Abstract
Background
Hyperglycemia is a risk factor for infarct expansion and poor outcome for both diabetic and non-diabetic patients. We aimed to study the prognostic value of stress hyperglycemia on the outcome of acute ischemic stroke patients as regards National Institutes of Health Stroke Scale (NIHSS) as a primary outcome.
Results
Patients with high random blood sugar (RBS) on admission showed significantly higher values of both median NIHSS score and median duration of hospital stay. There were significant associations between stress hyperglycemia and the risk of 30-day mortality (p < 0.001), the need for mechanical ventilation (p < 0.001) and vasopressors (p < 0.001), and the occurrence of hemorrhagic transformation (p = 0.001). The 24-h RBS levels at a cut off > 145 mg/dl showed a significantly good discrimination power for 30-day mortality (area under the curve = 0.809).
Conclusions
Stress hyperglycemia had a prognostic value and was associated with less-favorable outcomes of acute stroke patients. Therefore, early glycemic control is recommended for those patients.
Similar content being viewed by others
Background
Diabetes mellitus is a known risk factor for stroke. Diabetic patients are more likely to die or to have substantial neurologic disability after acute stroke compared to non-diabetic subjects (Candelise et al. 1985; Jorgensen et al. 1994). However, hyperglycemia per se is associated with increased morbidity and mortality in stroke patients (Umpierrez et al. 2002). Hyperglycemia has been documented in a considerably large number of patients admitted to hospitals (Heuschmann et al. 2004).
Additionally, 20 to 40% of patients admitted with ischemic stroke had been found hyperglycemic, usually without a previous diabetes mellitus (Kiers et al. 1992). Research studies suggest that the relative risk of death in hyperglycemic non-diabetic stroke patients is increased by 3.3% and is associated with poor outcome (Bruno et al. 1999; Capes et al. 2001). Stress hyperglycemia has been shown to influence prognosis and outcome of stroke (Talukder et al. 2018). Conclusions about how hyperglycemia affects outcomes are not consistent. This is because of the heterogeneous nature of previous studies as regards definition of hyperglycemia and timing of measurement, besides the retrospective design in most of them (Muir et al. 2011). Therefore, the objective of this study was to prospectively assess the prognostic value of stress hyperglycemia on the outcome of acute ischemic stroke patients as regards National Institutes of Health Stroke Scale (NIHSS) as a primary outcome.
Methods
Study design, setting, and date
This prospective observational cohort study was conducted at a Specialized Hospital from June 2018 to September 2019 on 80 patients with acute ischemic stroke (without other major comorbidities) within 24 h of onset of symptoms. The study was ethically conducted in accordance with Declaration of Helsinki, and we obtained ethical approval of our local ethics committee. An informed written consent was obtained from each patient or his first kin. The study was registered at ClinicalTrial.gov (registry number: NCT04408768).
Eligibility criteria
The study recruited patients of either gender, aged 40 to 70 years old, and with acute ischemic stroke within 24 h of onset of symptoms.
We excluded patients with any of the following: other potential differential diagnoses (such as subdural hematoma, transient ischemic attack, subarachnoid hemorrhage, hemorrhagic stroke); major comorbidities (e.g., end-stage liver and renal disease, malignant hypertension); hyperglycemia on admission that was controlled (random blood sugar (RBS) < 150 mg/dl) within 24 h with insulin therapy, diabetic ketoacidosis; or RBS less than 70 mg/dl on admission.
Data collection
All patients were subjected to full history taking and scoring according to the National Institutes of Health Stroke Scale (NIHSS). It is a 15-item neurologic examination stroke scale that provides an assessment of the stroke severity, both on admission and on discharge. It is a well-validated tool to evaluate the neurologic deficit in acute stroke patients. The NIHSS includes the following domains: level of consciousness, eye movements, integrity of visual fields, facial movements, arm and leg muscle strength, sensation, coordination, language, speech, and neglect. The total score ranges from 0 to 42. It is categorized into the following: no stroke symptoms (0), mild (1–4), moderate (5–15), moderate to severe (16–20), and severe (21–42) stroke (Kwah and Diong 2014).
In addition, patients received the standard protocol for management of stroke patients, which included computed tomography brain imaging with or without magnetic resonance imaging, carotid duplex, HbA1C, ECG, and serial measurements of random blood glucose levels during hospital stay either hourly if patients were on insulin infusion or every 4 h in patients with normal RBS. Patients were assessed and followed up for hemodynamics, respiratory parameters, hemorrhagic transformation, the length of hospital stay, 30-day mortality, the need for mechanical ventilation, and the need for vasopressors to maintain the mean arterial blood pressure between 70 and 100 mmHg.
On intensive care unit (ICU) admission, RBS was recorded and categorized as either normal (70–150 mg/dl) or high (more than 150 mg/dl). Patients were categorized into 2 groups: group A (with normal RBS on ICU admission and controlled blood sugar within 24 h) and group B (with high RBS on ICU admission and uncontrolled blood sugar during first 24 h). Both groups received the standard protocol for management of stroke patients.
Outcomes and sample size calculation
Based on Al-Weshahy et al. (Al-Weshahy et al. 2018) and using PASS program, setting alpha error at 5%, and power of 80%, the total sample size was estimated to be 80 cases.
The NIHSS was used as a primary outcome; 40 cases were recruited in each group. Secondary outcomes were hemorrhagic transformation, hospital stay duration, mechanical ventilation, need for vasopressors, and 30-day mortality.
Statistical analysis
Statistical analysis and presentation of data was conducted using SPSS (Statistical Package for the Social Sciences) version 22 computer program. Categorical variables were summarized as frequencies and percentages, and association between variables was tested using Χ2 tests (Pearson’s chi-square for independence or Fisher’s exact tests as appropriate). Numerical variables were checked for normality by Shapiro Wilk test. Normally distributed data were presented as mean ± standard deviation and were compared by independent T test. Skewed data with non-normal distribution were expressed as median and interquartile range (25th–75th percentile), and differences between the two groups were tested using Mann-Whitney U test. In addition, receiver operating characteristic curves were constructed to assess discriminating power (area under the curve), sensitivity, specificity, and accuracy of 24-h RBS levels in predicting 30-day mortality. Prognostic performance of 24-h RBS levels in predicting mortality was adjusted for age and severity (variables with p value < 0.1 in the univariate analysis and had an impact on mortality) by using a multivariable logistic regression analysis. A p value of < 0.05 was considered statistically significant.
Results
This study included eighty acute ischemic stroke patients who presented within 24 h of onset of symptoms. The patients were recruited so as 40 patients had normal (70–150 mg/dl) RBS on ICU admission and controlled blood sugar within 24 h (group A), while the other 40 patients had high RBS on ICU admission and uncontrolled blood sugar during first 24 h (group B).
Baseline characters of the studied groups were illustrated in Table 1. There was homogenous gender distribution in both groups, whereas the mean age of patients in group B was significantly higher than group B (66.8 ± 3.3 and 56.3 ± 6.5, respectively; p < 0.001). The median NIHSS score on ICU admission was significantly higher in group B (34.0) compared to group A (2.5).
Additionally, the mean RBS levels at admission and after 24 h were significantly higher in group B in comparison with group A (p < 0.001).
Table 2 shows absence of significant differences between the studied groups as regards comorbidities including diabetes mellitus, hypertension, ischemic heart disease, atrial fibrillation, smoking, old cardiovascular stroke, or transient ischemic attacks.
Table 3 shows the primary and secondary outcomes of the studied groups. Patients with high RBS showed significantly higher values of both median NIHSS score on discharge and median duration of hospital stay compared to those with normal RBS (2.0 vs 1.0 and 15.0 vs 10.0, respectively). There were significant associations between hyperglycemia and the risk of 30-day mortality (p < 0.001), the need for mechanical ventilation (p < 0.001) and vasopressors (p < 0.001), and the occurrence of hemorrhagic transformation (p = 0.001). Significantly higher percentage (75%) of patients in group B were expired, needed mechanical ventilation (82.5%) and vasopressors (85.0%), and showed hemorrhagic transformation of the infarct (30.0%).
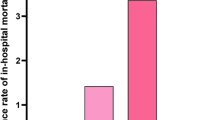
Receiver operating characteristics curve and univariate regression analysis revealed that 24-h RBS levels at a cut off > 145 mg/dl showed a significant good discrimination power for 30-day mortality (AUC = 0.809), correctly classified mortality by accuracy of 80.0%, and it carried a risk (odds ratio) for mortality of 1.023 as demonstrated in Table 4 and Fig. 1.
After adjustment for age and severity (NIHSS) by multivariable logistic regression analysis, the 24-h RBS levels’ risk (adjusted odds ratio) for 30-day mortality was 1.20. Moreover, the whole model showed excellent power of discrimination (AUC = 1) and accuracy of 100% (Table 5).
Discussion
This study explored that stress hyperglycemia encountered within the first 24 h of ischemic stroke onset reflected stroke severity and was associated with adverse clinical outcomes in the form of increased risk of 30-day mortality, prolonged duration of hospital stay, and increased need for mechanical ventilation as well as vasopressors. Additionally, patients with hyperglycemia exhibited more liability to hemorrhagic transformation of the infarct.
Hyperglycemia detected in the acute stroke phase—regardless the presence of diabetes mellitus—reflects a physiological stress and is a manifestation of relative insulin deficiency, which is related to increased lipolysis (Piironen et al. 2012a). In addition, hyperglycemia in stroke patients may result from an interaction between several hormones including glucagon, cortisol, cytokines, and growth hormone, which play a crucial role in blood glucose regulation (Yang et al. 2013).
Several mechanisms have been identified by which hyperglycemia could increase brain damage in ischemic stroke and thereby result in unfavorable outcomes. These include endothelial dysfunction, impaired fibrinolysis (MacDougall and Muir 2011), and increased tendency of red blood cells to form microaggregates (Lemkes et al. 2010). Moreover, hyperglycemia might result in a number of cellular derangements including loss of the blood-brain barrier integrity, increase of excitatory neurotransmitters production, enhancement of anaerobic glycolysis, and induction of oxidative stress. Persistent or poorly controlled hyperglycemia has been shown to reduce cerebral blood flow, increase intracranial pressure, and cause cerebral edema and neuronal death (Bar-Or et al. 2019).
An earlier large study recruited 811 consecutive patients with acute ischemic stroke. The study aimed at predicting unfavorable outcomes and 72-h and 7-day fatality. The researchers concluded that admission hyperglycemia was strongly associated with 72-h fatality (Nardi et al. 2012). Furthermore, hyperglycemia within the first 48 h was independently associated with higher mortality and poor functional outcome, with an absolute increase of 12.9% (Muir et al. 2011). Further strong evidence for the association of high glucose levels and deleterious effects during the acute phase of stroke has been concluded where blood glucose level was an independent predictor of larger infarct size, poor clinical outcome, and higher risk of mortality (Piironen et al. 2012b). Recently, random blood sugar obtained on admission had been found to be an independent predictor of in-hospital mortality in stroke patients (Atam et al. 2020).
Concerning the length of hospital stay, a prospective cohort study on ischemic stroke patients reported the impact of hyperglycemia on the duration of hospital stay where hyperglycemia was an independent predictor of prolonged hospitalization (Gofir et al. 2017). This agrees with the current study where patients with high RBS showed significantly higher duration of hospital stay compared to those with normal RBS. Recently, Hossain et al. (Hossain et al. 2020) reported that stroke patients with better glycemic control had significantly lower length of stay compared to those with higher admission blood sugars.
Increased risk of hemorrhagic transformation of the infarct was another adverse effect of hyperglycemia in this study. In agreement with this finding, Bruno et al. (Bruno et al. 2010) showed that hyperglycemia played a role in causing brain edema, increasing the risk of hemorrhagic transformation and brain herniation within the first 48 h of acute stroke.
In the present study, 24-h RBS levels carried a significant risk for 30-day mortality. Furthermore, at a cut off > 145 mg/dl, it showed a significant good power for discriminating patients at increased risk with an accuracy of 80.0%. In comparison, Farrokhnia et al. (Farrokhnia et al. 2005) found that 30-day mortality could be predicted in stroke, non-diabetic patients at blood glucose levels above 113 mg/dl. Another study reported that admission hyperglycemia ≥ 143 mg/dl was a strong predictor for 72-h fatality, especially in patients with no prior history of diabetes mellitus (Nardi et al. 2012). It is important to note that management of hyperglycemia is critical and cautious correction of glucose level is essential to avoid hypoglycemia, all of which can be damaging to the brain and affect the patient outcomes (Gaillard and Miller 2018). The selected 24 h RBS levels for prognostic purposes in this study agrees with Baird and colleagues (Baird et al. 2003) who reported that this time point is better than admission RBS levels in predicting outcome of ischemic stroke patients. Additionally, it has been stated that most cases of hyperglycemia appear throughout the first 24 h after stroke and only a low percentage of patients with sustained hyperglycemia show high RBS on admission (Yong and Kaste 2008). However, a continuous glucose monitoring in acute stroke revealed that blood glucose more than 8 mmol/L of during the initial 72 h were associated with death (Soriano-Tárraga et al. 2018).
The current study revealed a significant difference in the mean age of the studied groups. An earlier cohort study of predictors of mortality showed that age was associated with increased in-hospital mortality among acute ischemic stroke patients (Oliveira et al. 2019). Likewise, severity as assessed by NIHSS has been previously shown as an independent predictor of mortality (Wada et al. 2018). Therefore, multivariable logistic regression analysis was performed to show prognostic performance of 24-h RBS in predicting 30-day mortality after adjusting for age and severity. This revealed a model that could predict mortality with 100% accuracy, and the risk of 24-h RBS level was 1.20. A comparable study which included ischemic stroke patients and assessed blood glucose at the second day after admission concluded that stress hyperglycemia was not directly related to outcome of acute ischemic stroke where predictors of mortality included atrial fibrillation, diastolic blood pressure, age, and NIHSS at admission (Tziomalos et al. 2017).
Conclusion
Findings of the present study demonstrate significant deleterious effects of stress hyperglycemia on outcomes of patients with acute ischemic stroke. High random blood sugar levels obtained 24 h after the onset of stroke were associated with increased risk of 30-day mortality, prolonged duration of hospital stay, increased need for mechanical ventilation and vasopressors, and more liability to hemorrhagic transformation of the infarct. This emphasizes the importance of adequate glycemic control during acute phase of ischemic stroke.
Availability of data and materials
We intend to share the study protocol as well as the individual de-identified participants’ data. Data will be accessible through direct contact with the corresponding author, beginning 12 months and ending 36 months following the article publication.
Abbreviations
- ICU:
-
Intensive care unit
- NIHSS:
-
National Institutes of Health Stroke Scale
- RBS:
-
Random blood sugar
References
Al-Weshahy A, El-Sherif R, Selim K, Heikal A (2018) Short term outcome of patients with hyperglycemia and acute stroke. Egypt J Crit Care Med 5:93–98
Atam V, Majumdar A, Sawlani KK, Himanshu D (2020) Clinical profile and short-term mortality predictors in acute stroke with emphasis on stress hyperglycemia and THRIVE Score: An observational study. Int J Cur Res Rev 12:1
Baird TA, Parsons MW, Phan T, Butcher KS, Desmond PM, Tress BM et al (2003) Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 34:2208–2214
Bar-Or D, Rael LT, Madayag RM, Banton KL, Tanner AI, Acuna DL et al (2019) Stress Hyperglycemia in critically ill patients: insight into possible molecular pathways. Front Med 6:54
Bruno A, Biller J, Adams HP Jr, Clarke WR, Woolson RF, Williams LS et al (1999) Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology 52:280–284
Bruno A, Liebeskind D, Hao Q, Raychev R, Investigators US (2010) Diabetes mellitus, acute hyperglycemia, and ischemic stroke. Curr Treat Options Neurol 12:492–503
Candelise L, Landi G, Orazio EN, Boccardi E (1985) Prognostic significance of hyperglycemia in acute stroke. Arch Neurol 42:661–663
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC (2001) Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32:2426–2432
Farrokhnia N, Björk E, Lindbäck J, Terent A (2005) Blood glucose in acute stroke, different therapeutic targets for diabetic and non-diabetic patients? Acta Neurol Scand 112:81–87
Gaillard T, Miller E (2018) Guidelines for stroke survivors with diabetes mellitus. Stroke 49:e215–e217
Gofir A, Mulyono B, Sutarni S (2017) Hyperglycemia as a prognosis predictor of length of stay and functional outcomes in patients with acute ischemic stroke. Int J Neurosci 127:923–929
Heuschmann PU, Kolominsky-Rabas PL, Misselwitz B, Hermanek P, Leffmann C, Janzen RW et al (2004) Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: the German Stroke Registers Study Group. Arch Intern Med 164:1761–1176
Hossain MA, Wyczesany B, Jackie D, Patel S, Agarwal K, Soomro R et al (2020) Glycemic control of stroke patients and their outcomes in a comprehensive stroke center at a tertiary care hospital: a retrospective cohort. J Intern Med 8:133–137
Jorgensen H, Nakayama H, Raaschou HO, Olsen TS (1994) Stroke in patients with diabetes. The Copenhagen Stroke Study. Stroke 25:1977–1984
Kiers L, Davis SM, Larkins R, Hopper J, Tress B, Rossiter SC et al (1992) Stroke topography and outcome in relation to hyperglycaemia and diabetes. J Neurol Neurosurg Psychiatry 55:263–270
Kwah LK, Diong J (2014) National Institutes of Health Stroke Scale (NIHSS). J Physiother. 60:61
Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JC, Hoekstra JB (2010) Hyperglycemia: a prothrombotic factor? J Thromb Haemost 8:1663–1166
MacDougall NJJ, Muir KW (2011) Hyperglycaemia and infarct size in animal models of middle cerebral artery occlusion: systematic review and meta-analysis. J Cereb Blood Flow Metab 31:807–818
Muir KW, McCormick M, Baird T, Ali M (2011) Prevalence, predictors and prognosis of post-stroke hyperglycaemia in acute stroke trials: individual patient data pooled analysis from the virtual international stroke trials archive (VISTA). Cerebrovasc Dis Extra 1:17–22
Nardi K, Milia P, Eusebi P, Paciaroni M, Caso V, Agnelli G (2012) Predictive value of admission blood glucose level on short-term mortality in acute cerebral ischemia. J Diabetes Complications 26:70–76
Oliveira ADP, Andrade-Valença LPA, Valença MM (2019) Factors associated with in-hospital mortality in very elderly patients with ischemic stroke: a cohort study. J Stroke Cerebrovasc Dis 28:104281
Piironen K, Putaala J, Rosso C, Samson Y (2012a) Glucose and acute stroke: evidence for an interlude. Stroke. 43:898–902
Piironen K, Putaala J, Rosso C, Samson Y (2012b) Glucose and acute stroke: evidence for an interlude. Stroke 43:898–902
Soriano-Tárraga C, Giralt-Steinhauer E, Mola-Caminal M, Ois A, Rodríguez-Campello A, Cuadrado-Godia E et al (2018) Biological age is a predictor of mortality in ischemic stroke. Sci Rep 8:4148
Talukder RK, Uddin MJ, Battacharjee M, Akhter H, Pandit H, Pandit P et al (2018) S Stress hyperglycemia and stroke outcome in patients with acute stroke. Mymensingh Med J 27:685–692
Tziomalos K, Dimitriou P, Bouziana SD, Spanou M, Kostaki S, Angelopoulou SM et al (2017) Stress hyperglycemia and acute ischemic stroke in-hospital outcome. Metab Clin Exp 67:99–105
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE (2002) Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 87:978–982
Wada S, Yoshimura S, Inoue M, Matsuki T, Arihiro S (2018) Outcome prediction in acute stroke patients by continuous glucose monitoring. J Am Heart Assoc 7:e008744
Yang JH, Song PS, Song YB, Hahn JY, Choi SH, Choi JH (2013) Prognostic value of admission blood glucose level in patients with and without diabetes mellitus who sustain ST segment elevation myocardial infarction complicated by cardiogenic shock. Crit Care 17:R218
Yong M, Kaste M (2008) Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke 39:2749–2755
Acknowledgements
We would like to thank all our colleagues in El Zaitoun Specialized Hospital’s Intensive Care Units who performed the extensive data entry.
Funding
None.
Author information
Authors and Affiliations
Contributions
HE, MM, AA, and MN have full access to all the data in the study and take responsibility for the integrity of the data. Study concept and design were done by HE and AA. Acquisition of data was done by HE and MM. Analysis of data and critical revision of the manuscript were done by HE and MN. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study obtained approval from the Institutional Review Board of El Zaitoun Specialized Hospital (date of approval: 13-12-2017). The study was registered at ClinicalTrial.gov (registry number: NCT04408768). An informed written consent was obtained from each patient or his first kin.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Gendy, H.A., Mohamed, M.A., Abd-Elhamid, A.E. et al. Stress hyperglycemia as a prognostic factor in acute ischemic stroke patients: a prospective observational cohort study. Ain-Shams J Anesthesiol 13, 4 (2021). https://doi.org/10.1186/s42077-020-00122-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-020-00122-6