Abstract
Background
Stroke is a devastating condition, which not only affects patients’ activity, but also is a primary reason for the psychosocial impact on them, their caregivers, and the healthcare system. Transcranial direct current stimulation (tDCS) modulates cortical activity, encouraging neuro-modulation and motor recovery in stroke rehabilitation. Robotic therapy (RT) provides repetitive, high-intensity, interactive, task-specific intervention and can measure changes while providing feedback to people with stroke.
Objectives
This study aimed to evaluate and summarize the scientific literature systematically to investigate the combined effect of tDCS and RT in patients with stroke.
Methods
Four databases (MEDLINE, Web of Science, ScienceDirect, & PEDro) were searched for clinical trials investigating the effect of RT and tDCS in stroke patients with upper limb impairment. PEDro scale was used for the quality assessment of included studies.
Results
The search yielded 208 articles. A total of 213 patients with stroke who had upper limb impairment were studied. In the majority of the trials, RT combined with tDCS lead to positive improvement in various measures of upper limb function and spasticity.
Conclusions
RT along with tDCS is an effective mode of rehabilitation, although no additional effects of tDCS plus RT in comparison with RT alone were reported. Large, robust studies are needed, so that health care providers and researchers can make better decisions about merging tDCS and RT in stroke rehabilitation settings in the future.
Similar content being viewed by others
Introduction
Stroke is the second leading cause of death after ischemic heart disease, accounting for 9% of all fatalities worldwide [1]. The most common deficit after stroke is hemiparesis of the contralateral upper limb (UL) [2]. Stroke is one of the major reasons for disability [3]. Nearly, 33% of stroke survivors experience disability, particularly upper extremity (UE) disability [3, 4]. UE disability affects 50–80% of stroke patients in the acute phase [5,6,7] and 40–50% in the chronic phase [7, 8]. Paresis, abnormal tone of muscle, diminished somatosensation, and problems with coordination are common UE deficits after stroke [9]. UE motor impairment results in limitations of activities of daily living (ADL) [10] and lowered quality of life [10, 11].
Transcranial direct current stimulation (tDCS) is a non-invasive, safe, and relatively painless brain stimulation technique [12]. Constant weak electrical current is applied for few minutes via electrodes, placed in well-considered locations [13, 14]. Majority of the studies administer tDCS between 2 rubber electrodes 25–35 cm2, placed over the scalp using a low-current intensity, ranging from conventional 1–2 mA up to currents of 4 mA for 10–20 min [15,16,17]. It has been substantially scrutinized in cognitive and clinical neuroscience [18] and, is tolerated well by stroke patients with minimal and temporary adverse effects [19] when the protocol is performed according to the current safety guidelines [20]. Robot-mediated rehabilitation is a cutting-edge exercise-based therapy that uses robotic equipment to deliver extremely repetitive, rigorous, adaptable, and quantified physical training [21]. By offering complicated but controlled multimodal stimulation [22], robotic therapy (RT) has been presented as a potential strategy for the rehabilitation of the UE, as a way to increase the amount and intensity of therapy [22] and standardize the treatment [23]. Furthermore, robotic devices can provide quantitative measures of the user's dexterity due to their built-in technology in terms of sensors and actuators [24].
A considerable number of research papers on robot-assisted stroke rehabilitation have been published, examining the effects of robotics alone [25,26,27,28,29,30] and in combination with traditional therapy [31,32,33]. A Cochrane review published in 2012 [34] found that rehabilitation-assisted electro mechanics and robotics may assist in improving the function of the arm after stroke, so there might be an improvement in daily living activities, muscle strength, and arm function. Although, results of treatment depend on practice intensity, training amount and duration, treatment type, type of device, and characteristics of participants as they are potential drivers for efficacious motor rehabilitation interventions after stroke [35]. Extensive research has been done on the effects of tDCS on arm function and motor learning in stroke patients but conclusions drawn from these studies reported mixed inferences [36]. To this point in time, no systematic review has been conducted which determines the effect of combined tDCS and RT in different types of strokes. This review aims to fill this slot in literature to date. Therefore, the purpose of the present review was to comprehensively and systematically evaluate clinical trials to investigate the effect of tDCS in combination with RT in UE impairments in patients with stroke.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [37]. The protocol of this review was registered on PROSPERO (International Prospective Register of Systematic Reviews) and can be accessed by registration number CRD42021238334.
Study selection criteria
The inclusion criteria for the selection of studies were: full-text randomized controlled trials published in the English language; people clinically diagnosed with acute, subacute, or chronic stroke (ischemic/hemorrhagic) and having UE impairment; with age > 18 years; studies administering a combination of tDCS and RT; studies having at least two of the validated clinical outcome measure. Trials including patients with cognitive impairment, having preceding epilepsy or intracranial metal implant impairments and studies done on animals were excluded.
Search strategy
MEDLINE (accessed by PubMed), Web of Science (Web of Science Core Collection), ScienceDirect, and PEDro were searched for relevant literature between inception to February 2021. Search terms used were a combination of keywords, “transcranial direct current stimulation, robotics, upper limb, and stroke”. Boolean operators ‘AND’ or ‘OR’ were used. Three authors (AR, SP, and FB) independently searched and then compiled all the records. The titles and abstracts of records identified were analysed. Full-text articles were assessed to meet the eligibility criteria after removing the redundant studies.
Quality assessment of studies
Two reviewers (AR and SP) independently performed the quality assessment of selected studies using PEDro scale which is an 11-point scale to determine the quality of clinical trials. Any disagreement that remains unresolved was taken to the third reviewer (MN). To reduce ambiguity in responses, each criterion was rated either yes (score = 1) or no (score = 0). Summing all of the replies yielded a total score for each included study's methodological quality (maximum score = 10). Based on the total score, studies were classified as poor (score of 4), fair (score of 4 or 5), good (score of 6–8), and excellent (score of > 8) quality [38].
Results
Study selection
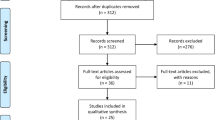
A sum of 208 articles was identified after a systematic search of databases. A total of 55 duplicates were removed. The remaining 153 articles were then screened by reading their title and abstract. Of these, 136 articles were excluded after screening. The remaining 17 articles were identified and were assessed for eligibility by three reviewers (AR, SP, and FB). A total of six studies were considered for qualitative analysis (Fig. 1).
Characteristics of study
Participants
Six papers included in this review had a total of 213 patients with stroke. Their age ranged from 18 to 90 years. The sample size ranged from 21 participants [39] to 82 participants [40]. Details of sample size calculation were reported in only one study [41], where the sample size was determined using the PS Power and sample size calculation software (version 3.1.2, department of biostatistics, Vanderbilt University, USA). In other studies, the method of calculation was not mentioned [3, 4, 36, 39, 40]. Two studies included patients with subacute stroke [3, 41], two with chronic stroke [39, 40], and two trials included both subacute and chronic stroke patients [4, 36]. All the studies included both males and female in different proportions (Table 1).
Intervention
In three studies, a current of 2 mA [3, 40, 41] was used and the rest of the studies used 1 mA [4, 36, 39]. Only one study used a single session of RT and tDCS, while the rest of the trials used multiple sessions of RT and tDCS, which ranged from 2 sessions [4] to 36 sessions [40] per week. The duration of the treatment session varied from 20 min [39] to 1 h and 20 min [4, 40] (Table 2).
Outcome measures
Four studies used box and block test (BBT) for gross and fine manual dexterity [3, 36, 39, 41]. Four studies used Fugl–Meyer assessment scale (FMAS) [3, 4, 36, 40, 41], two studies used motricity index (MI) [3, 41], and two studies employed motor activity log (MAL) [4, 36]. For the assessment of spasticity, modified Ashworth scale for the wrist (MAS/w) was used in two trials [3, 41]. Only one study used action research arm test (ARAT) [4] (Table 1).
Quality assessment of included trials
The average PEDro score for all the included studies was 8.5 (excellent quality). Four trials scored 8/10 [3, 36, 39, 41], one scored 9/10 [4], and one scored 10/10 [40]. Three studies lacked allocation concealment [36, 39, 41]. All the included trials reported blinding of the participants. Blinding of the therapist was reported in only one trial [40]. There was a blinding of assessors in two studies [3, 41]. All the studies scored well on reporting between-group differences, random allocation, point estimation, and variability reporting. All the studies applied to intention to treat analysis of drop-outs (Table 3).
Effect of RT and tDCS on UE function and spasticity
Five studies found positive improvement in arm motor recovery when measured with FMAS-UE [3, 4, 36, 40, 41]. Two studies showed no significant positive change in MAS/w score [3, 41]. Five studies showed an increase in BBT score, out of which only three studies reported a significant increase in BBT score [3, 36, 41]. No significant change was reported in scores of MAL in two trials [4, 36]. Two studies showed positive significant change in MI [3, 41]. Two studies administered tDCS first followed by RT [4, 40]. Three studies administered tDCS simultaneously with RT [3, 39, 41]. One trial did not make it clear whether RT was administered first or tDCS or both were delivered simultaneously to the patient [36].
Discussion
This is the first systematic review providing information on major findings, characteristics, and quality of studies investigating the combined effect of RT and tDCS on UE motor function and spasticity in people with stroke. Although the direct pooled analysis was limited due to the variability of the outcome measures, the conclusion obtained from the existing evidence implies that stroke patients receiving RT combined with tDCS had a favourable effect on UE motor function of stroke patients having UE impairment as indicated by positive changes in FMAS-UE, MI, and BBT score.
The studies provided considerable motor improvements, evaluated by clinical scales, although, no superiority of real tDCS was seen over sham tDCS. Straudi and her colleagues administered 10 sessions of RT and real tDCS in patients with acute and chronic stroke for 2 weeks. When these patients were compared with stroke patients receiving RT and sham tDCS, no significant difference was reported in measures of hand function [36]. Similar findings were reported by Edward et al. [40], Mazzoleni et al. [3], Triccas et al. [4], and Mazzoleni et al. [41]. Dehem et al. [39] showed a significant difference in scores of BBT, after patients received RT + real tDCS. All studies reported no significant difference in scores of FMAS-UE between groups receiving RT + tDCS and RT + sham tDCS. A possible explanation for the similarity between the groups could be the relative timing of delivering the stimulation or the effectiveness of tDCS overlapped due to the effect of high-intensity RT [41]. The BBT was used in four studies to assess UE gross motor function [3, 36, 39, 41]. It was found that both treatment arms improved BBT score and the patient was able to move the box to a greater distance. The MAL tool was used in 2 trials [4, 36], and researchers found significant differences from baseline to post-intervention without any significant difference between the groups. Two studies used MAS/w, where one study reported only a slight decrease in spasticity in both groups [3]. In another study, the score of the scale remained unchanged in both groups [41].
The effect of lesion location does not affect the intervention outcome. This was examined in a study [40], where the administration of RT plus tDCS in patients with cortical and subcortical lesions does not influence the clinical improvement. Two studies investigated the combined effect of RT and tDCS in subacute and chronic stroke patients [4, 36]. According to Straudi et al., bilateral tDCS along with RT was more effective and suitable in chronic stroke patients [36]. Conversely, Triccas et al. showed that the percentage improvement of motor impairment was more in subacute stroke patients [4]. This can be explained by the spontaneous recovery in the subacute phase which involves cortical reorganization [42, 43].
Only two studies examined the sustained effect of RT + tDCS and evaluated the participants for follow-up [4, 40]. The treatment effect does persist for a longer period of time, as seen in one study, where significant changes were reported in scores of FMAS-UE between baseline and follow-up at 3 months; however, no significant difference was shown in scores of FMAS-UE between groups receiving RT + tDCS and RT + sham tDCS [4]. Similar findings were reported in another study, where a 6-month follow-up was performed. Significant changes were observed in measures of UE motor function between baseline and 6-month follow-up but no significant mean difference between groups was found [40].The current amplitude used by three studies was 1 mA [4, 36, 39], while the rest of the studies included in this review used 2 mA of current [3, 40, 41]. No change was observed in clinical improvement when studies used different stimulation intensities.
The essential process driving improvement in functional outcomes after a stroke is neuroplasticity [44]. As a result, one of the most essential goals of stroke therapy is to make good use of neuroplasticity for functional recovery. Goal planning, high-intensity practice, multidisciplinary team care, and task-specific training are some of the principles of stroke rehabilitation [43]. As a result, for stroke recovery, high-dose rigorous training and repetitive practice of functional tasks are critical [45]. All these requirements make stroke rehabilitation a labor-intensive process. The tDCS [13, 46] and RT [47] are two promising therapies in rehabilitation used for stroke patients. It is unlikely for tDCS to bring any optimal functional change on its own but it augments other rehabilitation strategies by enhancing brain plasticity. The RT has been proven to improve functional recovery after stroke [26]. Both of these treatments aim to influence brain plasticity; therefore, there is a case to be made for seeing if combining the two could provide an additive effect. However, the optimal approach for combined therapy of tDCS and RT, and whether the outcomes of the combination of these two are cooperative or conflicting, is yet to be determined. The tDCS and RT are synergistic therapy approaches which was evident by an increase in corticospinal excitability resulting from the administration of tDCS followed by RT [40].
This systematic review analyzed the effect of integrated therapies using RCTs, has good methodological qualities, and certifies a significant level of evidence, so the results are considered reliable. However, there are certain limitations of this review. First, this review lacks studies administering tDCS and RT in acute cases of stroke. The second limitation of this study is the number of patients in the included studies was quite small to be analyzed with full potential. Third, a follow-up in four out of six studies was not done. This could provide us with a sustained effect of desired treatment after completing the proposed sessions.
RT can be used as an adjunct in the management of UE impairment in stroke patients. Future research should involve administering tDCS + RT with more complex tasks with different stimulation time. A large sample size in future studies could help generalize data over the population. Trials on examining carryover effects can also be done.
Conclusion
Motor improvement was observed in the affected UE after taking combined tDCS and RT intervention in both groups. However, there is no added benefit of real direct current stimulation compared to sham, justifying robotic therapy as the main influencer of recovery. A probable reason for this could be the administration of robotic training at high intensity. The sample size was too small in the included trials and the effects of the two treatments were overlapping, making it difficult to detect potential benefits solely from stimulation. The treatment was seen to be more effective in sub-acute cases rather than chronic, and the treatment program used in the studies is safe and well-tolerated by patients, with no or minimal adverse events.
Availability of data and materials
Not applicable.
Abbreviations
- UL:
-
Upper limb
- UE:
-
Upper extremity
- tDCS:
-
Transcranial direct current stimulation
- RT:
-
Robotic therapy
- ADL:
-
Activities of daily living
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- RCTs:
-
Randomized Controlled Trials
- BBT:
-
Box and Block Test
- FMAS:
-
Fugl–Meyer Assessment Scale
- MI:
-
Motricity Index
- MAS/w:
-
Modified Ashworth Scale for wrist
- MAL:
-
Motor Activity Log 28
- ARAT:
-
Action Research Arm Test
- FM-UE:
-
Upper sub-section of Fugl–Meyer Assessment Scale
References
Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349(9061):1269–76.
Hatem SM, Saussez G, della Faille M, Prist V, Zhang X, Dispa D, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci. 2016. https://doi.org/10.3389/fnhum.2016.00442.
Mazzoleni S, Tran VD, Iardella L, Dario P, Posteraro F. Randomized, sham-controlled trial based on transcranial direct current stimulation and wrist robot-assisted integrated treatment on subacute stroke patients: Intermediate results. IEEE Int Conf Rehabil Robot. 2017;2017:555–60.
Triccas LT, Burridge JH, Hughes A, Verheyden G, Desikan M, Rothwell J. A double-blinded randomised controlled trial exploring the effect of anodal transcranial direct current stimulation and uni-lateral robot therapy for the impaired upper limb in sub-acute and chronic stroke. NeuroRehabilitation. 2015;37(2):181–91.
Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32(6):1279–84.
Persson HC, Alt Murphy M, Danielsson A, Lundgren-Nilsson Å, Sunnerhagen KS. A cohort study investigating a simple, early assessment to predict upper extremity function after stroke-a part of the SALGOT study. BMC Neurol. 2015;15:92. https://doi.org/10.1186/s12883-015-0349-6.
Jørgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Støier M, Olsen TS. Outcome and time course of recovery in stroke. Part I: outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76(5):399–405.
Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21(8):357–64.
Coupar F, Pollock A, Rowe P, Weir C, Langhorne P. Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin Rehabil. 2012;26(4):291–313.
Kang N, Lee RD, Lee JH, Hwang MH. Functional balance and postural control improvements in patients with stroke after noninvasive brain stimulation: a meta-analysis. Arch Phys Med Rehabil. 2020;101(1):141–53. https://doi.org/10.1016/j.apmr.2019.09.003.
Baker K, Barrett L, Playford ED, Aspden T, Riazi A, Hobart J. Measuring arm function early after stroke: is the DASH good enough? J Neurol Neurosurg Psychiatry. 2016;87(6):604–10.
Ang KK, Guan C, Phua KS, Wang C, Teh I, Chen CW, et al. Transcranial direct current stimulation and EEG-based motor imagery BCI for upper limb stroke rehabilitation. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:4128–31. https://doi.org/10.1109/EMBC.2012.6346875.
Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527 Pt 3(Pt 3):633–9.
Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127(2):1031–48.
Khadka N, Borges H, Paneri B, Kaufman T, Nassis E, Zannou AL, et al. Adaptive current tDCS up to 4 mA. Brain Stimul. 2020;13(1):69–79.
Aparício LVM, Guarienti F, Razza LB, Carvalho AF, Fregni F, Brunoni AR. A systematic review on the acceptability and tolerability of transcranial direct current stimulation treatment in neuropsychiatry trials. Brain Stimul. 2016;9(5):671–81.
Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53.
Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14(8):1133–45.
Russo C, Souza Carneiro MI, Bolognini N, Fregni F. Safety review of transcranial direct current stimulation in stroke. Neuromodulation. 2017;20(3):215–22.
Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmöller J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol. 2021;132(1):269–306. https://doi.org/10.1016/j.clinph.2020.10.003.
Duret C, Grosmaire A-G, Krebs HI. Robot-assisted therapy in upper extremity hemiparesis: overview of an evidence-based approach. Front Neurol. 2019. https://doi.org/10.3389/fneur.2019.00412.
Masiero S, Armani M, Rosati G. Upper-limb robot-assisted therapy in rehabilitation of acute stroke patients: focused review and results of new randomized controlled trial. J Rehabil Res Dev. 2011;48(4):355–66.
Gassert R, Dietz V. Rehabilitation robots for the treatment of sensorimotor deficits: a neurophysiological perspective. J Neuroeng Rehabil. 2018;15(1):46.
Germanotta M, Cruciani A, Pecchioli C, Loreti S, Spedicato A, Meotti M, et al. Reliability, validity and discriminant ability of the instrumental indices provided by a novel planar robotic device for upper limb rehabilitation. J Neuroeng Rehabil. 2018;15(1):39.
Housman SJ, Scott KM, Reinkensmeyer DJ. A randomized controlled trial of gravity-supported, computer-enhanced arm exercise for individuals with severe hemiparesis. Neurorehabil Neural Repair. 2009;23(5):505–14.
Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362(19):1772–83.
Klamroth-Marganska V, Blanco J, Campen K, Curt A, Dietz V, Ettlin T, et al. Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomised trial. Lancet Neurol. 2014;13(2):159–66.
Orihuela-Espina F, Roldán GF, Sánchez-Villavicencio I, Palafox L, Leder R, Sucar LE, et al. Robot training for hand motor recovery in subacute stroke patients: a randomized controlled trial. J Hand Ther. 2016;29(1):51–7 (quiz 7).
Vanoglio F, Bernocchi P, Mulè C, Garofali F, Mora C, Taveggia G, et al. Feasibility and efficacy of a robotic device for hand rehabilitation in hemiplegic stroke patients: a randomized pilot controlled study. Clin Rehabil. 2017;31(3):351–60.
Conroy SS, Whitall J, Dipietro L, Jones-Lush LM, Zhan M, Finley MA, et al. Effect of gravity on robot-assisted motor training after chronic stroke: a randomized trial. Arch Phys Med Rehabil. 2011;92(11):1754–61.
Fazekas G, Tavaszi I. The future role of robots in neuro-rehabilitation. Expert Rev Neurother. 2019;19(6):471–3.
McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2015;96(6):981–90.
Palermo E, Hayes DR, Russo EF, Calabrò RS, Pacilli A, Filoni S. Translational effects of robot-mediated therapy in subacute stroke patients: an experimental evaluation of upper limb motor recovery. PeerJ. 2018;6: e5544.
Mehrholz J, Hädrich A, Platz T, Kugler J, Pohl M. Electromechanical and robot-assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. 2012;6:Cd006876.
Morone G, Cocchi I, Paolucci S, Iosa M. Robot-assisted therapy for arm recovery for stroke patients: state of the art and clinical implication. Expert Rev Med Devices. 2020;17(3):223–33.
Straudi S, Fregni F, Martinuzzi C, Pavarelli C, Salvioli S, Basaglia N. tDCS and robotics on upper limb stroke rehabilitation: effect modification by stroke duration and type of stroke. BioMed Res Int. 2016. https://doi.org/10.1155/2016/5068127.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097.
de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–33.
Dehem S, Gilliaux M, Lejeune T, Delaunois E, Mbonda P, Vandermeeren Y, et al. Effectiveness of a single session of dual-transcranial direct current stimulation in combination with upper limb robotic-assisted rehabilitation in chronic stroke patients: a randomized, double-blind, cross-over study. Int J Rehabil Res. 2018;41(2):138–45.
Edwards DJ, Cortes M, Rykman-Peltz A, Chang J, Elder J, Thickbroom G, et al. Clinical improvement with intensive robot-assisted arm training in chronic stroke is unchanged by supplementary tDCS. Restor Neurol Neurosci. 2019;37(2):167–80.
Mazzoleni S, Tran VD, Dario P, Posteraro F. Effects of transcranial direct current stimulation (tDCS) combined with wrist robot-assisted rehabilitation on motor recovery in subacute stroke patients: a randomized controlled trial. IEEE Trans Neural Syst Rehabil Eng. 2019;27(7):1458–66.
O’Dell MW, Lin CC, Harrison V. Stroke rehabilitation: strategies to enhance motor recovery. Annu Rev Med. 2009;60:55–68.
Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–702.
Pekna M, Pekny M, Nilsson M. Modulation of neural plasticity as a basis for stroke rehabilitation. Stroke. 2012;43(10):2819–28.
Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet. 1999;354(9174):191–6.
Butler AJ, Shuster M, O’Hara E, Hurley K, Middlebrooks D, Guilkey K. A meta-analysis of the efficacy of anodal transcranial direct current stimulation for upper limb motor recovery in stroke survivors. J Hand Ther. 2013;26(2):162–70 .
Volpe BT, Huerta PT, Zipse JL, Rykman A, Edwards D, Dipietro L, et al. Robotic devices as therapeutic and diagnostic tools for stroke recovery. Arch Neurol. 2009;66(9):1086–90.
Acknowledgements
Authors are thankful to Jamia Millia Islmia, New Delhi, India for providing the access to various databases for study.
Funding
No funding has been received for this study.
Author information
Authors and Affiliations
Contributions
AR: methodology, investigation, data curation, writing—original draft. SP: methodology, investigation, data curation, writing—original draft, writing—review and editing. FB: methodology, investigation, data curation. MNN: methodology, investigation, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
The type of study was systamatic review and no patient records were used.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rizvi, A., Parveen, S., Bazigha, F. et al. Effect of transcranial direct current stimulation in combination with robotic therapy in upper limb impairments in people with stroke: a systematic review. Egypt J Neurol Psychiatry Neurosurg 59, 41 (2023). https://doi.org/10.1186/s41983-023-00640-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00640-8