Abstract
Background
Oxidative stress has a significant influence in the initiation and progression of epileptic seizures. It was reported that inhibiting oxidative stress could protect against epilepsy. The aim of the current research is to estimate some biomarkers that reflect the oxidative stress in epileptics, its relation to seizure control as well as to study the impact of antiepileptic drugs (AEDs) on these biomarkers. This case–control study included 62 epileptic patients beside 62 age and gender-matched healthy controls. The epileptic patients subjected to detailed history taking with special regards to disease duration, seizure frequency, and the current AEDs. Laboratory evaluation of serum malondialdehyde (a lipid peroxidation byproduct) and superoxide dismutase (an endogenous antioxidant) were done.
Results
Malondialdehyde (MDA) was significantly higher, and superoxide dismutase (SOD) was lower in epileptic patients than in the controls (p < 0.001). Seizure frequency was directly correlated with MDA (r = 0.584, p < 0.001) while inversely correlated with SOD (r = − 0.432, p = 0.008). High MDA and low SOD were recorded in epileptic patients receiving polytherapy as compared to monotherapy (p < 0.001).
Conclusions
Epileptic patients had higher oxidative stress biomarkers than healthy individuals. Frequent seizures, long disease duration, and AEDs were associated with higher MDA and lower SOD that reflects an imbalance in the oxidant–antioxidant status among these patients.
Similar content being viewed by others
Introduction
Epilepsy is a neurological illness affecting over 70 million people worldwide. It is characterized by a continuous tendency for spontaneous (unprovoked) seizures that carries multiple behavioral, cognitive, and psychosocial consequences [1, 2]. The previous experimental and clinical studies had demonstrated the influence of oxidative stress on epilepsy pathogenesis. It was reported that oxidative stress can impact seizure initiation and recurrence [3,4,5].
Oxidative stress refers to an imbalance between generation and degradation of reactive oxygen and nitrogen species [6]. The reactive oxygen species that are generated during cellular metabolism can be neutralized by either endogenous antioxidant enzymes, for example, superoxide dismutase (SOD) [7, 8] or nonenzymatic pathway through molecules with scavenging properties, for example, vitamin E, melatonin, and glutathione [9].
Superoxide dismutase, an intracellular antioxidant enzyme, belongs to metalloenzymes. It stimulates the conversion of superoxide radical to hydrogen peroxide [4]. The SOD level is considered a biomarker that reflects the antioxidant status in various studies [10,11,12]
Lipid peroxidation process denotes to the damage of polyunsaturated fatty acids that caused by oxidative stress and leads to irreversible damage of cell membrane [13,14,15] and changing membrane permeability leading to hyperexcitability [16].
Malondialdehyde is considered as an important byproduct of lipid peroxidation which is formed by oxidation of polyunsaturated lipids [17]. Malondialdehyde was vastly employed as a marker of oxidative stress among many studies [12, 18, 19].
This study aimed to assess SOD and MDA levels among epileptic patients and compare them with controls. In addition, studying the impact of seizure frequency, disease duration, and AEDs on these biomarkers.
Methods
Study design and patients
A case–control study that included a total of one hundred and twenty-four subjects; 62 epileptic patients beside 62 age and gender matched healthy individuals as controls. The epileptic group consisted of patients aged 18 to 45 years old. These patients were selected from the outpatient’s epilepsy clinic, Neurology Department, Zagazig University from April to December 2020. Epilepsy was diagnosed according to the International League Against Epilepsy (ILAE) 2017 classification system [20, 21].
Inclusion criteria patients aged 18 to 45 years old, on regular antiepileptic drugs either monotherapy or polytherapy. Exclusion criteria: smoking, pregnancy, breastfeeding, psychiatric comorbidity, acute, or chronic medical illnesses, malignancies, and metabolic disorders. Patients who take medications other than AEDs were also excluded from the study.
The research protocol was approved by the Ethics Committee of our institution (ZU-IRB # 6007/9-3-2020). Written consent was taken out from all included subjects.
Clinical and laboratory assessment
All patients were subjected to thorough examination and history taking including disease duration, seizure frequency, seizure control (either controlled or uncontrolled according to the response to AED treatment), and the current antiepileptic medications. Patients who were seizure free during the last year prior to the study were considered controlled while the uncontrolled patients were considered when adequate trials of two tolerated, properly selected antiepileptic drugs (whether used separately or in combination) with proper doses failed to control patient’s seizure [22]. Regarding the AEDs treatment, patients who were on single AED were categorized as monotherapy and those on more than one drug were considered polytherapy recipients.
Electroencephalography (EEG) using EB Neuro machine (Italy), according to 10- 20 system of electrode placement and magnetic resonance imaging (MRI) of brain by 1.5 Tesla MR imager (Achieva, Philips Medical System) were done at the time of recruitment to all patients.
Blood sample collection
Venous blood samples were obtained from participants by venipuncture from the antecubital vein using a disposal plastic syringe and collected without using an anticoagulant. After clotting, the blood was centrifuged at 4000 rpm for 15 min. Serum was separated from the blood and stored at – 20 °C until chemical analysis.
Measurement of serum MDA and SOD levels were done at Medical Biochemistry and Molecular Biology Department by calorimetric method according to Ohkawa et al. [23] for MDA (nmol/ml), and to Nishikimi et al. [24] for SOD (U/ml).
Principle of MDA The methododology is based on the reaction of MDA with thiobarbituric acid in acidic medium at temperature of 95 °C for 30 min to form thiobarbituric acid reactive product. The absorbance of the resulting product can be measured spectrophotometrically at 534 nm [23].
Estimation of malondialdhyde
Principle of SOD This test depends on the capability of SOD to inhibit the phenazine methosulphate-mediated reduction of nitro blue tetrazolium dye. The change in the absorbance over 5 min was measured at 560 nm for control (Δcontrol) and for sample (Δsample) at 25 °C. 1.5 U/assay of the purified enzyme produced 80% inhibition [24]
Calculation of SOD
Statistical analysis
Data analysis was done using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). Qualitative data were expressed as number and percentage, Quantitative data were expressed as mean and standard deviation. Chi-square, Student’s t test, and Pearson correlation coefficient were used when appropriate. For multiple group comparisons, we used analysis of variance (ANOVA) followed by Tukey’s Post Hoc test. p value was set at ≤ 0.05 for significant results.
Results
Sixty-two epileptic patients (36 males and 26 females) and 62 controls (35 males and 27 females) were recruited. The mean age (± SD) was 27.98 ± 6.44 for epileptic patients and 26.63 ± 6.06 years for controls. In the epileptic group, 21 patients with focal and 41 patients with generalized seizures. The mean age of disease onset was 17.85 ± 4.64 years, the mean disease duration was 13.11 ± 8.19 years. 41.9% of patients were well-controlled by AEDs while 58.1% were uncontrolled. Regarding the AEDs, there were 27 (43.5%) patients on monotherapy and 35 patients (56.5%) on polytherapy (Table 1).
Malondialdehyde was significantly higher (p < 0.001) among the epileptic group (5.83 ± 1.46 nmol/ml) than controls (4.80 ± 0.62 nmol/ml). Superoxide dismutase (SOD) was significantly lower (7.59 ± 0.85 U/ml) among epileptics than controls (11.98 ± 1.39 U/ml) (p < 0.001) (Table 1).
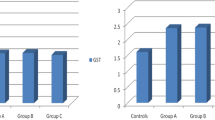
There was a direct correlation between seizure frequency and MDA (p < 0.001), and an inverse correlation between seizure frequency and SOD (p = 0.008) (Fig. 1). Regarding the relation of disease duration with laboratory parameters, low SOD and high MDA concentrations were observed among patients with disease duration > 5 years than those ≤ 5 years. No significant difference in levels of MDA or SOD was observed between patients with focal and generalized seizures (Table 2).
Regarding AEDs, lower SOD and higher MDA were demonstrated in patients receiving polytherapy than those on monotherapy and healthy controls (Table 3). Nonsignificant difference was detected between individual AEDs as regard MDA or SOD levels (Table 4).
Discussion
Oxidative stress attracts great interest in the pathogenesis of epilepsy [25]. It could aid in recognizing individuals at risk of developing epilepsy. Hence, it might facilitate the clinical trials concerned with antiepileptogenesis [26].
In the current study, the oxidant status was studied through assessment of MDA as a byproduct of lipid peroxidation, while the antioxidant status was assessed by measuring SOD as an endogenous antioxidant. There was a significant increase in mean values of MDA in epileptic group than controls. This is in consistence with many previous human [11, 12, 18, 19, 27,28,29,30] and experimental studies [25, 31]. A significant (p < 0.001) reduction in mean values of SOD was demonstrated among epileptic group than controls as observed in the previous studies [10,11,12, 29, 32] in which the SOD level among epileptic patients was significantly less than controls.
In contrary, other studies had demonstrated no significant alteration in SOD values between the epileptics and controls [30, 33,34,35]. Moreover, Ercegovac et al. [36] observed a significant elevation of SOD among patients with first seizure. They explained this rise in SOD level as an adaptive mechanism to face the increased free radical generation during seizure.
From the above data, we observed that epileptic patients had an imbalance in the oxidant–antioxidant status. It could be explained as recurrent epileptic seizures can cause oxidative stress and free radicals formation leading to macromolecular damage, neuroinflammation and neurotoxicity [37]. The seizure, as a brain insult, produces free radicals that disturb the mitochondrial function and energy metabolism and lead to enhancement of lipid peroxidation, gliosis and abnormal rearrangements of neural circuits that promote the formation of hyperexcitable networks [38, 39].
On studying the relation between biomarkers of oxidative stress and seizure profile, we found a direct correlation between seizure frequency and MDA. However, an indirect correlation was observed between SOD and seizure frequency. Similarly, Maes et al. [40] found that highly frequent seizures were associated with high levels of oxidative stress markers as MDA.
On stratifying our epileptic patients based on disease duration (> 5 years and ≤ 5 years duration), there was significantly high values of MDA and lower values of SOD in patients with disease duration > 5 years. In contrast, Turkdogan et al. [41] found no relation between antioxidant enzymes or MDA levels and disease duration.
Regarding seizure type of our patients, no significant difference in MDA or SOD values could be observed between patients with focal and generalized seizures. Similarly, Yis et al. [42] found no difference in oxidant and antioxidant biomarkers between patients with generalized and focal epilepsy. This denotes that epilepsy type does not disturb oxidant/antioxidant status in different ways.
In this study, we compared patients on single drug (monotherapy) with those on multiple drugs (polytherapy) to study the effect of antiepileptic drugs, if any, on MDA and SOD. we observed higher MDA and lower SOD levels in polytherapy than monotherapy patients and healthy controls. This finding suggests that currently used antiepileptic drugs did not improve the antioxidant status in those patients and an additional oxidative stress could be induced by AEDs.
Similarly, Iwuozo et al. [43] demonstrated that patients on AED polytherapy had significantly higher MDA and lower SOD levels than AED naïve patients as well as patients on monotherapy. Also, Ethemoglu et al. [37] found higher oxidant and lower antioxidant biomarkers in polytherapy in comparison to monotherapy groups. They believed that the patients on monotherapy consisted of patients with controlled seizures, while patients receiving polytherapy had higher seizure frequency and being uncontrolled. While Menon et al. [18, 44] and Guler et al. [11] recorded no significant alterations in the biomarkers of oxidant–antioxidant status between patients receiving monotherapy and polytherapy.
The oxidative stress induced by AEDs was explained as many conventional AEDs are metabolized to active metabolites able to combine with vital molecules such as lipids and proteins and resulting in impairment of cellular function and structure rather than having a neuroprotective effect [45].
Accumulating evidence suggests that new generations of AEDs are superior on the conventional AEDs in terms of neuroprotection and antioxidant effects by scavenging-free radicals [3, 46]. To address this point, 27 epileptic patients on monotherapy were tested in the current study for MDA and SOD. We found no significant changes between individual AED groups as regard MDA and SOD concentrations. This could be attributed to small number of patients in each individual AED group. Therefore, the superiority of an individual AED on antioxidant status could not be inferred from the results of this study and future studies with large sample size recruiting patients receiving different AEDs including the newer and old AEDs might reveal this issue.
Conclusions
In this study, we observed that epileptic patients had an imbalance in the oxidan–antioxidant status as we found higher MDA and lower SOD levels in patients than healthy individuals. Types of epilepsy did not affect oxidative status and antioxidant enzyme activities. Poor seizure control impaired the oxidant–antioxidant regulatory system. AEDs did not improve the antioxidant status in epileptic patients and an additional oxidative stress could be induced by AEDs. Future research should focus on novel drug treatments that can modify the development and progression of epilepsy through having antioxidant effect.
Availability of data and materials
The data results generated or analyzed during this study are included in this published article.
Abbreviations
- AEDs:
-
Antiepileptic drugs
- SOD:
-
Superoxide dismutase
- MDA:
-
Malondialdehyde
- ILAE:
-
International league against epilepsy
- EEG:
-
Electroencephalography
- MRI:
-
Magnetic resonance imaging
- CBZ:
-
Carbamazepine
- VPA:
-
Valproate
- LMG:
-
Lamotrigine
- LEV:
-
Levitracetam
- OXC:
-
Oxcarbazepine
References
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82.
Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. The Lancet. 2019;393(10172):689–701.
Shin EJ, Jeong JH, Chung YH, Kim WK, Ko KH, Bach JH, et al. Role of oxidative stress in epileptic seizures. Neurochem Int. 2011;59(2):122–37.
Aguiar CC, Almeida AB, Araújo PV, Abreu RN, Chaves EM, Vale OC, et al. Oxidative stress and epilepsy: literature review. Oxid Med Cell Longev. 2012;2012: 795259. https://doi.org/10.1155/2012/795259.
Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Exp Neurol. 2013;1(244):11–21.
Fujii H, Nakai K, Fukagawa M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Ther Apher Dial. 2011;15(2):125–8.
Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology. 2012;17(4):311–21.
Yasui K, Baba A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm Res. 2006;55(9):359–63.
Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018;63(1):68–78.
Al-Muhammadi MO, Al-Tameemi KM, Kadhim HM. Role of superoxide dismutase enzyme in patients with epilepsy. Med J Babylon. 2015;12(4):1015–9.
Guler SK, Aytac B, Durak ZE, Cokal BG, Gunes N, Durak I, et al. Antioxidative–oxidative balance in epilepsy patients on antiepileptic therapy: a prospective case–control study. Neurol Sci. 2016;37(5):763–7.
Prasad DK, Satyanarayana U, Shaheen U, Prabha TS, Munshi A. Oxidative stress in the development of genetic generalised epilepsy: an observational study in southern Indian population. J Clin Diagn Res JCDR. 2017;11(9):BC05.
Yoshida Y, Umeno A, Shichiri M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J Clin Biochem Nutr. 2013;52(1):9–16.
Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014: 360438. https://doi.org/10.1155/2014/360438.
Nigar S, Pottoo FH, Tabassum N, Verma SK, Javed MN. Molecular insights into the role of inflammation and oxidative stress in epilepsy. J Adv Med Pharm Sci. 2016;10(1):1–9. https://doi.org/10.9734/JAMPS/2016/24441.
Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys J. 2007;93(12):4225–36.
Sapkota M, Wyatt TA. Alcohol, aldehydes, adducts and airways. Biomolecules. 2015;5(4):2987–3008.
Menon B, Ramalingam K, Kumar RV. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure. 2012;21(10):780–4.
Das A, Sarwar MS, Hossain MS, Karmakar P, Islam MS, Hussain ME, et al. Elevated serum lipid peroxidation and reduced vitamin C and trace element concentrations are correlated with Epilepsy. Clin EEG Neurosci. 2019;50(1):63–72.
Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):522–30.
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21.
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies [published correction appears in Epilepsia. 2010 Sep; 51(9):1922]. Epilepsia. 2010;51(6):1069–77. https://doi.org/10.1111/j.1528-1167.2009.02397.x.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46(2):849–54.
Pauletti A, Terrone G, Shekh-Ahmad T, Salamone A, Ravizza T, Rizzi M, et al. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain. 2019;142(7): e39. https://doi.org/10.1093/brain/awz130.
Simonato M, Agoston DV, Brooks-Kayal A, Dulla C, Fureman B, Henshall DC, et al. Identification of clinically relevant biomarkers of epileptogenesis—a strategic roadmap. Nat Rev Neurol. 2021;17(4):231–42.
Hamed SA, Abdellah MM, El-Melegy N. Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients. J Pharmacol Sci. 2004;96(4):465–73.
Hamed SA, Hamed EA, Hamdy R, Nabeshima T. Vascular risk factors and oxidative stress as independent predictors of asymptomatic atherosclerosis in adult patients with epilepsy. Epilepsy Res. 2007;74(2–3):183–92.
Nemade ST, Melinkeri RR. Oxidative and antioxidative status in epilepsy. Pravara Med Rev. 2010;2(4):8–10.
Saad K, Hammad E, Hassan AF, Badry R. Trace element, oxidant, and antioxidant enzyme values in blood of children with refractory epilepsy. Int J Neurosci. 2014;124(3):181–6.
Dal-Pizzol F, Klamt F, Vianna MM, Schröder N, Quevedo J, Benfato MS, et al. Lipid peroxidation in hippocampus early and late after status epilepticus induced by pilocarpine or kainic acid in Wistar rats. Neurosci Lett. 2000;291(3):179–82.
Ben-Menachem E, Kyllerman M, Marklund S. Superoxide dismutase and glutathione peroxidase function in progressive myoclonus epilepsies. Epilepsy Res. 2000;40(1):33–9.
Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. 2001;303(1–2):19–24.
Verrotti A, Basciani F, Trotta D, Pomilio MP, Morgese G, Chiarelli F. Serum copper, zinc, selenium, glutathione peroxidase and superoxide dismutase levels in epileptic children before and after 1 year of sodium valproate and carbamazepine therapy. Epilepsy Res. 2002;48(1–2):71–5.
Peker E, Oktar S, Arı M, Kozan R, Doğan M, Çağan E, et al. Nitric oxide, lipid peroxidation, and antioxidant enzyme levels in epileptic children using valproic acid. Brain Res. 2009;22(1297):194–7.
Ercegovac M, Jovic N, Simic T, Beslac-Bumbasirevic L, Sokic D, Djukic T, et al. Byproducts of protein, lipid and DNA oxidative damage and antioxidant enzyme activities in seizure. Seizure. 2010;19(4):205–10.
Ethemoglu O, Ay H, Koyuncu I, Gönel A. Comparison of cytokines and prooxidants/antioxidants markers among adults with refractory versus well-controlled epilepsy: a cross-sectional study. Seizure. 2018;1(60):105–9.
Cardenas-Rodriguez N, Huerta-Gertrudis B, Rivera-Espinosa L, Montesinos-Correa H, Bandala C, Carmona-Aparicio L, et al. Role of oxidative stress in refractory epilepsy: evidence in patients and experimental models. Int J Mol Sci. 2013;14(1):1455–76.
Puttachary S, Sharma S, Stark S, Thippeswamy T. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed Res Int. 2015;20(2015): 745613. https://doi.org/10.1155/2015/745613.
Maes M, Supasitthumrong T, Limotai C, Michelin AP, Matsumoto AK, de Oliveira SL, et al. Increased oxidative stress toxicity and lowered antioxidant defenses in temporal lobe epilepsy and mesial temporal sclerosis: associations with psychiatric comorbidities. Mol Neurobiol. 2020;57:3334–48.
Turkdogan D, Toplan S, Karakoc Y. Lipid peroxidation and antioxidative enzyme activities in childhood epilepsy. J Child Neurol. 2002;17(9):673–6.
Yiş U, Seçkin E, Kurul SH, Kuralay F, Dirik E. Effects of epilepsy and valproic acid on oxidant status in children with idiopathic epilepsy. Epilepsy Res. 2009;84(2–3):232–7.
Iwuozo EU, Obiako OR, Ejiofor JI, Kehinde JA, Abubakar SA. Effect of epilepsy and antiepileptic drugs therapy on erythrocyte malondialdehyde and some antioxidants in persons with epilepsy. West Afr J Med. 2019;36(3):211–6.
Menon B, Ramalingam K, Kumar RV. Low plasma antioxidant status in patients with epilepsy and the role of antiepileptic drugs on oxidative stress. Ann Indian Acad Neurol. 2014;17(4):398.
Grewal GK, Kukal S, Kanojia N, Saso L, Kukreti S, Kukreti R. Effect of oxidative stress on ABC transporters: contribution to epilepsy pharmacoresistance. Molecules. 2017;22(3):365.
Martinc B, Grabnar I, Vovk T. The role of reactive species in epileptogenesis and influence of antiepileptic drug therapy on oxidative stress. Curr Neuropharmacol. 2012;10(4):328–43.
Acknowledgements
We would like to acknowledge the excellent technical assistance of staff members of Central Research Lab, Medical Biochemistry and Molecular Biology Department, Faculty of Medicine, Zagazig University.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
All authors were involved in crafting the study topic and design. All authors read and approved the final manuscript. NS analyzed and interpreted the data, wrote, and prepared the final manuscript. AEK supervised clinical/laboratory work, interpreted results, and participated in manuscript drafting. ES recruited the patients, carried out clinical/laboratory investigation, collected data and submitted manuscript. MHE supervised clinical/laboratory work, and participated in manuscript drafting. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of the faculty of Medicine, Zagazig University. The reference number is (ZU-IRB # 6007/9-3-2020). The purpose of the study was explained, and an informed written consent was taken before taking any data or doing any investigations. The participants were informed that their participation was voluntary and that they could withdraw from the study at any time without consequences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shehta, N., Kamel, A.E., Sobhy, E. et al. Malondialdehyde and superoxide dismutase levels in patients with epilepsy: a case–control study. Egypt J Neurol Psychiatry Neurosurg 58, 51 (2022). https://doi.org/10.1186/s41983-022-00479-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-022-00479-5