Abstract
Background
Despite marked progress in endovascular treatment (EVT) techniques and devices, calcified lesions remain one of the toughest obstacles to EVT success. Moreover, because the common femoral artery (CFA) is known as a “non-stenting zone,” endovascular strategies for this area are controversial.
Case presentation
Here we describe the technical tips for a novel, less invasive, and effective debulking strategy for severe nodular calcification using an endovascular maneuver. This technique was demonstrated in a 73-year-old man with severe calcified stenosis of the CFA. To complete a stent-less strategy for CFA, we conducted aggressive debulking of the nodular calcification, established a bidirectional approach from the radial artery and the superficial femoral artery (SFA), and inserted a balloon-guiding catheter in the SFA. Under distal protection provided by this catheter, we crushed the nodular calcification 43 times using myocardial biopsy forceps. After achieving a volume reduction of nodular calcification through this maneuver, we completed the procedure by inflating a 6-mm drug-coated balloon catheter. Final angiography demonstrated a reduced filling defect of the contrast medium in the CFA and favorable blood flow as far as the ankle. The puncture site on the SFA was closed with a vascular suture assisted by balloon inflation inside the vessel, which allowed the patient to be ambulatory immediately after the procedure without requiring bed rest.
Conclusions
Severely calcified lesions in the CFA are usually difficult to treat using an endovascular strategy, but our novel and less invasive method may become a promising technique for managing these lesions.
Similar content being viewed by others
Introduction
Although marked progress has been made in the field of endovascular treatment (EVT) for peripheral artery diseases, calcified lesions remain a critical issue to obtain optimal EVT results (Cunnane et al. 2015). Atherectomy devices have recently become adjunctively important to debulk plaque within vessels, especially heavily calcified plaques, and greater plaque volume reduction usually results in more efficient balloon angioplasty and/or stenting. Furthermore, lesions in the common femoral artery (CFA) are usually treated with endarterectomy by vascular surgeons. However, some drawbacks of common femoral endarterectomy (CFE), such as wound complications, have been reported (Nguyen et al. 2015).
Case presentation
A 73-year-old man with silent myocardial ischemia, hypertension, and dyslipidemia presented with claudication of the right foot (Rutherford class 3) after walking for 100 m. He was taking drugs for comorbidities and dual antiplatelet therapy for former coronary interventions. The right and left ankle-brachial pressure index (ABI) was 0.52 and 0.88, respectively. Ultrasound of the lower-extremity arteries showed a severe acoustic shadow in the right CFA, while a pulsed Doppler assessment distal to the CFA revealed an impaired flow pattern. Guidelines suggest CFE as the first-line treatment for this lesion type (Aboyans et al. 2017), but the patient desired less invasive EVT instead. Therefore, we performed EVT for the CFA lesions with severe nodular calcifications.
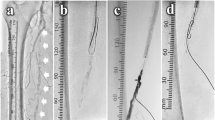
We used the left radial artery (RA) because of heavy calcification at the puncture site in the contralateral CFA. A 7-Fr Glidesheath Slender (Terumo Corp., Tokyo, Japan) was inserted in the left RA. Immediately thereafter, an intra-arterial bolus of 5,000 units of unfractionated heparin was administered, and the activated clotting time was controlled to > 250 s. A 150-cm-long R2P SlenGuide guiding catheter (Terumo Corp., Tokyo, Japan), was placed just proximal to the left CFA. Initial angiography showed a clear filling defect of the contrast medium in the CFA (Fig. 1 A, B). Next, we punctured the proximal superficial femoral artery (SFA) under duplex ultrasound guidance and inserted a balloon-guiding catheter (OPTIMO PPI; Tokai Medical Products, Inc., Aichi, Japan) to enable ipsilateral retrograde access. After retrogradely advancing the floppy guidewire and avoiding the nodular calcification, we performed intravascular ultrasonography (IVUS) and angioscopy, which showed a mass of calcification protruding into the vascular lumen (Fig. 1 C, D). We crunched crushed the nodular calcification 43 times using myocardial biopsy forceps under distal protection provided by the OPTIMO PPI (Fig. 2 A, B; Videos 1, 2). We fully aspirated the debris trapped by the OPTIMO balloon through the catheter every 10 crushes. The calcified mass was notably shrunk on fluoroscopy, and IVUS showed favorable luminal enlargement (Fig. 2 C). Nodular calcification volume reduction was observed on angioscopy (Fig. 2D).
Finally, we inflated a 6-mm drug-coated balloon catheter after inflating a 6-mm scoring balloon catheter (Fig. 3 A). The SFA puncture site was closed with a Perclose ProGlide Suture-Mediated Closure System (Abbott Laboratories, Lake Bluff, IL, USA) assisted by balloon inflation (Senri; Terumo Corp., Tokyo, Japan) inside the vessel for 3 min (Fig. 3B), which allowed the patient to be ambulatory immediately after the procedure without requiring bed rest. The final angiography demonstrated a reduced filling defect of contrast medium in the CFA (Fig. 3 C) and favorable blood flow below the ankle (Fig. 3D). The collected specimens had various sizes and rigidities, and the maximum sample diameter was approximately 3.2 mm (Fig. 4 A). Histopathology with hematoxylin and eosin staining showed calcified plaques mixed with fibrin deposition to various extents (Fig. 4B). After treatment, the right ABI recovered to 0.89 and the claudication resolved completely (Rutherford class 0). After discharge, the patient continued the same prescriptions. One month later, the right ABI remained at 0.95, and no symptoms of claudication were observed (Rutherford class 0).
A Inflation of a 6-mm drug-coated balloon catheter in the common femoral artery (CFA). B Hemostasis of the puncture site of the superficial femoral artery with vascular suture assisted by balloon inflation inside the vessel. Final angiography image demonstrating a reduced filling defect of contrast medium in the CFA (C) and favorable blood flow as far as below the ankle (D)
Discussion
Here we demonstrated a less invasive and successful EVT procedure for severe nodular calcification in CFA. Atherectomy devices are adjunctively important for debulking heavily calcified plaques (Panaich et al. 2016), and a greater plaque volume reduction usually results in more efficient balloon angioplasty and/or stenting (Böhme et al. 2021; Hassan et al. 2021). Various types of debulking devices are now available in most countries, but unfortunately, none designed for EVT are covered by insurance in our country. Therefore, we devised this original method to obtain favorable vessel dilation for severe nodular calcifications in the CFA as an alternative to conventional atherectomy devices.
Our strategy has three strengths. First, the use of myocardial biopsy forceps enabled repetitive and effective debulking despite the presence of a hard calcified mass. However, vessel rupture and distal embolism are major complications requiring prevention. In this regard, owing to the closer position between the balloon-guiding catheter and the targeted plaque, we easily delivered the forceps just distal to the target, intentionally targeting the plaque and optimizing their use. To ensure safe debulking, clinicians should occasionally assess the lesion using intravascular imaging modalities and determine the maneuver’s endpoint. Of course, we must acknowledge that our method’s use of biopsy forceps is technically off-label.
Second, the OPTIMO PPI balloon-guiding catheter completely prevented distal embolization. As this balloon-guiding catheter was developed to prevent thromboembolism, applying the system in this case was reasonable. However, we must consider the risk of balloon rupture of this catheter during procedure because continuous friction occurs between the balloon and vessel wall due to the repetitive debulking maneuvers.
Third, avoiding puncturing the bilateral CFA allowed the patient to be ambulatory immediately after the procedure without requiring bed rest. Severe calcification of the CFA often occurs bilaterally, and the contralateral femoral approach was not feasible in our case.
In such circumstances, we can use the R2P system (Terumo Corp., Tokyo, Japan), an EVT device specialized for the trans-radial approach (Shinozaki et al. 2019).
We emphasize that this strategy is very patient-oriented since it leaves nothing behind, the patient does not require postoperative bed rest, and maximum debulking of nodular calcification is achieved. Only one other case report to date described the use of myocardial biopsy forceps for debulking nodular calcification in CFA (Tanaka et al. 2020). However, our method is distinct from that of the previous report since it combines use of the R2P system and the OPTIMO catheter in the proximal SFA, which can contribute to patient comfort and more efficient debulking. We named this procedure the CRUNCH (CRUsh Nodular Calcification with easy Hemostasis management) technique.
Conclusions
A severely calcified lesion in the CFA is usually difficult to treat using an endovascular strategy, but our novel less invasive and effective debulking strategy using myocardial biopsy forceps -called the CRUNCH technique- may become a promising technique for such situations.
Availability of data and materials
Not applicable.
Abbreviations
- ABI:
-
Ankle-brachial pressure index
- CFA:
-
Common femoral artery
- CFE:
-
Common femoral endarterectomy
- EVT:
-
Endovascular treatment
- IVUS:
-
Intravascular ultrasonography
- PAD:
-
Peripheral artery disease
- RA:
-
Radial artery
- SFA:
-
Superficial femoral artery
References
Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T et al (2017) ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS)
Böhme T, Romano L, Macharzina RR, Noory E, Beschorner U, Jacques B et al (2021) Outcomes of directional atherectomy for common femoral artery disease. EuroIntervention 17:260–266
Cunnane EM, Mulvihill JJ, Barrett HE, Healy DA, Kavanagh EG, Walsh SR et al (2015) Mechanical, biological and structural characterization of human atherosclerotic femoral plaque tissue. Acta Biomater 11:295–303
Hassan AE, Nakao M, Katsumata H, Inagaki Y, Tanaka K, Otsuki H et al (2021) Clinical outcomes after balloon angioplasty with crosser device for heavily calcified common femoral and popliteal artery disease. Heart Vessels. doi: https://doi.org/10.1007/s00380-021-01816-z
Nguyen BN, Amdur RL, Abugideiri M, Rahbar R, Neville RF, Sidawy AN (2015) Postoperative complications after common femoral endarterectomy. J Vasc Surg 61:1489–1494
Panaich SS, Arora S, Patel N, Patel NJ, Patel SV, Savani C et al (2016) In-hospital outcomes of atherectomy during endovascular lower extremity revascularization. Am J Cardiol 117:676–684
Shinozaki N, Ikari Y (2019) Superficial femoral artery stenting via radial access using R2P Misago stents: first-in-human report of the new R2P(R) system. SAGE Open Med Case Rep 7:2050313 × 19847348
Tanaka K, Nagai H, Okamura A, Iwamoto M, Watanabe S, Sumiyoshi A et al (2020) Calcified nodules in the superficial femoral artery confirmed by intravascular ultrasound, angioscopy, and histology. J Am Coll Cardiol Case Rep 2:1862–1866
Acknowledgements
We thank Editage (www. Editage.com) for the English language editing.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TN was the primary author of this study. IO, ST, KO, YS, KS, and NW made substantial editorial revisions to this manuscript. NW contributed significantly to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were performed in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration. Informed consent was obtained from the patient.
Consent for publication
The authors confirm that written consent for the submission and publication of this case report, including images and associated text, was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Video 1.
Debulking nodular calcification performed with forceps under distal protection using a balloon-guiding catheter
Additional file 2: Video 2.
Difficulty detaching a calcified hard mass on a large scale using this procedure
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nomura, T., Ota, I., Tasaka, S. et al. Percutaneous debulking strategy for severe nodular calcification in common femoral artery. CVIR Endovasc 5, 25 (2022). https://doi.org/10.1186/s42155-022-00301-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42155-022-00301-6