Abstract
Background
Postoperative pain after laparoscopic cholecystectomy still remains a fairly common challenge for the anesthesiologist. Commonly used drugs such as NSAIDs and opioids have their own side effects requiring a need to substantially evaluate the use of non-pharmacological methods like TENS as adjuvants to conventional methods of pain control. We studied the effect of transcutaneous electrical nerve stimulation (TENS) on postoperative pain in laparoscopic cholecystectomy. The primary objective was to compare postoperative analgesia between TENS and placebo groups using visual analog scale (VAS). The secondary objectives were to compare the requirement and dosage of rescue analgesia, hemodynamic parameters (blood pressure and heart rate), and incidence of nausea and emesis between placebo and TENS groups. A total of 64 patients of age group 18 years to 60 years of either gender posted for elective laparoscopic cholecystectomy were randomly allocated into two groups using a computer-generated sequence of random numbers: Group P (n= 32): placebo TENS and Group A (n= 32): active TENS. Statistical analysis was performed by the SPSS program for Windows, version 17.0 (SPSS, Chicago, Illinois). Unpaired t test, Mann-Whitney U test, chi-square test, and Fisher’s exact test were used for statistical analysis.
Results
The two groups were statistically similar in terms of age, gender, and weight. The VAS scores were significantly lower in patients in the active TENS group for up to 2 h after surgery, and the total weighted dose of diclofenac consumed over 8 h in the active TENS group was significantly lower as compared to the placebo TENS group. Patients who received TENS showed significantly less rise in blood pressure and heart rate and remained hemodynamically stable. Total episodes of nausea and emesis though less in the active TENS group were statistically insignificant.
Conclusion
We conclude that TENS is an effective adjuvant non-pharmacologic modality for postoperative pain relief after laparoscopic cholecystectomy.
Similar content being viewed by others
Background
Laparoscopic cholecystectomy is indicated for various diseases of the gallbladder and is one of the most commonly performed procedures all over the world [Javid and Brooks 2004]. It is a widely preferred procedure over open cholecystectomy by both surgeons and patients due to its various advantages such as lesser postoperative pain, faster recovery time, and shorter hospital stay. But like any other surgical procedure, it comes with its own challenges which are dealt by the anesthesiologist. One such challenge happens to be postoperative pain whose origins and nature are yet to be fully understood. Nausea and vomiting are the most commonly reported postoperative complaint after pain (Lee et al. 2001). Interestingly, post-laparoscopic cholecystectomy pain does not resemble the postoperative pain that is reported after other laparoscopic procedures (Bisgaard et al. 2001a, b, c; Bisgaard et al. 2001a, b, c; Wills and Hunt 2000). Acute pain after laparoscopic cholecystectomy has three components: incisional (somatic), deep abdominal (visceral), and shoulder pain (presumably referred visceral pain) (Bisgaard et al. 2001a, b, c). The causative factors range from the stretching of the diaphragm and peritoneum due to gas insufflation, to irritants like blood, bile, and pus (O’Hanlon et al. 2002). Treating postoperative pain with drugs like opioids is the common practice which in turn causes adverse effects like sedation, respiratory depression, urinary retention, nausea, and vomiting (White 1995; Za´rate 2001). This problem gets confounded as pain itself plays a role in increasing postoperative nausea and vomiting (PONV) in these patients and is the main reason for prolonged hospital stay (Bisgaard et al. 2001a, b, c; Lau and Brooks 2001) and convalescence (Bisgaard et al. 2001a, b, c; Bisgaard et al. 2001a, b, c). Intense acute pain after the procedure has also been associated with the development of chronic pain and post-laparoscopic cholecystectomy syndrome (Bisgaard et al. 2001a, b, c). Non-steroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase -2 (COX-2) inhibitor treatment resulted in higher opioid sparing (approximately 30%) compared to other classes of drugs and significantly reduced PONV and sedation by approximately 30%, though the effects on urinary retention and respiratory problems were inconclusive. The benefits of opioid-sparing in certain cases were outweighed by the adverse effects such as bleeding with NSAIDs and cardiovascular complications in certain high-risk patients with COX-2 inhibitors. Non-pharmacological methods have been successfully used in both chronic and acute pain, including postoperative pain. The mechanism involves modulation of neural conduction as described in Melzak and Wall’s gate control theory as well as the release of endogenous opioids at the level of the central nervous system, the spinal cord specifically (Melzak and Wall 1965; Rushdon 2002). This study evaluated not only the effectiveness of TENS for pain relief and hemodynamic stability but also its potential to decrease the use of pharmacologic analgesic methods along with its effect on nausea and vomiting in patients. Transcutaneous electrical nerve stimulation (TENS) has been shown to have definite benefits as a complementary therapy in managing acute postoperative pain and has no side effects, unless and until contraindicated in particular patients(Javid and Brooks 2004; Lee et al. 2001 ) Effective TENS analgesia can facilitate early post-operative recovery and reduce hospital stay.
Methods
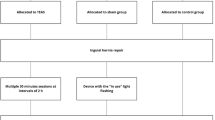
After due clearance from the hospital ethical committee, the prospective interventional comparative study was conducted in our institution as per the study protocol submitted to the board. Informed and written consent was obtained from all patients for inclusion in the study. A total of 64 American Association of Anesthesiologists (ASA) Grade 1 and 2 patients in the age group 18 years to 60 years of either gender posted for elective laparoscopic cholecystectomy were randomly allocated to one of the two groups using a computer-generated sequence of random numbers as follows: Group P (n= 32): Patients receiving sham TENS or placebo TENS and Group A (n= 32): Patients receiving active TENS for 45 min at predetermined amplitude and at 100 Hz. Patients with intra-surgical complications, history of seizures, motion sickness, neurological disease, neuropathy, and pacemakers were excluded from the study. Patients were informed about the study procedure and that the postoperative pain would be measured using a visual analog scale (VAS). The VAS consisted of a 10-cm horizontal line with the words “no pain” written on the left end of the scale and the words “worst pain possible” written on the right end of the scale. The patient was asked to mark a line at a place corresponding to his or her pain level. Subjects were informed that two types of TENS therapy will be tested, one in which little or no sensation will be felt and one in which a strong sensation will be felt. Standard general anesthetic technique was used with injection fentanyl (2mcg/kg), injection propofol (2mg/kg), and injection vecuronium (0.08mg/kg) followed by intubation with appropriate-sized endotracheal tube. Following intubation, HR, ECG, SpO2, and BP were monitored throughout the surgery. Maintenance of anesthesia was achieved with N2O (66%), O2 (33%), and sevoflurane (1–2%). Residual neuromuscular blockade was reversed with neostigmine 0.05mg/kg and glycopyrrolate 0.01mg/kg at the end of surgery. Just prior to extubation all patients participating in the study were given injection paracetamol 1000mg intravenously. In TENS Group A, electrodes were placed at the site of pain in the recovery room and the TENS equipment was applied for 45 min. The generator was connected by lead wires to two sterile electrodes with a pre-gelled contact surface. These electrodes were placed parallel to and on either side of the incisions through which trocars were inserted by the surgical team, approximately 2 to 3 in. away. This distance was recommended by Mannheimer and Lampe, as most pain from the surgical incision is from trauma to tissue and muscle (as a result of surgical retraction) and not at the incision site itself (Mannheimer and Lampe 1984). The intensity of TENS was increased if the patient informed us about any accommodation. In the placebo TENS Group P, electrodes were placed in a similar manner as described for Group A but no electrical current was applied. In all patients, blood pressure, heart rate, and VAS scores were measured at 0 min (T1) and after 45 min (T2) of TENS. Subsequent data was collected at 2, 4, 6, and 8 h, i.e., T3, T4, T5, and T6, respectively. Pain assessment VAS scores, hemodynamic parameters, and rescue analgesia dose at T1, T2, T3, T4, T5, and T6 were noted. Rescue analgesia with injection of diclofenac 75 mg IM was given after TENS if any patient gave a score of more than 3 on VAS at any point of time during the next 8 h (Fig. 1).
Statistical analysis
The sample size was determined based on a previous study, assuming a difference of 1 in postoperative VAS between the two groups as clinically significant. A sample size of minimum of 28 patients per group was calculated with an effect size of 0.67, at an alpha error of 0.05 and power of 90% (Silva et al. 2012 ). Statistical analysis was performed by the SPSS program for Windows, version 17.0 (SPSS, Chicago, Illinois). Continuous variables were presented as mean ± SD, and categorical variables were presented as absolute numbers and percentage. Data was also checked for normality before applying tests for statistical analysis. Normally distributed continuous variables were compared using the unpaired t test. Variables not normally distributed were tested with the Mann-Whitney U test. Chi-square test or Fisher’s exact test was used to analyze categorical variables. A P value <0.05 was considered statistically significant.
Results
The two groups were statistically similar in terms of age, gender (female predominance), and weight (Table 1).
The mean systolic blood pressure was significantly reduced at 45 min and 2 h post-TENS application in Group A as compared to Group P (p<0.001) (Table 2 and Fig. 2).
The mean diastolic blood pressure also followed a similar trend with Group P demonstrating significantly reduced mean diastolic pressure at 45min, 2 h, and 4h (Table 3 and Fig. 3).
Compared to Group P, the heart rate was lower after applying TENS at T2, T3, and T4 in Group A with p values of 0.012, 0.017, and <0.001, respectively (Table 4 and Fig. 4).
The VAS scores were significantly lower in patients in the active TENS group for up to 2 h after surgery, and the total weighted dose of diclofenac consumed over 8 h in the active TENS group was significantly lower as compared to the placebo TENS group. Total episodes of nausea and emesis though less in the active TENS group were statistically insignificant (Table 5, Fig. 5, Table 6 and Fig. 6).
Discussion
Laparoscopic cholecystectomy is one of the most commonly performed surgical procedures all over the world and still remains a major cause of postoperative pain. Our objective was to assess if something as simple and non-invasive like TENS can benefit patients undergoing laparoscopic cholecystectomy. Kara et al. observed significantly lower pain scores in patients who received both TENS and patient-controlled analgesia (PCA) as compared to PCA alone (p<0.05) (Kara et al. 2011). Eidy et al. in 2016 evaluated the effects of preoperative TENS on post-inguinal hernia repair pain and showed that the mean estimated postoperative pain intensity was significantly reduced at 2 and 4 h after the surgery (3.54± 1.48 and 5.12±1.41 (p < 0.001), respectively) (Eidy et al. 2016). In our study, we observed significantly lower VAS scores in patients who received TENS at 45 min and 2 h after surgery. We found that the differences were statistically significant with p values of 0.002 at both 45 min and at 2 h. Subsequent VAS scores were also lower till 8 h, but they were not statistically significant. Hamza et al. observed that morphine requirements were reduced down to 53 % using mixed frequency TENS when compared to the placebo group. They also noted a decrease in morphine-related side effects (Hamza et al. 1991). Bjordal et al. conducted a meta-analysis to analyze if acupuncture like TENS (ALTENS) could reduce analgesic consumption in surgery. They found the mean reduction in analgesic consumption after TENS/ALTENS to be 26.5% (range −6 to +51%) better than the placebo groups in all trials. In nine of the trials, mean weighted analgesic consumption was 4.1% (range −10 to +29%) which was in favor of ALTENS while eleven trials reported a mean weighted reduction in analgesic consumption of 35.5% (range 14–51%) (Bjordal et al. 2003). We also noticed a lower requirement of rescue analgesia doses of diclofenac at 45 min and 2 h. The total dose of diclofenac for the active TENS group was much lower over 8 h (11.72 ± 27.67) when compared to the placebo group (39.84 ± 42.53). This difference was significant with a p value of 0.003. TENS has anti-nociceptive effects similar to acupuncture which can last for much later as was found by Sjolund et al. in 1976 (Sjolund et al. 1976). Oncel et al. concluded that for mild pain after minor rib fractures, TENS can be more effective than NSAIDs (Oncel et al. 2002). TENS is not only a viable adjuvant therapy with pharmacological analgesia but can be the only or main therapy for mild surgical or post-surgical pain, with NSAIDS and opioids reserved for rescue analgesia. Benedetti too underlined the lack of side effects of TENS when compared with conventional opioid and non-opioid analgesics which have side effects like nausea and vomiting. These adverse effects add to the general discomfort of postoperative patients who are already dealing with postoperative pain (Benedetti 1997). TENS itself has the effect of reducing nausea and vomiting as Silva et al. found in their study (Silva et al. 2012). We also found that complaints of nausea and emesis were fewer among patients receiving active TENS (14.3%) as compared with the placebo TENS group (emesis, 23.8%; nausea, 38.1%). Also, the relative risk of nausea and emesis was 2.17 times greater in the placebo TENS group. Although in our active TENS group, the mean of total incidences of nausea or emesis was lower (0.25 ± 0.76) than that in the placebo group (0.56 ± 1.27), but this difference was statistically not significant with a p value of 0.4253. Chandra et al. found that patients who underwent posterolateral thoracotomy for decortication of the lung, reported significantly lesser VAS scores in group II (TENS group) at 2, 4, 6, and 8 h (p < 0.01, p < 0.05, p < 0.05, p < 0.05, respectively). Similarly, the systolic blood pressure was found to be significantly lesser in group II at 2, 4, and 6 h after surgery, with a p < 0.02, p < 0.01, and p < 0.01, respectively (Chandra et al. 2010).
In our study, the mean of systolic blood pressure changes was significant at 45 min and 2 h with p values of <0.001 and <0.001, respectively, but not on subsequent measurements at 4, 6, and 8 h. Mean diastolic pressure in the study group also remained significantly lower than the placebo group at 45 min, 2 h, and 4 h measurements, with p values of <0.001, .004, and <0.001, respectively. The heart rate is another indicator of hemodynamic stability and can also be a cue for pain. We found that heart rate in the active TENS group remained significantly lower than the placebo TENS group till 4 h after application of TENS with p values of 0.012, 0.017, and less than 0.001 at 45 min, 2 h, and 4 h, respectively. Patients who received TENS had heart rates closer to their baseline values, and there was no statistically significant difference at 6 and 8 h in the active TENS group. The average heart rates of patients in the placebo group became comparable with their baseline heart rate after 8 h had passed. The change in heart rate in the active TENS group over time was not statistically significant with a p value of 0.478, but the heart rate varied significantly from baseline in the placebo group with a p value less than 0.001 which demonstrates the benefit of TENS when it comes to hemodynamic stability. The limitation of our study was that we tested TENS on only ASA grade I and II patients and further studies need to be done on a wider range of patients. Also, larger studies need to be conducted to ascertain its role in the general wellbeing of the patients in the postoperative period.
Conclusions
We conclude that TENS is an effective adjuvant non-pharmacologic modality for postoperative pain relief after laparoscopic cholecystectomy with significant pain relief in the early postoperative period and reduced pharmacological analgesia requirement. We recommend the use of TENS in post-surgical recovery zone for patients undergoing laparoscopic cholecystectomy.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TENS:
-
Transcutaneous electrical nerve stimulation
- VAS:
-
Visual analog scale
- PONV:
-
Postoperative nausea and vomiting
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- COX-2 inhibitors:
-
Cyclooxygenase-2 inhibitors
- ALTENS:
-
Acupuncture like TENS
References
Benedetti F, Amanzio H, Casadio C, Cavallo A, Cianci R, Giobbe R et al (1997) Control of postoperative pain by transcutaneous electrical nerve stimulation after thoracic operations. Ann Thorac surg 63:773–776
Bisgaard T, Kehlet H, Rosenberg J (2001) Pain and convalescence after laparoscopic cholecystectomy. Ann R Coll Surg Engl 167:84–96
Bisgaard T, Klarskov B, Rosenberg J, Kehlet H (2001) Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain 90:261–9
Bisgaard T, Klarskov B, Rosenberg J, Kehlet H (2001) Factors determining convalescence after uncomplicated laparoscopic cholecystectomy. Arch Surg 136:917–921
Bjordal JM, Johnson MI, Ljunggreen AE (2003) Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A metaanalysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain 7:181–188
Chandra A, Banavaliker JN, Das PK, Hasti S (2010) Use of transcutaneous electrical nerve stimulation as an adjunctive to epidural analgesia in the management of acute thoracotomy pain. Indian Journal of Anaesthesia 54(2):116
Eidy M, Fazel MR, Janzamini M, Razaei MH, Moravveji AR (2016) Preemptive analgesic effects of transcutaneous electrical nerve stimulation (TENS) on postoperative pain: a randomized, double blind, placebo-controlled trial. Iran Red Crescent Med 18:14–26
Hamza MA, White PF, Ahmed HE, Ghoname EA (1991) Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anaesthesiology 91:1232–1238
Javid PJ, Brooks DC (2004) Cholecystectomy. In: Johnson LR (ed) Encyclopedia of Gastroenterology, 1st edn. San Diego, Elesevier, p 317
Kara B, Baskurt F, Acar S, Karadibak D, Ciftci L, Erbayraktar S et al (2011) The Effect of TENS on pain, function, depression, and analgesic consumption in the early postoperative period with spinal surgery patients. Turkish Neurosurgery 21:618–624
Lau H, Brooks DC (2001) Predictive factors for unanticipated admissions after ambulatory laparoscopic cholecystectomy. Arch Surg 136:1150–3
Lee IO, Kim SH, Kong MH et al (2001) Pain after laparoscopic cholecystectomy: the effect and timing of incisional and intraperitoneal bupivacaine. Can J Anaesth 48:545–550
Mannheimer JS, Lampe GN (1984) Clinical transcutaneous electrical nerve stimulation. Philadelphia, PA
Melzack R, Wall P (1965) Pain mechanisms: a new theory. Science 150:971–979
O’Hanlon DM, Colbert S, Ragheb J et al (2002) Intraperitoneal pethidine versus intramuscular pethidine for relief of pain after laparoscopic cholecystectomy: randomized trial. World J Surg 26:1432–6
Oncel M, Sencan S, Yildiz H, Kurt N (2002) Transcutaneous electrical nerve stimulation for pain management in patients with uncomplicated minor rib fractures. Eur J Cardiothorac surg 22(1):7–13
Rushton DN (2002) Electrical stimulation in the treatment of pain. Ann Disabil Rehabil 24:407–415
Silva MB, de Melo PR, de Oliveira NML et al (2012) Analgesic effect of transcutaneous electrical nerve stimulation after laparoscopic cholecystectomy. Am J Phys Med Rehabil 91(8):652–657
Sjolund BH, Eriksson MBE (1976) Electro-acupuncture and endogenous morphine. Lancet 2:1085
White PF (1995) Management of postoperative pain and emesis. Can J Anaesth 42:1053–5
Wills VL, Hunt DR (2000) Pain after laparoscopic cholecystectomy. Br J Surg 87:273–84
Za´rate E, Mingus M, White PF, et al (2001) The use of transcutaneous acupoint electrical stimulation for preventing nausea and vomiting after laparoscopic surgery. Anesth Analg 92:628–35
Acknowledgements
Not applicable.
Funding
The study did not require any funding from any source. All equipment used was readily available in the Department of Anaesthesiology and Department of Physiotherapy.
Author information
Authors and Affiliations
Contributions
The concept, design, and definition of the intellectual content for the study were undertaken by ST, SS, and AC. Literature search was done by ST and SS. The clinical study was done by SS and AK, and the observations were noted by ST. Data acquisition was done by ST and DR, and the data analysis was done by all the authors. The statistical analysis was also done by all the authors. The manuscript was prepared by SS and ST, and the manuscript editing was done by AC and DR. The manuscript was reviewed and approved by all the authors. The guarantor of the manuscript is SS and AC. All four authors agree to be personally accountable for their work. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Due clearance from the Institutional Ethical Committee, North DMC Medical College and Hindu Rao Hospital was taken. S. No of Ethics Committee: ECR/979/Inst/DL/2017/RR-17.
(IEC submission No: IEC/NDMC/2020/50).
Written informed consent was obtained from all for inclusion in the study.
Consent for publication
Written informed consent was obtained from all participants. Patient’s identity was not revealed in any way for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tara, S., Sharma, S., Chandra, A. et al. Evaluation of the role of transcutaneous electrical nerve stimulation for postoperative pain relief in laparoscopic cholecystectomy: a prospective comparative study. Ain-Shams J Anesthesiol 15, 55 (2023). https://doi.org/10.1186/s42077-023-00352-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-023-00352-4