Abstract
Background
Coronavirus was primarily discovered in December 2019, causing pneumonia and severe acute respiratory syndrome. It was reported several neurological symptoms associated with COVID-19. Both the central and peripheral nervous systems could be affected which might result in a higher mortality rate in hospitalized patients. This study aimed to determine the spectrum of neurological clinical presentations among patients admitted to Fayoum University Hospital before, during, and after the COVID-19 era and to examine the influence of COVID-19 vaccines mandated by the Egyptian government on neurological disorders.
Methods
This is a historical cohort study that was conducted on patients admitted to the Neurology Department at Fayoum University Hospital before, during, and after COVID-19 outbreaks from January 1st, 2018, to July 31, 2022. All participants had undergone thorough history taking and neurological examination and the necessary investigations according to the suspected diagnosis. All hospitalized patients during the COVID-19 pandemic were positive for the virus, as determined by either a positive rapid antigen test or a positive real-time reverse transcription polymerase chain reaction (RT-PCR).
Results
It was shown that the patients hospitalized during the COVID-19 era were notably older, smokers, and diabetic in comparison to other groups. Cerebrovascular disorders were more prevalent in the COVID-19 pandemic. Surprisingly, compared to prior times, individuals with autoimmune-mediated neurological diseases had higher hospitalization rates than those with other neurological disorders. Patients who were not vaccinated reported more vascular complications than those who got them. However, patients who received vaccination exhibited significantly higher neurological complications as regards, exacerbation of paroxysmal disorders.
Conclusion
It was concluded that the frequency of hospitalizations with cerebrovascular disorders and autoimmune-mediated illnesses was significantly influenced during the pandemic era. Although COVID-19 vaccinations have potential adverse effects, they have played a crucial role in preventing serious neurological problems.
Similar content being viewed by others
Introduction
A new strain of coronavirus was discovered in December 2019, causing severe acute respiratory syndrome in Wuhan, China. Currently known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it has spread to over 200 countries, infecting more than 3 million patients and leading to the COVID-19 pandemic [1]. The World Health Organization broadcast the outbreak in March 2020 [2].
COVID-19 can cause symptoms related to the heart, kidneys, eyes, skin, and more. Various studies have reported a range of neurological symptoms associated with COVID-19. Both the central and peripheral nervous systems can be affected. It is important to note that the presence of neurological symptoms and/or syndromes like delirium and coma has been linked to a fivefold increase in the probability of death in the hospital [3].
Neurologists face uncertainty as more data and clinical aspects of COVID-19 emerge. However, they accept ambiguity and work toward gradual resolution of these issues [4]. Brain is often the target organ in various infectious diseases either as a direct injury or as a subsequent effect of infection. In addition, the peripheral nervous system is vulnerable during immune-mediated illnesses as well as the central nervous system [5]. The purpose of this study was to determine the spectrum of clinical neurological presentations among patients admitted to Fayoum University Hospitals’ Neurology Department during the COVID-19 pandemic and compare it to the pre- and post-pandemic periods. In addition, the study aimed to examine the influence of COVID-19 vaccines mandated by the Egyptian government on neurological disorders.
Patients and methods
This is a historical cohort study that was conducted on patients admitted to the Neurology Department at Fayoum University Hospital before, during, and after COVID-19 outbreaks from January 1, 2018, to July 31, 2022.
The patients were divided into three groups based on the periods in which they were admitted to University Hospitals’ neurology departments. Group 1 consisted of patients admitted in 2018 and 2019, before the Covid pandemic. Group 2 included patients admitted in 2020 and 2021, during the pandemic. And Group 3 included patients admitted in the first 7 months of 2022, after the pandemic.
According to the Centers for Disease Control and Prevention (CDC), guidelines [6] for confirming COVID-19 infection, patients with confirmed COVID-19 infection had positive real-time reverse transcription polymerase chain reaction (RT-PCR) tests for SARS-CoV-2 via naso/oropharyngeal swabs or a positive Rapid antigen test. Because nasopharyngeal PCR was unavailable early in the COVID-19 era due to its high cost, we relied on the Rapid antigen test, which was performed for patients presenting with symptoms indicative of COVID-19 infection during the pandemic. If the test came out negative, it was repeated 48 h later to confirm the negative outcome.
The patient was suspected or considered “probable” to have COVID-19 infection if exhibited typical clinical characteristics such as fever, respiratory symptoms, and loss of taste and smell that are consistent with COVID-19 infection. However, the RT-PCR and/or antigen test result was negative. The radiological lesions, if present, may show ground-glass opacities (GGO), which is a common imaging feature of COVID-19 in the lungs and the patient may have had contact with a confirmed or suspected COVID-19 case within the last 2 weeks [7].
Post-COVID-19 syndrome, also known as Long COVID-19, is defined by the National Institute for Health and Care Excellence (NICE) [8] as the persistence of neurological symptoms or the development of consequences caused by SARS-CoV-2 infection beyond 3 or 4 weeks from the onset of acute COVID-19 symptoms. This is because the presence of replication-competent SARS-CoV-2 becomes almost zero after 3 to 4 weeks, and these symptoms cannot be explained by any other diagnoses. As per the CDC’s report on the Clinical Spectrum of SARS-CoV-2 Infection [9], COVID-19 patients in the current study were categorized into three groups—mild, moderate, and severe. Patients with critical illness, who experienced respiratory failure, septic shock, and/or multiple organ dysfunction, were not included in the study.
All participants underwent a complete neurological examination, which included gathering a detailed medical history, with emphasis on COVID-19 infection, symptoms, duration of illness, and comorbidities during the pandemic era. Various laboratory tests were conducted, including complete blood counts (CBC), erythrocyte sedimentation rates (ESR), C-reactive protein (CRP), serum ferritin, liver and kidney function tests, fasting and postprandial blood sugar levels, lipid and immunological profiles, and d-dimer. To confirm the diagnosis of SARS-COV2 infection, nasopharyngeal swabs from the patients were analyzed using RT-PCR during the COVID-19 pandemic, or antigen tests were performed that identify the presence of certain viral proteins. A positive test result indicates a current infection.
The Radiology Department used a Toshiba Aquilion Prime 160-slice CT scanner from Japan to perform a computed tomography (CT) scan of the chest to check for GGO, which could indicate concomitant pneumonia in COVID-19 patients. Individuals who had lesions thought to be structural central lesions underwent magnetic resonance imaging (MRI). The Radiology Department evaluated the outcomes of 1.5 Tesla MRI scans conducted using a Toshiba Scanner Activion from Japan, which included the acquisition of axial and coronal diffusion, T1, T2, and images with FLAIR weighted images. Skilled radiologists evaluated those patients, and those with suspected cerebral venous sinus thrombosis underwent MR venography, while those with suspected arterial ischemic stroke received magnetic resonance angiography (MRA).
The Department of Neurology used the Nihon Koden device to identify disorders of the peripheral nervous system through nerve conduction and electromyography studies. Each study was conducted in a standard setting. As well, Electroencephalogram (EEG) was also conducted on patients presented with seizures using 32-channel Nihon Kohden equipment from Japan. During the EEG collection, hyperventilation and photic stimulation were used as stimuli in a typical environment. The EEG data were examined for epileptogenic and background activity. When encephalopathy was suspected, CSF samples were taken from patients to check for cell pleocytosis.
Statistical analysis
Data were coded to facilitate data manipulation. The data were analyzed using SPSS software version 22, running on Windows 7. Simple descriptive analysis was done by percentages and numbers for qualitative data, arithmetic means for assessing central tendency, and standard deviations for quantifying dispersion for parametric quantitative data. An independent samples test is performed. In the case of comparing quantitative measurements across more than two distinct sets of quantitative data, the one-way ANOVA test is used. For comparing qualitative information between two or more groups, the Chi-square analysis is implemented. Any p values below 0.05 should be considered statistically significant.
Results
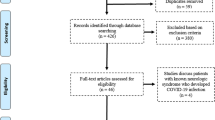
This study included 1405 patients enrolled in Fayoum University’s neurology department between January 2018 and July 2022. 441 patients enrolled in the study from January 2018 to February 2019 (Group 1). There were 679 patients from March 2019 to December 2021 (Group 2). Furthermore, from January 2022 to July 2022 (Group 3), 285 patients were included as shown in Fig. 1. It was shown that the patients hospitalized during the COVID-19 era were notably older, smokers, and diabetic in comparison to the other groups. There were no significant differences between the groups in terms of the severity of early presenting respiratory symptoms (p = 0.1) as shown in Table 1.
It was discovered that cerebrovascular disorders were more prevalent than other neurological disorders in the COVID-19 pandemic lockdown. Surprisingly, individuals with autoimmune-mediated neurological diseases in the group (2) had higher hospitalization rates than the other groups. Moreover, the autoimmune diseases had the highest prevalence in those with a confirmed COVID-19 diagnosis, 65 (45.5%), compared to those with suspected COVID cases, 36 (25.2%), and non-COVID cases, 42 (29.4%) (p = 0.001). In addition, the majority of neurological cases were discovered to be a result of acute COVID-19 infection rather than a result of a post-COVID-19 consequence as shown in Table 2. Furthermore, there were no significant differences in death rates in comparison among the three groups; however, the COVID-19 era had the greatest proportion of mortality when compared to the other periods, but it did not reach to significant level.
In terms of vaccination’s effect, it was demonstrated that 283 patients (29.14%) got vaccination. However, 298 (31%) patients did not receive vaccine. Patients who did not get vaccinations reported significantly greater neurological complications in the form of vascular complications than those who got them. However, it was found that it was shown that patients who received vaccination exhibited significantly higher neurological complications as regards immune-mediated disorders, and exacerbation of paroxysmal disorders such as epilepsy and headache than those who did not receive any vaccination (p < 0.001)as shown in Fig. 2. Regarding the neurological complication of vaccination, there was no significant difference between the different vaccinations as shown in Table 3.
Discussion
COVID-19 is a complex disease, affecting various organs including the respiratory system, kidneys, liver, heart, skin, and brain, with a wide range of clinical symptoms including neurological signs caused by SARS-CoV-2 [10], which compelled us to investigate the effects of this pandemic on patients with neurological presentations hospitalized at Fayoum University Hospital in light of the possibility that COVID-19 might increase the frequency of cases with neurological complications secondary to COVID-19 infection in comparison to the years before this era, as well as what are the most common neurological complications secondary to COVID-19 vaccinations.
The patients with confirmed COVID-19 infection who were hospitalized at the Neurology Department of Fayoum University Hospital accounted for 44.91% of the study's participants, which was similar to findings from earlier multicentric studies conducted in Egypt [11] as well as Brazil [12]. Contrary to these findings, a retrospective study of a large group in Northern Italy [13] found that the percentage of COVID-19 patients hospitalized with neurological problems was approximately 20%. The difference in percentages might be explained by rachial and ethnic susceptibility to SARS-CoV-2 infection and its consequences.
Regarding their clinical characteristics, patients hospitalized with neurological disorders during the pandemic era tended to be middle-aged, diabetic, and smokers in comparison to pre- and post-pandemic era, which was consistent with earlier findings [3, 11, 14]. Diabetes mellitus and smoking were thought to make COVID-19 infection worse, making patients more likely to require hospitalization and develop neurological sequelae. SARS-Cov-2 infection was reported to worsen diabetes glycemic control by escalating inflammation and changing the immune system’s response with an increased risk of complications, which can result in thromboembolism [15]. It is well-known that cigarette smoke causes abnormal inflammatory activation since the production of various pro-inflammatory cytokines (such as Il-6, TNF-, and KC) is increased and potentiates further morbidity and mortality [16].
It is important to note that during the COVID-19 period, cerebrovascular neurological diseases accounted for 60.2% of hospitalizations, making them the most common neurological illnesses requiring hospitalization. These findings are consistent with [17, 18] who hypothesized that individuals with COVID-19 may experience acute cerebrovascular events, including ischemic and hemorrhagic strokes and cerebral venous thrombosis. A hypercoagulable condition, cytokine storms and inflammation, endothelial dysfunction, and SARA-CoV-2 binding to endothelial ACE-2 are possible causes for the underlying processes. These disorders ultimately lead to vasoconstriction, oxidative stress, inflammation, and thrombogenesis [18]. Although cerebrovascular events were the most common among our COVID-19 patients, they were still less common than prior to the pandemic. The COVID-19 outbreak had a significant impact on cerebrovascular care, including particularly hospital care, resulting in a substantial decrease in admissions, thrombolytic therapy and interventional therapies as well as the lack of awareness combined with the terror of the virus imposed on patients with vascular disorders much less likely to request assistance. The focus on social isolation may have excessively conducted acute stroke patients to resist getting medical help in person. A patient's increased social isolation may have made it more difficult for friends and relatives to notice that they were experiencing a stroke [19].
In this study, it was surprising that the individuals with autoimmune-related diseases such as transverse myelitis and Guillain–Barré syndrome (GBS) were found to have a higher rate of hospital admissions during the pandemic era compared to before (34.2% versus 15.2%). Moreover, the confirmed COVID-19 diagnosis had the highest prevalence of autoimmune illnesses (45.5%) compared to the suspected (25.2%) cases and non-COVID (29.4%) It is significant to note that the literature may have underestimated the prevalence of this condition in COVID-19 patients since it is technically challenging to conduct an adequate neurological assessment in an intensive-care setting [20]. Several mechanisms, including both direct viral and immune-mediated pathogenicity, may play a role in the development of neurological damage associated with COVID-19. Evidence suggested the existence of immune-mediated inflammatory pathways, as demonstrated by the ongoing presence of CSF markers of inflammation [20, 21].
It has been observed that almost 60% of COVID-19 patients experienced neurological syndromes during the active infection, rather than as a post-COVID-19 infection consequence. The post-COVID-19 syndrome can lead to various neurological symptoms, including anosmia, ageusia, encephalopathy, encephalitis, myelitis, and post-infectious sequelae such as Guillain–Barré Syndrome or plexopathies [22]. COVID-19 has four stages, namely acute respiratory distress syndrome, cytokine storm, acute hypercoagulable state, and autonomic dysfunction [23]. Researchers have suggested that SARS-CoV-2 may remain dormant in the central nervous system of recovered individuals for an extended period, making it possible to reactivate and cause neurological disorders. Previous studies have shown that the intensity and duration of COVID-19 infection can result in various neurological complications [24]. On the contrary, recent research on COVID-19 has shown no associations with the severity of the original infection [25, 26].
Although the pandemic period witnessed a higher mortality rate compared to the pre-pandemic period, there was no noticeable difference in mortality between the durations before and after the outbreak in this study. Previous studies [27, 28] suggested that the existence of neurological conditions could predict an increased COVID-19 mortality rate, but our research findings are consistent with studies [12, 13] that showed no correlation between the increase in COVID-19 mortality and neurological disorders. According to Travi et al. (2021) [13], patients with primary neurological illnesses had a lower fatality rate, indicating that such illnesses could progress differently than respiratory syndromes. Although patients with intermediate COVID-19 severity experienced more neurological involvement compared to severe or critical patients, their increased mortality rate was likely due to respiratory deterioration rather than neurological issues. This hypothesis supports the finding that only 3 (9.1%) patients experienced severe respiratory symptoms during the pandemic era in our study, which in turn explained the lower mortality incidence observed in our study.
The COVID-19 pandemic prompted the launch of the first vaccinations in early 2021. Since then, approximately 68.2% of the global population has been fully immunized against this illness. There are four basic strategies used in producing COVID-19 vaccines: nucleic acid-based vaccines (DNA–mRNA), viral vectors (replication–non-replication), live inactivated vaccines and protein (spike protein or its subunits). After completing phase 3 clinical trials, the World Health Organization authorized 11 candidate vaccines for COVID-19 for widespread immunization in November 2021 [29]. In Egypt, the national authorities enforced the use of just five candidate vaccinations, including Sinopharm, Sinovac, AstraZeneca, Johnson, and Pfizer.
In contrast to earlier studies [30, 31], patients who did not receive immunization had a higher incidence of cerebrovascular disorders. According to a study by Marckus in 2021 [32], the occurrence of vascular adverse effects due to immunization is infrequent and less prevalent than the incidence of both cerebral venous thrombosis and ischemic stroke caused by COVID-19 infection. The increased risk of cerebrovascular disorders in COVID-19 patients is primarily due to a prothrombotic condition found in those patients, which activates the coagulation system and raises d-dimer and fibrinogen levels. This condition, known as sepsis-induced coagulopathy, is linked to infection-induced systemic inflammation [32]. There have been reports of vascular complications in the brain caused by certain COVID-19 vaccines, particularly those based on adenovirus. These complications are thought to be triggered by the immune system's response to the vaccine, which can result in thrombocytopenia and cerebral venous sinus thrombosis. It is believed that the creation of IgG antibodies against platelet factor 4 is responsible for activating platelets and causing blood clots in large venous arteries, which leads to thrombocytopenia [31].
Our study found that the most common adverse effects of COVID-19 immunization were headaches, which is consistent with findings from previous study [33]. Post-vaccination headaches can also be caused by stress and vascular spasm. Among the different types of vaccines, mRNA and adenovirus vaccines were found to be more likely to cause headaches [34]. Another common adverse effect was the exacerbation of pre-existing neurological conditions such as epilepsy. Convulsions triggered by COVID-19 vaccination can be attributed to the release of spike proteins, which cause acute inflammation and hyperthermia. As a result, hyperthermia increases glial cell activity and the permeability of the blood–brain barrier. This leads to the entry of peripheral blood cells and albumin into the brain, which alters the osmotic equilibrium [35].
During the investigation, it was found that COVID-19 vaccinations might cause immune-mediated neurological illnesses such as transverse myelitis and GBS, which have been previously reported [36, 37]. The two main mechanisms for vaccine toxicity and how these consequences emerge are ectopic immune reactivity and molecular mimicry [37]. Moreover, this study did not identify significant differences between different vaccinations despite previously reported negative effects.
Conclusion
Finally, it has been concluded that the COVID-19 pandemic is a significant event in modern history that has had a major impact on public health, especially for those who have suffered from neurological complications. The frequency of hospitalizations in neurological departments with cerebrovascular disorders and autoimmune-mediated illnesses has greatly increased. Although COVID-19 vaccinations have potential adverse effects, they have played a crucial role in preventing serious neurological problems.
Limitations
The study had some limitations that should be taken into consideration. First, nasopharyngeal PCR tests for confirmation of COVID-19 diagnosis were not always available due to their high cost in the early months of the pandemic. So we relied on Rapid antigen tests and clinical, laboratory, and imaging findings that could raise suspicion of COVID-19 infection. During the period between March and October 2020, as Fayoum University Hospital was converted into an isolation hospital, there was a shortage of availability of neurological ICUs. So, cases that required ICU hospitalization were missed. In addition, individuals with severe and critical respiratory symptoms who required ICU admission were discounted, which prevented us from studying the influence of respiratory manifestation severity on the presentation of neurologic sequelae. Finally, this study did not include COVID-19 patients who experienced mild neurological symptoms such as tiredness, headache, dizziness, anosmia, and ageusia. These symptoms were not accompanied by any neurological syndromes and did not require hospitalization. As a result, they were not recorded in the hospital’s files.
Availability of data and materials
The dataset cannot be publicly available due to institutional rules. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The figures used in this manuscript are original.
Abbreviations
- ACE:
-
Angiotensin converting enzyme
- CBC:
-
Complete blood counts
- CDC:
-
Centers for disease control and prevention
- COVID-19:
-
Coronavirus disease
- CRP:
-
C-reactive protein
- CSF:
-
CerebroSpinal fluid
- CT:
-
Computed tomography
- EEG:
-
Electroencephalogram
- ESR:
-
Erythrocyte sedimentation rate
- FLAIR:
-
Fluid attenuated inversion recovery
- GBS:
-
Guillain–Barré syndrome
- GGO:
-
Ground–glass opacities
- MRI:
-
Magnetic resonance imaging
- NICE:
-
National institute for health and care excellence
- RT-PCR:
-
Real-time reverse transcription polymerase chain reaction
- SARS-COV2:
-
Severe acute respiratory syndrome coronavirus 2
References
Tsivgoulis G, Palaiodimou L, Katsanos AH, Caso V, Köhrmann M, Molina C, et al. Neurological manifestations and implications of COVID-19 pandemic. Ther Adv Neurol Disord. 2020;13.
Coronavirus disease (COVID-19): Variants of SARS-COV-2 [Internet]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus2019/question-and-answers-hub/q-a-detail/coronavirus-disease-%28covid-19%29-variants-of-sars-cov-. Accessed online on September 10th, 2023.
Misra S, Kolappa K, Prasad M, Radhakrishnan D, Thakur KT, Solomon T, et al. Frequency of neurologic manifestations in COVID-19. Neurology. 2021;97(23):E2269–81.
Shellhaas RA. Neurologists and COVID-19: a note on courage in a time of uncertainty. Neurology. 2020;94(20):855–7.
Stevens RD, Nyquist PA. Types of brain dysfunction in critical illness. Neurol Clin. 2008;26(2):469–86.
Overview of Testing for SARS-CoV-2, the virus that causes COVID-19 | CDC [Internet]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed online on September 11th, 2023.
Raveendran AV. Long COVID-19: challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab Syndr. 2021;15(1):145–6.
Overview | COVID-19 rapid guideline: managing the long-term effects of COVID-19 | Guidance | NICE. Available from: https://www.nice.org.uk/guidance/ng188. Accessed online on September 11th, 2023.
Clinical Spectrum | COVID-19 Treatment Guidelines [Internet]. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ Accesed online on September 11th, 2023.
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
Mekkawy DA, Hamdy S, Abdel-Naseer M, Shehata HS, Halfawy Al A, Shalaby NM, et al. Neurological manifestations in a cohort of Egyptian patients with COVID-19: a prospective, multicenter, observational study. Brain Sci. 2022;12(1):74.
Marcolino MS, Anschau F, Kopittke L, Pires MC, Barbosa IG, Pereira DN, et al. Frequency and burden of neurological manifestations upon hospital presentation in COVID-19 patients: findings from a large Brazilian cohort. J Neurol Sci. 2022;15:443.
Travi G, Rossotti R, Merli M, D’Amico F, Chiappetta S, Giussani G, et al. Neurological manifestations in patients hospitalized with COVID-19: a retrospective analysis from a large cohort in Northern Italy. Eur J Neurosci. 2021;53(8):2912–22.
Jassat W, Mudara C, Vika C, Welch R, Arendse T, Dryden M, et al. A cohort study of post-COVID-19 condition across the Beta, Delta, and Omicron waves in South Africa: 6-month follow-up of hospitalized and nonhospitalized participants. Int J Infect Dis. 2023;128:102–11.
Gęca T, Wojtowicz K, Guzik P, Góra T. Increased risk of COVID-19 in patients with diabetes mellitus—current challenges in pathophysiology, treatment and prevention. Int J Environ Res Public Health. 2022;19(11):6555.
Xie J, Zhong R, Wang W, Chen O, Zou Y. COVID-19 and smoking: what evidence needs our attention? Front Physiol. 2021;18:12.
Fraiman P, Godeiro Junior C, Moro E, Cavallieri F, Zedde M. COVID-19 and cerebrovascular diseases: a systematic review and perspectives for stroke management. Front Neurol. 2020;5(11): 574694.
Ghasemi M, Umeton RP, Keyhanian K, Mohit B, Rahimian N, Eshaghhosseiny N, et al. SARS-CoV-2 and acute cerebrovascular events: an overview. J Clin Med. 2021;10(15):3349.
Zhao J, Li H, Kung D, Fisher M, Shen Y, Liu R. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke. 2020;51(7):1996–2001.
Canavero I, Ravaglia S, Valentino F, Micieli G. Guillain Barrè syndrome and myelitis associated with SARS-CoV-2 infection. Neurosci Lett. 2021;8(759): 136040.
Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. 2020;16(11):636–44.
Ahmed JO, Ahmad SA, Hassan MN, Kakamad FH, Salih RQ, Abdulla BA, et al. Post COVID-19 neurological complications; a meta-analysis. Ann Med Surg (Lond). 2022;1:76.
Yamamoto V, Bolanos JF, Fiallos J, Strand SE, Morris K, Shahrokhinia S, et al. COVID-19: review of a 21st century pandemic from etiology to neuro-psychiatric implications. J Alzheimer’s Dis. 2020;77(2):459–504.
Carlos Duran J, Pablo DJ. Post COVID-19 neurological syndrome: a prospective study at 3600 m above sea level in La Paz Bolivia. J Neurol Sci. 2021;1(429): 119820.
Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE. 2020;15(11):e0240784.
Moreno-Pérez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jiménez J, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–83.
Romagnolo A, Imbalzano G, Artusi CA, Balestrino R, Ledda C, De Rosa FG, et al. Neurological comorbidities and COVID-19-related case fatality: a cohort study. J Neurol Sci. 2021;9(428): 117610.
Carlos CR, Gerardo MM, Jaime OG, Isauro GHL, Dios APJ, Wilmar CS, et al. Prevalence of neurological manifestations in COVID-19 and their association with mortality. Neurol Perspect. 2021;1(1):11–6.
Hosseini R, Askari N. A review of neurological side effects of COVID-19 vaccination. Eur J Med Res. 2023;28(1):1–8.
Schulz JB, Berlit P, Diener HC, Gerloff C, Greinacher A, Klein C, et al. COVID-19 vaccine-associated cerebral venous thrombosis in Germany. Ann Neurol. 2021;90(4):627–39.
Iba T, Levy JH, Warkentin TE. Recognizing vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2022;50(1):E80-86.
Markus HS. Ischaemic stroke can follow COVID-19 vaccination but is much more common with COVID-19 infection itself. J Neurol Neurosurg Psychiatry. 2021;92(11):1142.
Ghiasi N, Valizadeh R, Arabsorkhi M, Hoseyni TS, Esfandiari K, Sadighpour T, et al. Efficacy and side effects of Sputnik V, Sinopharm and AstraZeneca vaccines to stop COVID-19; a review and discussion. Immunopathologia Persa. 2021;7(2):e31–e31.
Ekizoglu E, Gezegen H, Yalınay Dikmen P, Orhan EK, Ertaş M, Baykan B. The characteristics of COVID-19 vaccine-related headache: clues gathered from the healthcare personnel in the pandemic. Cephalalgia. 2022;42(4–5):366–75.
Assiri SA, Althaqafi RMM, Alswat K, Alghamdi AA, Alomairi NE, Nemenqani DM, et al. Post COVID-19 vaccination-associated neurological complications. Neuropsychiatr Dis Treat. 2022;18:137–54.
Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13(3):215–24.
Kohli S, Varshney M, Mangla S, Jaiswal B, Chhabra PH, Kumar V, et al. Guillain–Barré syndrome after COVID-19 vaccine: should we assume a causal link? Int J Med Pharm Case Reports. 2021;1:20–4.
Acknowledgements
Given how much suffering they endured during the epidemic era, all patients who took part in this study deserve to be acknowledged.
Funding
No funding resources were declared for this research.
Author information
Authors and Affiliations
Contributions
SS: responsible for analyzing, and interpreting the data of the work, and approving the final reversion of the work. ME: responsible for analyzing, and interpreting the data of the work. MA: responsible for analyzing the data of the work. FM: responsible for data collection, sharing in writing the article. AE: responsible for statistical analysis and interpretation of the collected data. LD: responsible for drafting the work, analyzing, and interpreting the data, writing the article, and communicating with the journal during the manuscript submission, peer review, and publication process. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Faculty of Medicine, Fayoum University Ethical Committee had approved the study written informed consent was obtained from all the patients and control volunteers before study initiation. The research was approved by the ethical committee, Faculty of Medicine, Fayoum University on June 12, 2022 with code (M-603).
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of financial interest to declare concerning this study. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sayed, S.S., El-Khatib, M.ES., Abdelghaffar, M. et al. The pattern of neurological disorders requiring hospitalization during the COVID-19 era: an experience from Fayoum University Hospital, Egypt. Egypt J Neurol Psychiatry Neurosurg 60, 58 (2024). https://doi.org/10.1186/s41983-024-00831-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-024-00831-x