSFI:
-
Steady-state fast inactivation
- Δ9-THC:
-
Δ9-Tetrahydrocannabinol
- THCA:
-
Δ9-Tetrahydrocannabinolic acid
- THCV:
-
Δ9-Tetrahydrocannabidvarin
- THCVA:
-
Δ9-Tetrahydrocannabidvarinic acid
- V0.5 act:
-
Voltage-dependence of half-activation
- V0.5 inact:
-
Voltage-dependence of half-inactivation
References
Anderson LL, Absalom NL, Abelev SV, Low IK, Doohan PT, Martin LJ, et al. Coadministered cannabidiol and clobazam: preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia. 2019a;60(11):2224–34.
Anderson LL, Low IK, Banister SD, McGregor IS, Arnold JC. Pharmacokinetics of phytocannabinoid acids and anticonvulsant effect of cannabidiolic acid in a mouse model of Dravet syndrome. J Nat Prod. 2019b;82(11):3047–55.
Bakas T, van Nieuwenhuijzen PS, Devenish SO, McGregor IS, Arnold JC, Chebib M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol Res. 2017;119:358–70.
Berecki G, Bryson A, Terhag J, Maljevic S, Gazina EV, Hill SL, et al. SCN1A gain of function in early infantile encephalopathy. Ann Neurol. 2019;85(4):514–25.
Berecki G, Howell KB, Deerasooriya YH, Cilio MR, Oliva MK, Kaplan D, et al. Dynamic action potential clamp predicts functional separation in mild familial and severe de novo forms of SCN2A epilepsy. Proc Natl Acad Sci United States America. 2018;115(24):E5516–25.
Berkovic SF, Mulley JC, Scheffer IE, Petrou S. Human epilepsies: interaction of genetic and acquired factors. Trends Neurosci. 2006;29(7):391–7.
Blanchard MG, Willemsen MH, Walker JB, Dib-Hajj SD, Waxman SG, Jongmans MC, et al. De novo gain-of-function and loss-of-function mutations of SCN8A in patients with intellectual disabilities and epilepsy. J Med Genet. 2015;52(5):330–7.
Brunklaus A, Zuberi SM. Dravet syndrome--from epileptic encephalopathy to channelopathy. Epilepsia. 2014;55(7):979–84.
Capano A, Weaver R, Burkman E. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: a prospective cohort study. Postgrad Med. 2020;132(1):56–61.
Cheah CS, Westenbroek RE, Roden WH, Kalume F, Oakley JC, Jansen LA, et al. Correlations in timing of sodium channel expression, epilepsy, and sudden death in Dravet syndrome. Channels (Austin, Tex.). 2013;7(6):468–72.
Chen YJ, Shi YW, Xu HQ, Chen ML, Gao MM, Sun WW, et al. Electrophysiological Differences between the Same Pore Region Mutation in SCN1A and SCN3A. Mol Neurobiol. 2015;51(3):1263–70.
de Kovel CG, Meisler MH, Brilstra EH, van Berkestijn FM, van’t Slot R, van Lieshout S, et al. Characterization of a de novo SCN8A mutation in a patient with epileptic encephalopathy. Epilepsy Res. 2014;108(9):1511–8.
Depienne C, Trouillard O, Saint-Martin C, Gourfinkel-An I, Bouteiller D, Carpentier W, et al. Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet. 2009;46(3):183–91.
Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet. Neurol. 2016;15(3):270–8.
Djemie T, Weckhuysen S, von Spiczak S, Carvill GL, Jaehn J, Anttonen AK, et al. Pitfalls in genetic testing: the story of missed SCN1A mutations. Mol Genet Genomic Med. 2016;4(4):457–64.
Duan Y, Liao C, Jain S, Nicholson RA. The cannabinoid receptor agonist CP-55,940 and ethyl arachidonate interfere with [(3) H]batrachotoxinin A 20 alpha-benzoate binding to sodium channels and inhibit sodium channel function. Comparative biochemistry and physiology. Toxicol Pharmacol. 2008;148(3):244–9.
Epi4K. De novo mutations in epileptic encephalopathies. Nature. 2013;501(7466):217–21.
Estacion M, O'Brien JE, Conravey A, Hammer MF, Waxman SG, Dib-Hajj SD, et al. A novel de novo mutation of SCN8A (Nav1.6) with enhanced channel activation in a child with epileptic encephalopathy. Neurobiol Dis. 2014;69:117–23.
Gazina EV, Richards KL, Mokhtar MB, Thomas EA, Reid CA, Petrou S. Differential expression of exon 5 splice variants of sodium channel alpha subunit mRNAs in the developing mouse brain. Neuroscience. 2010;166(1):195–200.
Ghovanloo MR, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J Biol Chem. 2018;293(43):16546–58.
Gray RA, Stott CG, Jones NA, Di Marzo V, Whalley BJ. Anticonvulsive Properties of Cannabidiol in a Model of Generalized Seizure Are Transient Receptor Potential Vanilloid 1 Dependent. Cannabis Cannabinoid Res. 2020;5(2):145–9.
Gray RA, Whalley BJ. The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord. 2020;22(S1):10–5.
Holland KD, Kearney JA, Glauser TA, Buck G, Keddache M, Blankston JR, et al. Mutation of sodium channel SCN3A in a patient with cryptogenic pediatric partial epilepsy. Neurosci Lett. 2008;433(1):65–70.
Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12(8):996–1002.
Huntsman RJ, Tang-Wai R, Alcorn J, Vuong S, Acton B, Corley S, et al. Dosage related efficacy and tolerability of cannabidiol in children with treatment-resistant epileptic encephalopathy: preliminary results of the CARE-E study. Front Neurol. 2019;10:716.
Kaplan JS, Stella N, Catterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2017;114(42):11229–34.
Knapp O, Nevin ST, Yasuda T, Lawrence N, Lewis RJ, Adams DJ. Biophysical properties of Na(v) 1.8/Na(v) 1.2 chimeras and inhibition by microO-conotoxin MrVIB. Bri J Pharmacol. 2012;166(7):2148–60.
Lamar T, Vanoye CG, Calhoun J, Wong JC, Dutton SBB, Jorge BS, et al. SCN3A deficiency associated with increased seizure susceptibility. Neurobiol Dis. 2017;102:38–48.
Leal-Cardoso JH, da Silva-Alves KS, Ferreira-da-Silva FW, dos Santos-Nascimento T, Joca HC, de Macedo FH, et al. Linalool blocks excitability in peripheral nerves and voltage-dependent Na+ current in dissociated dorsal root ganglia neurons. Eur J Pharmacol. 2010;645(1-3):86–93.
Miller LL, Pellock JM, DeLorenzo RJ, Meyer JM, Corey LA. Univariate genetic analyses of epilepsy and seizures in a population-based twin study: the Virginia Twin Registry. Genet Epidemiol. 1998;15(1):33–49.
Nuutinen T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur J Med Chem. 2018;157:198–228.
Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27(22):5903–14.
Okada Y, Imendra KG, Miyazaki T, Hotokezaka H, Fujiyama R, Zeredo JL, et al. Biophysical properties of voltage-gated Na+ channels in frog parathyroid cells and their modulation by cannabinoids. J Exp Biol. 2005;208(Pt 24):4747–56.
Parrini E, Marini C, Mei D, Galuppi A, Cellini E, Pucatti D, et al. Diagnostic targeted resequencing in 349 patients with drug-resistant pediatric epilepsies identifies causative mutations in 30 different genes. Hum Mutat. 2017;38(2):216–25.
Perucca P, Perucca E. Identifying mutations in epilepsy genes: impact on treatment selection. Epilepsy Res. 2019;152:18–30.
Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Therapeut. 2017;175:133–50.
Richards KL, Milligan CJ, Richardson RJ, Jancovski N, Grunnet M, Jacobson LH, et al. Selective NaV1.1 activation rescues Dravet syndrome mice from seizures and premature death. Proc Natl Acad Sci U S A. 2018;115(34):E8077–85.
Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: no “strain,” no gain. Front Plant Sci. 2019;9:1969. https://doi.org/10.3389/fpls.2018.01969. eCollection 2018.
Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Bri J Pharmacol. 2011;163(7):1344–64.
Speed D, O'Brien TJ, Palotie A, Shkura K, Marson AG, Balding DJ, et al. Describing the genetic architecture of epilepsy through heritability analysis. Brain. 2014;137:2680–9.
Stein RE, Kaplan JS, Li J, Catterall WA. Hippocampal deletion of NaV1.1 channels in mice causes thermal seizures and cognitive deficit characteristic of Dravet Syndrome. Proc Natl Acad Sci U S A. 2019;116(33):16571–6.
Sun Y, Dolmetsch RE. Investigating the therapeutic mechanism of cannabidiol in human induced pluripotent stem cell (iPSC)-based model of Dravet syndrome. 2018. https://doi.org/10.1101/sqb.2018.83.038174.
Suraev A, Lintzeris N, Stuart J, Kevin RC, Blackburn R, Richards E, et al. Composition and use of cannabis extracts for childhood epilepsy in the Australian community. Sci Rep. 2018;8(1):10154.
Suraev AS, Todd L, Bowen MT, Allsop DJ, McGregor IS, Ireland C, et al. An Australian nationwide survey on medicinal cannabis use for epilepsy: History of antiepileptic drug treatment predicts medicinal cannabis use. Epilepsy Behav. 2017;70(Pt B):334–40.
Turkanis SA, Partlow LM, Karler R. Delta-9-tetrahydrocannabinol depresses inward sodium current in mouse neuroblastoma cells. Neuropharmacology. 1991;30(1):73–7.
Vanoye CG, Gurnett CA, Holland KD, George AL Jr, Kearney JA. Novel SCN3A variants associated with focal epilepsy in children. Neurobiol Dis. 2014;62:313–22.
Veeramah KR, O'Brien JE, Meisler MH, Cheng X, Dib-Hajj SD, Waxman SG, et al. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am J Hum Genet. 2012;90(3):502–10.
Watkins AR. Cannabinoid interactions with ion channels and receptors. Channels (Austin, Tex.). 2019;13(1):162–7.
Whitaker WR, Faull RL, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Brain Res Mol Brain Res. 2001;88(1-2):37–53.
Zhang HB, Bean BP. Cannabidiol inhibition of murine primary nociceptors: tight binding to slow inactivated states of Nav1.8 channels. J Neurosci. 2021;41(30):6371–87.
Acknowledgements
The authors gratefully acknowledge Barry and Joy Lambert for their continued support of the Lambert Initiative for Cannabinoid Therapeutics. In addition, we thank Katelyn Lambert for inspiring our work on novel cannabinoid therapies for childhood epilepsy.
Funding
This study was supported by the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded centre for medicinal cannabis research at the University of Sydney.
Author information
Authors and Affiliations
Contributions
ISM, MTB, JCA, SDB and SP conceived of the study. CJM and SP designed the experiments. CJM performed the functional experiments and analysed the data. CJM, JCA and LLA prepared the manuscript. MTB and SDB provided edits. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
Associate Professor Jonathon Arnold is Deputy Academic Director of the Lambert Initiative. He has served as an expert witness in various medicolegal cases involving cannabis and cannabinoids and served as a temporary advisor to the World Health Organization on their review of cannabis and cannabinoids. Associate Professor Arnold has received consulting fees from Creo Inc. and Medicinal Cannabis Industry Australia (MCIA). Professor Iain McGregor is Academic Director of the Lambert Initiative for Cannabinoid Therapeutics. He has served as an expert witness in various medicolegal cases involving cannabis, has received honoraria from Janssen, is currently a consultant to Kinoxis Therapeutics and has received research funding and fellowship support from the Lambert Initiative, NHMRC and Australian Research Council. He currently sits on medical advisory board of BOD Australia and holds a variety of patents for non-cannabinoid therapeutics. Associate Professor Arnold and Professor McGregor hold patents on cannabinoid therapies (PCT/AU2018/05089 and PCT/AU2019/050554). The nutraceutical product tested in this study was provided by Ananda Professional Pty Ltd, a subsidiary of Ecofibre Limited. Ananda Professional Pty Ltd and Ecofibre Limited played no part in the design, conduct, analysis, writing or decision to publish this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Ananda Hemp 600 certificate of analysis.
Additional file 2.
Effects of hemp seed oil on voltage-dependent sodium channel currents.
Additional file 3.
Effect of hemp seed oil on activation and steady-state fast inactivation (SSFI).
Additional file 4.
NP does not affect the time constant of fast steady-state inactivation.
Additional file 5.
The effect of hemp seed oil on the time constant of fast inactivation.
Additional file 6.
Effect of hemp seed oil on recovery from fast inactivation.
Additional file 7.
Concentration-response curves for hemp seed oil.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Milligan, C.J., Anderson, L.L., Bowen, M.T. et al. A nutraceutical product, extracted from Cannabis sativa, modulates voltage-gated sodium channel function. J Cannabis Res 4, 30 (2022). https://doi.org/10.1186/s42238-022-00136-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42238-022-00136-x



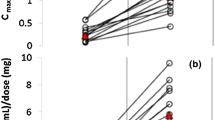




 ; n=6), NaV1.2 (
; n=6), NaV1.2 (
 ; n=6), NaV1.3 (
; n=6), NaV1.3 (
 ; n=6), NaV1.4 (
; n=6), NaV1.4 (
 ; n=6), NaV1.5 (
; n=6), NaV1.5 (
 ; n=6), NaV1.6 (
; n=6), NaV1.6 (
 ; n=7), NaV1.7 (
; n=7), NaV1.7 (
 ; n=8) and NaV1.8 (
; n=8) and NaV1.8 (
 ; n=6). Data points are mean±SEM
; n=6). Data points are mean±SEM
 ; n=9), NaV1.2 (
; n=9), NaV1.2 (
 ; n=6), NaV1.6 (
; n=6), NaV1.6 (
 ; n=7) and NaV1.7 (
; n=7) and NaV1.7 (
 ; n=7). Data points are mean±SEM
; n=7). Data points are mean±SEM