Abstract
Background
Specific treatment for Guillain–Barre syndrome is based on plasma exchange and intravenous immunoglobulin (IvIg). In developing countries such as Morocco, we are often confronted with constraints in terms of price and availability of substitutes. Comparative studies of these two therapeutic modalities have been conducted particularly in severely extensive forms.
Results
Our study compared small-volume plasmapheresis (SVP) with intravenous Immunoglobulin over a nine-year period in the neurology department of the University Hospital Center of Marrakech in terms of efficacy and safety in Moroccan patients with GBS of varying degrees of severity.
We included 76 patients who were hospitalized for GBS. Forty-six patients were treated with SVP and 30 were treated with IvIg. The therapeutic choice depended on contraindications, socioeconomic considerations, patient choice, and availability of treatment. The clinical and paraclinical evaluations of the two groups were statistically comparable, including factors that may influence the prognosis (p > 0.05). The efficacy of IvIg and SVP did not show a statistically significant difference except for a longer neurology department stay with plasmapheresis (p < 0.001). This efficacy is evaluated by the evolution of the Hughes and MRC sum scores one month after treatment, length of hospital stay, use of mechanical ventilation and its duration, and mortality rate.
Conclusion
The results selected further encourage the use of SVP because of its efficacy and safety, which are comparable to those of IvIg. And the review of the literature confirms our recommendations.
Similar content being viewed by others
Background
Guillain–Barre syndrome (GBS) is an acute polyradiculoneuropathy of autoimmune origin. Its incidence is around 0.5 to 2 cases per 100 000 inhabitants [1]. It is an emergency with neurological and medical complications whose gravity can justify hospitalization in intensive care.
The treatment of Guillain–Barré syndrome remained symptomatic until the 1990s, and the aim of specific measures was to limit the spread of paralysis, promote motor recovery, and reduce the neurological sequelae [2]. Intravenous immunoglobulin (IvIg) and plasma exchange (PE) are immunomodulatory treatments that have been proven effective in accelerating recovery of motor function [3, 4]. Small-volume plasma exchange (SVPE) may be an effective alternative treatment for GBS, particularly in developing countries where cost is the limiting factor for the prescription options [5].
The aim of our study is to compare the two components of GBS specific treatment; small-volume plasmapheresis and intravenous immunoglobulin, based on efficacy and tolerance in the neurology department at the Mohamed VI University Hospital in Marrakech in order to share our experience and discuss a possible therapeutic variant that merits further research.
Methods
This is a single-center study comparing small-volume plasmapheresis and intravenous immunoglobulin in two groups (A and B) for the treatment of Guillain–Barre syndrome in terms of safety, and efficacy.
Treatment allocation: The choice of treatment depends on contraindications, socioeconomic considerations, patient choice, and availability of treatment.
Conventional plasma exchange aims to remove pathological agents from the circulation, this will allow non-selective removal of plasma proteins, including albumin and immunoglobulins. It requires the use of an extracorporeal system, which returns the blood to the cell separation system and, after plasma extraction and the addition of colloidal replacement solution, to the patient. In this technique, large volumes of plasma (3 to 5 L) are removed from a patient requiring a large quantity of substitute fluid.
While plasmapheresis is based on the concept of apheresis as a method of separating different blood components without the need for a replacement fluid, given the small volume.
With conventional plasma exchange, plasma can be separated from blood by either continuous or discontinuous centrifugation, or by filtration. In our study, the small-volume plasmapheresis uses nanofiltration technique.
Group A received 5 sessions of small-volume plasmapheresis (SVP) (by "HEMOFENIX") over 10 to 14 days. This device consists of flat, porous nano-membrane filtration (consisting of the superposition of several porous membranes covered by nano pores) allowing separation of the plasma during the passage of the blood through the filters.
The "HEMOFENIX" system is suitable mainly for cell separation for the purpose of donation but, if necessary, also lends itself to simpler purification therapies. It is a technique with a small filling volume of the extracorporeal circuit (10–70 ml) and small-volume variability (9 ml). The operation of the device is based on membrane plasmapheresis with a single needle via a sterile disposable extracorporeal circuit. Since the device operates at normal speed, the lack of volume restored increases with time, so the plasma must be compensated periodically (after lifting 100–600 ml; depending on the patient's weight, hemodynamic status, and other indications). The total volume of blood exchanged during GBS is half of the total plasma volume.
Patients included in the study received SVPE and not conventional plasma exchange. Patients who received conventional plasma exchange were excluded (among the fifteen patients who did not receive neither of the two therapeutic modalities studied).
Group B received 0.4 g/kg/day of intravenous immunoglobulin for 5 days.
Patient selection: The study concerns a series of patients who presented with an acute polyradiculoneuritis, hospitalized at the neurology department of the Mohammed VI University Hospital Center in Marrakech, for Guillain–Barre syndrome from 2010 to August 2019.
The selection of patients included was performed using the hospitalization registries of the department. A search was then performed to retrieve medical records where terms "Guillain–Barre syndrome" or "acute polyradiculoneuritis" or "polyradiculoneuritis" were included in the discharge diagnosis.
We included all patients diagnosed with GBS with high level of certainty according to the Brighton criteria (The sensitivity of the Brighton criteria correlates with the levels of the criteria) [6] and who are treated with small-volume plasmapheresis or intravenous immunoglobulin.
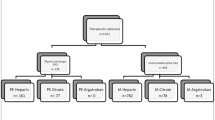
Over the study period, 111 patients with polyradiculoneuritis were detected on preliminary screening (Fig. 1).
We excluded from this study: Any acute polyradiculoneuritis secondary to infectious, toxic, or other inflammatory causes. And patients with incomplete records or insufficient data were also excluded.
Data collection and statistical methodologies: We have adopted a standardized datasheet for all our patients, in order to obtain the necessary demographic (age, sex, origin, past medical history, preceding infection), clinical (the different symptoms and clinical signs and their onset), and para clinical information including nerve conduction studies and cerebrospinal fluid study as well as therapeutic and outcomes using Hughes and MRC scores furthermore the occurrence of adverse events.
In this study, we used SPSS and EXCEL software. The data collected were entered and analyzed using SPSS software (software version 16.0).
The study was descriptive and comparative. Means and standard deviations were calculated for continuous variables and frequencies and percentages for categorical variables. A comparison of percentages was performed using the Chi-square test and the Fisher’s exact test. The comparison of means was performed by the Mann–Whitney test.
The degree of statistical significance was set at 0.05 (p-value significant if < 0.05).
Ethical considerations: The study was conducted without any commercial or financial relationship that could be interpreted as a potential conflict of interest.
Data collection was carried out with respect to patient anonymity and data confidentiality.
Results
Homogeneity of the two groups: Our study involved 76 patients; all of them received symptomatic measures associated with the specific treatment. Small-volume plasmapheresis (SVP) was performed on 46 patients and intravenous immunoglobulin (IvIg) was administered to 30 patients.
The mean age of the patients was 42.4 ± 18.1 with a range of 7 to 82 years. The difference between the two groups was statistically non-significant (p = 0.18).
There was a male predominance; 53.9% (n: 41) of male and females represented 46% (n: 35), sex ratio of 1.17. Male predominance remains marked in the different age groups; the difference between the two groups was statistically non-significant (p = 0.13).
In the four weeks prior to the onset of neurological signs, an infectious event was reported in 30.3% of patients and this history was dominated by respiratory 55.6% (18.4% of all patients), and digestive 24.2% (6.6% of all patients) infections. Other infections included tonsillitis, otitis, isolated fever, and urinary tract infection. The difference between the two groups was statistically non-significant (p = 0.96).
The total time management (from the onset of the 1st neurological signs to the start of treatment) was studied and evaluated in days. It ranged from 1 to 33 days with a mean of 13.6 days. This delay was mainly related to the time before the consultation, which varied from 1 to 30 days with a mean of 10.3 days ± 7.7. The difference between the two groups was statistically non-significant (p = 0.15).
All our patients had a motor deficit (100%) which was predominantly symmetrical (in 94.5% of patients), stretch reflexes were abolished in all cases (100%) and hypotonia was objectified in 88% of our patients. The difference between the two groups was statistically non-significant (p > 0.05).
In this study, 46.1% of the patients had a sensory disorder. The difference between the two groups was statistically non-significant (p > 0.05).
Cranial nerve damage was present in 48.7% of cases. It was essentially the 7th bilateral cranial pair which was affected, causing bi paresis or bifacial palsy (in 44% (n = 17) of cases), followed by the bulbar nerves: X, IX, XI, and XII which were affected in 41%, and ophthalmoplegia in 15% (n = 6) of cases, for whom the diagnosis of Miller Fisher Syndrome was retained. These patients had ataxia in the foreground associated with ophthalmoplegia and areflexia while the motor deficit was mild, the anti-GQ1b antibody assay was performed in only two patients and was positive.
In our series, 47.8% of patients treated with SVP and 50% of those treated with Immunoglobulin had damage to one or more cranial nerves. The difference between the two groups is statistically non-significant (p = 0.85).
The distribution of other clinical signs including swallowing disorders, respiratory distress, ataxia, sphincter disorders; urinary disorders, constipation, and diarrhea, was comparable between the two groups. The two most frequent signs were respiratory distress in 26.3% of cases and swallowing disorders in 21.1% of GBS cases. The difference between the two groups was statistically non-significant (p > 0.05).
Electromyography was performed in all patients. There was a predominance of the acute inflammatory demyelinating polyradiculoneuropathy (AIDP), and the acute motor axonal neuropathy (AMAN) forms with equal proportions (33.6%). The difference between the two groups is statistically non-significant (p > 0.05).
Lumbar puncture was performed in all patients. Cerebrospinal fluid proteins measured ranged from 0.2 g/l to 4.3 g/l, with a mean of 1.2 g/l. The difference between the two groups is statistically non-significant (p = 0.69).
Evolution of patients presenting GBS: The evolution of the scores was similar in the two groups. At one month after treatment there was an improvement in The Medical Research Council score (MRC) of the upper limbs (UL) with a score of 5 in 50% of patients treated with SVP and 48% of patients treated with Iv Ig (Table 1), and in the lower limbs (LL); 21% of patients had a score of 5 in SVP group and 17% in Iv Ig group (Table 1). The mean MRC sum score increased from 29.8 ± 13.1 to 45.4 ± 15.3 in SVP group, and from 31 ± 15.2 to 45.7 ± 12.6 in IvIg group (Fig. 2, Table 1). The mean Hughes score in SVP group patients decreased from 3.7 ± 1.0 to 2.1 ± 1.6 and from 4.0 ± 1.0 to 2.3 ± 1.4 in IvIg group patients (Fig. 3, Table 2). Patients in both groups improved one or more grades on Hughes and MRC scores after one month of progression (Table 2). The improvement in the MRC sum score was 15.6 ± 14.3 in the SVP group and 14.7 ± 13.6 in the IV Ig group. The evolution of the different functional scores for the two groups is statistically comparable (p > 0.05).
The duration of stay in the neurology department for patients in our series ranged from 6 to 30 days with a mean of 13.7 ± 5.3 days. In SVP group the mean duration of hospital stay was 15.4 ± 5.1 days, and in Iv Ig Group it was 11.2 ± 4.6 days. The difference between the two groups was statistically significant (p < 0.001). About 32.9% of patients (n: 25) required hospitalization in an intensive care unit. The length of stay in intensive care unit ranged from 1 to 29 days with a mean of 10.4 ± 8.8 days (Tables 3, 4). The duration of ventilation ranged from1 to 21 days and 20 patients (26.3%) required ventilation during a mean of 9.8 ± 6.6 days (Tables 3, 4).
Post-treatment side effects were noted in 15 cases (21.4%) (Table 5). The onset of recovery ranged from 2 to 30 days, and a mean of 11.9 days ± 6.8 (Table 4). Recovery was complete in 73% of the cases, while 9.2% of the cases retained sequelae (17.8% lost to follow-up). Neurological sequelae: (Hughes Score > 2): 9.2% (n: 9) of our patients retained disabling sequelae; 6 cases (13.0%) in group SVP and 3 cases (10%) in Iv Ig group, it is dominated by footdrop gait.
There were four deaths (5.3%); three deaths (6.5%) in the plasmapheresis group and one death (3.3%) in the IvIg group. These patients had a severe respiratory distress at admission.
Cost analysis in a country such as Morocco is necessary, since overall health budgets are low, and most of these costs are covered directly by individuals.
The cost of plasmapheresis is essentially attributable to the price of filters and single-use equipment, which costs 3,000 MAD (3000 per device, such as a total of 15,000 MAD for five procedures). There is no need for a replacement product such as albumin, which can be purchased for around 1,096 MAD for a 100-ml dose, weight-dependent, and difficult to obtain for a blood-based product that can only be produced in limited quantities.
For immunoglobulins, the price per unit varies from 226 MAD to 570.2 MAD, or a total dose of 27,120–68,424 MAD for a patient weighing 60kg.
And considering that the only significant difference between the results of the two groups was the length of hospital stay. An analysis of the cost of hospitalization was carried out, using the pricing system for acts and services rendered by hospitals and departments under the authority of the Ministry of Health, which showed that longer hospital stays had no influence on the cost of GBS treatment.
The average cost of hospitalization in neurology for plasmapheresis was 1926 ± 639 MAD and for immunoglobulins 1400 ± 580 MAD, with a non-significant difference (p > 0.05).
Discussion
The treatment of GBS: The ultimate goal in any therapeutic approach is the improvement of GBS patients therefore, it becomes necessary to identify which treatment results in optimizing profit in a short time with few complications and minimal cost. For this reason, a comparative analysis of the two immunomodulatory treatments for GBS remains an important tool for the production of a context-based therapeutic strategy.
The specific treatment of Guillain–Barre Syndrome is based on immunotherapy, which is represented by IvIg and Plasma exchange. This immunotherapy has long proven its efficacy, particularly in severe extensive forms, by limiting the extension of paralysis and allowing an early recovery and reduction in the rate of sequelae [7,8,9].
Plasma exchanges are accepted by the American Apheresis Society Committee as first-line therapy for GBS and are recognized as grade A (good quality evidence). The strategy is to exchange 1–1.5 volumes of plasma 5–6 times over 10–14 days, with albumin or fresh-frozen plasma as replacement fluid [10].
The literature search has found a difference in the volume of plasma collected and the optimal number of plasma exchanges, depending on the trials. Many studies use the North American trial protocol in which a total of 200 to 250 ml/kg was exchanged over seven to 10 days [11].
In India, low-volume plasma exchange was used by Tharakan and colleagues with satisfactory results [12]. They used 15 ml/kg body weight/day to continue until disease progression was stopped or recovery began.
A recent study conducted at the hospital in Dhaka, Bangladesh to assess the safety and feasibility of SVPE in 20 GBS patients [13]. SVPE is based on the same principle as a conventional plasma exchange but uses a simple new technique at a lower cost. It involves the sedimentation of blood cells in a blood bag, removal of the supernatant plasma, and the blood cells are transfused again. This procedure has been repeated three to six times a day for eight consecutive days; fresh-frozen plasma (FFP) and saline were used as replacement fluids [13].
The majority of comparative studies of plasma exchange and intravenous Immunoglobulin have used a technique of 200–250 ml/kg/day for 4–5 days by centrifugation or filtration [14, 15]. A study published by an Indian team comparing the three therapeutic modalities (high-volume plasma exchange, intravenous immunoglobulin, and small-volume plasma exchange); the authors of this study concluded that there is no difference between small and large volume plasma exchanges) [16].
Factors that may influence therapeutic response: Some clinical outcomes and severity scales have been reported in the literature to predict the prognosis of the disease in the short and long term [17, 18]. Advanced age, the presence of gastroenteritis as a precursor infection, diarrhea, high Hughes score on admission, a decreased MRC sum score, respiratory failure, cranial nerve involvement, and electrophysiological type have been recognized in several studies as prognostic factors that will influence therapeutic response [17, 19,20,21,22,23].
In our series, the two groups were comparable in terms of variables that may influence therapeutic response with a p-value of < 0.05, which implies a statistically similar pattern of the degree of disease at the start of treatment [17, 19,20,21,22,23].
Evolution after treatment: The GBS disability scale is currently the reference scale for treatment indications. One month after treatment, the mean Hughes score in SVP group patients decreased from 3.7 ± 1.0 to 2.1 ± 1.6 and in group B patients from 4.0 ± 1.0 to 2.3 ± 1.4. The difference between the two groups was non-significant. These results are similar to those reported by Maheshwari and colleagues, Hughes and colleagues, and Bril and colleagues but differ from those of Kishore who found a result in support of plasma exchanges [14, 15, 24, 25].
One month after treatment the mean MRC sum score in patients of group A increased from 29.8 ± 13.1 to 45.4 ± 1 5.3 and in group B from 31.0 ± 15.2 to 45.7 ± 12.6. The difference between the two groups was not meaningful. This is consistent with data from the study by El Bayoumi and colleagues [26] and Y. Ye and colleagues [27].
The difference in the duration of mechanical ventilation between the two groups of our series is non-significant, which is in line with the results of Salara, Netto, and Vasjar and colleagues. However, Charaa and colleagues found a shorter duration with IVIG, and in the El Bayoumi study plasma exchanges shortened this duration [16, 26, 28,29,30]. In general, the duration of mechanical ventilation with both treatment modalities is less than in other studies, which may be explained by the severity of the disease in the patients included in these studies.
If intensive care measures were used; the mean length of stay in the intensive care unit was 8.9 ± 7.8 days in group A and 11.8 ± 9.8 days in group B with a p-value of 0.4. This result is comparable to the results of Maheshwari, Netto, El Bayoumi and Hughes, but differs from the results of Salara, Charaa, Walker and Alshekle who found a longer hospital stay in the intensive care unit with plasma exchanges [16, 23, 25, 26, 28, 29, 31, 32]. The average of our series remains lower than those of the other studies, which can be explained by the relative benignity of the disease of the patients included in our study compared to other studies carried out in intensive care units, as well as the organizational aspect of the university hospital center involving the minimization of the length of hospitalization, mainly in high-demand departments such as the intensive care unit.
The average length of stay in the neurology department was 15.4 ± 5.1 days in Group A and 1.2 ± 4.6 days in Group B (p < 0.001). This increase in duration in Group A is primarily related to the rhythm of treatment administration. Patients in the other studies remain hospitalized as long as possible for further rehabilitation, whereas our patients, due to lack of space, were obliged to leave the ward once they had passed the severe stage, by making an appointment for physiotherapy.
A meta-analysis published by Hughes and colleagues revealed no difference in complications related to treatment [9]. The overall side effects were 21.4% in our study, with 9.2% in group A, and 12.1% in group B, respectively. Most complications were mild, easily treated in both groups and without significant difference.
Mortality of GBS ranges from 3 to 13% in the first year. The main risk factor for mortality in the acute phase is hospitalization in intensive care units and especially the need for invasive ventilation. Death is due to an unfavorable evolution of the initial respiratory failure but especially to the appearance of pneumopathy and septic shock. Rarely is caused by the dysautonomic syndrome. In our study, there were 3/46 deaths in the group treated with plasmapheresis and 1/30 deaths in the group treated with intravenous Immunoglobulin, a number that remains higher than that of other studies (Van der Mech: 2/73–1/74, El Bayoumi 0/21–0/20 and Bril: 0/24–0/26) [14, 16, 26]. These deaths are explained by the severity of the GBS symptoms at admission including severe respiratory distress.
About 30% of patients retain residual weakness after 3 years, and about 3% suffer a relapse of muscle weakness and tingling sensations many years after the initial attack, and about 15% of GBS individuals do not fully recover [33]. In our series, nine patients (9.2%) had disabling sequelae; 6 cases (13%) in group A and 3 cases (10%) in group B. This is in line with the data in the literature 19/114 (16.6%) in the plasmapheresis group and 21/129 (16.2%) in the group treated with intravenous immunoglobulin [15].
Regarding the comparison of the average cost of the two therapy modalities, our study shows that SVP is about a quarter of the cost of IvIg.
Nagpal's meta-analysis of the direct costs of the two methods showed that IvIg was 60% more expensive than plasma exchange (PE: $6204, IVIG: $10,165) [34], 53% more expensive in an Indian study [24] and twice as expensive in the Winters study [35].
Our comparative study illustrates the efficacy and safety of both types of specific treatment. The results further encourage the use of small-volume plasmapheresis given the efficacy, safety which are comparable to those of intravenous Immunoglobulin and the lower cost even with the practically long hospital stay objectified in patients treated with plasmapheresis.
Limitations
The study is retrospective, patients were not randomized, but the two groups were comparable for different characteristics. The impact of the heterogeneity of the pathways adopted by patients from diagnosis to the last rehabilitation visits, as well as the indirect costs, were not studied. The unavailability of conventional plasma exchange throughout the study phase made a comparison of plasmapheresis and conventional plasma exchange unfeasible, despite its relevance. This will be the subject of future study.
Availability of data and materials
Available at the research database in the department of neurology Arrazi. The datasets used and/or analyzed during the current study are freely available and could be obtained on written request to the corresponding author.
Abbreviations
- IvIg:
-
Intravenous immunoglobulin
- SVP:
-
Small-volume plasmapheresis
- GBS:
-
Guillain–Barre syndrome
- PE:
-
Plasma exchange
- SVPE:
-
Small-volume plasma exchange
- MRC:
-
Medical Research Council
- n:
-
Number
- AIDP:
-
Acute inflammatory demyelinating polyradiculoneuropathy
- AMAN:
-
Acute motor axonal neuropathy
- UL:
-
Upper limbs
- LL:
-
Lower limbs
- FFP:
-
Fresh-frozen plasma
- MAD:
-
Moroccan dirham
References
Peter D, Donofrio FAAN. Guillain-Barre syndrome. Continuum J. 2017;23(5):1295–309.
Léger J-M, Haghi B, Guimarães-Costa R. Guillain-Barre syndrome: therapeutic management. Bull Acad Natl Med. 2016;200(6):1101–13.
Raphaël J-C. Present treatment of Guillain-Barre syndrome. Bull Acad Nat Med. 2004;188(1):87–95.
Verboon CH, Doets AY, Galassi G, Davidson A, Waheed W, Péréon Y, et al. Current treatment practice of Guillain-Barré syndrome. Neurology. 2019;93:1.
Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019;15(11):671–83.
Ghazanfar H, Qazi R, Ghazanfar A, Iftekhar S. Significance of Brighton criteria in the early diagnosis and management of Guillain-Barré syndrome. Cureus. 2020;12(5):e8318. https://doi.org/10.7759/cureus.8318.
Esmail S. An overview of Guillain-Barré syndrome. Neurophysio Rehab. 2019;42-6.
Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2017;2(2):CD001798. https://doi.org/10.1002/14651858.CD001798.pub3.
Hughes RAC, Swan AV, Raphael J-C, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barre syndrome: a systematic review. Brain. 2007;130(9):2245–57.
Padmanabhan A, Connelly‐Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice—evidence‐based approach from the writing committee of the American society for apheresis: the eighth special issue. J Clin Apher. 2019;34(3):171‑354.
Meena AK, Khadilkar SV, Murthy JMK. Treatment guidelines for Guillain-Barré syndrome. Ann Indian Acad Neurol. 2011;14(1):S73.
Tharakan J, Jayaprakash PA, Iyer VP. Small-volume plasma exchange in Guillain-Barre syndrome: experience in 25 patients. J Assoc Physicians India. 1990;38(8):550–3.
Islam B, Islam Z, Rahman S, Endtz HP, Vos MC, van der Jagt M, et al. Small-volume plasma exchange for Guillain-Barré syndrome in resource-limited settings: a phase II safety and feasibility study. BMJ Open. 2018;8(8):e022862.
Bril V, Ilse WK, Pearce R, Dhanani A, Sutton D, Kong K. Pilot trial of immunoglobulin versus plasma exchange in patients with Guillain-Barré syndrome. Neurology. 1996;46:100.
Kishore CK, Vijayabhaskar J, Vishnu Vardhan R, Sainaresh VV, Sriramnaveen P, Sridhar A, et al. Management of Guillain-Barré syndrome with plasmapheresis or immunoglobulin: our experience from a tertiary care institute in South India. Ren Fail. 2014;36(5):732–6.
Netto AB, Kulkarni GB, Taly AB, Rao GU, Periyavan S, Rao S. A comparison of immunomodulation therapies in mechanically ventilated patients with Guillain Barre syndrome. J Clin Neurosci. 2012;19(12):1664–7.
Ceylan M, Sonkaya A. The investigation of Guillain-Barre syndrome and prognosis. Ann Med Res. 2019; 763.
Doets AY, Jacobs BC, van Doorn PA. Advances in management of Guillain-Barré syndrome. Curr Opin Neurol. 2018;31(5):541–50. https://doi.org/10.1097/WCO.0000000000000602.
Tian C, Li Z, Li L. Electrophysiological subtypes and prognostic factors of Guillain-Barre syndrome in Northern China. Front Neurol. 2019;10:714.
Estrade S. Prognostic factors for the sequelae and severity of Guillain-Barré syndrome in children. Muscle Nerve. 2019;60(6):716–23.
Çetiner M, Seyit M, Akdağ G, Demirbaş H, Temel O, et al. Factors associated with prognosis in patients with Guillain-Barré syndrome. Turk J Neuro. 2019;25(3):140–5.
Thy P. Nguyen; Roger S. Taylor. Guillain Barre Syndrome– StatPearls,NCBI Bookshelf 2019. https://www.ncbi.nlm.nih.gov/books/NBK532254/
Konuşkan B, Okuyaz Ç, Taşdelen B, Kurul SH, Anlar B. Electrophysiological subtypes and prognostic factors of childhood Guillain-Barré syndrome. Noro Psikiyatr Ars. 2018;55(3):199–204.
Maheshwari A, Sharma RR, Prinja S, Hans R, Modi M, Sharma N, et al. Cost-minimization analysis in the Indian subcontinent for treating Guillain Barre Syndrome patients with therapeutic plasma exchange as compared to intravenous immunoglobulin. J Clin Apher. 2018;33(6):631–63.
Hughes RAC. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet Janv. 1997;349(9047):225–30.
El-Bayoumi MA, El-Refaey AM, Abdelkader AM, El-Assmy MM, Alwakeel AA, El-Tahan HM. Comparison of intravenous immunoglobulin and plasma exchange in treatment of mechanically ventilated children with Guillain Barré syndrome: a randomized study. Crit Care. 2011;15(4):R164.
Ye Y, Li SL, Li YJ. Comparison on therapeutic effect of plasma exchange and intravenous immunoglobulin for Guillain-Barre syndrome. Transfus Med. 2015;25(2):79–84.
Shunmuga sundaram K, Sarala G, Lakshmi narasimhan R, Balasubramanian S, Krishnamoorthy K .Comparative efficacy of ivig and plasma exchange in management of Guillain Barre syndrome. IJAR 2019. 9(3)
Charra B, Hachimi A, Benslama A, Motaouakkil S. Intravenous immunoglobulin versus plasma exchange in treatment of mechanically ventilated adults with Guillain-Barré syndrome. Pan Afr Med J. 2014;18:35. https://doi.org/10.11604/pamj.2014.18.35.2911.
Vajsar J, Sloane A, Wood E, Murphy G. Plasmapheresis versus intravenous immunoglobulin treatment in childhood Guillain-Barré Syndrome. JAMA Pediatrics Arch Pediatr Adolesc Med. 1994;148(11):1210–2.
Oczko-Walker M, Manousakis G, Wang S, Malter JS, Waclawik AJ. Plasma exchange after initial intravenous immunoglobulin treatment in Guillain-Barré syndrome: critical reassessment of effectiveness and cost-efficiency. J Clin Neuromuscul Dis déc. 2010;12(2):55–61.
Alshekhlee A, Hussain Z, Sultan B, Katirji B. Immunotherapy for Guillain-Barré syndrome in the US hospitals. J Clin Neuromuscul Dis sept. 2008;10(1):4–10.
Palmer SJ. A new clinical tool for diagnosis and management of Guillain-Barré syndrome. Br J Neurosci. 2019;15(6):2052–800.
Nagpal S, Benstead T, Shumak K, Rock G, Brown M, Anderson DR. Treatment of Guillain-Barré. J Clin Apher. 1999;14(3):107–13.
Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C. Cost-minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain-Barré syndrome. BMC Health Serv Res. 2011;11:101.
Acknowledgements
Not applicable.
Funding
No source of funding.
Author information
Authors and Affiliations
Contributions
KB collected, researched and wrote the manuscript. NL proposed the research topic, drafted the design, and made the modifications and follow-up throughout the study. LA studied the homogeneity of the two groups, analyzed and interpreted the statistical data. MC, AH and AB corrected and judged the work. NK took care of the general course of the study as well as the inclusion of the patients in the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Ethics Committee of the Faculty of Medicine and Pharmacy of Marrakesh in accordance with the Declaration of Helsinki on January 1, 2002, The committee’s date: 4th October 2019 (Reference number: 33/2020). The requirement of patient consent was waived by the ethics committee seeing as data were gathered from department archives and did not involve intimate details of any patient in particular.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balili, K., Louhab, N., Adarmouch, L. et al. Guillain–Barre syndrome: small-volume plasmapheresis versus intravenous immunoglobulin—3rd level hospital experience. Egypt J Neurol Psychiatry Neurosurg 60, 45 (2024). https://doi.org/10.1186/s41983-024-00820-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-024-00820-0