Abstract
Background
In this study, an entomogenous, fungus was isolated from the Egyptian mealybug, Icerya aegyptiaca (J.) (Hemiptera: Monophlebidae) on the parasol leaf tree, Macaranga tanarius, in China where evaluated as a biocontrol fungus to reduce the population of the target insect. The strain was identified as Aspergillus parasiticus by morphological and phylogenetic analysisand named ZHKUAP1. The biological characteristics, pathogenicity, and field control effect of the strain were determined.
Results
The most suitable medium for the mycelial growth of strain ZHKUAP1 was PPDA medium, with an optimum temperature of 30 °C and pH 7, in addition to glucose and peptone as carbon and nitrogen sources. The optimum sporulation conditions were the PPDA medium at 30 °C and pH 6, using the soluble starch and beef extract as carbon and nitrogen sources. The mycelial growth and spore production of strain ZHKUAP1 were stopped at 70 °C and above, indicating that it was not resistant to high temperatures. High concentrations of spore suspension, against young insect age, resulted high corrected mortality, as well as decreased the median lethal time. When the spore concentration was 1 × 108 cfu/ml, the corrected mortality of the second nymph was 88.33%, and the LT50 was 0.66 day. After 10 days of inoculation, the LC50 of the second instar nymph was the smallest, reaching 4.07 × 104 cfu/ml. On the 10th day of the field experiment, the corrected mortality was 76.45%, indicating that the A. parasiticus strain ZHKUAP1 had strong pathogenicity on I. aegyptiaca population.
Conclusions
The indoor toxicity of the strain to I. aegyptiaca was determined, and the field control effect of the pathogen was explored on this basis. The results have important application prospects in the biological control of I. aegyptiaca.
Similar content being viewed by others
Background
The Egyptian mealybug, Icerya aegyptiaca, belongs to Arthropoda, Insecta, Hemiptera, Monophlebidae, and the Aegyptiaca genus (Unruh and Gullan 2008). It is a garden plant pest widely distributed in tropical and subtropical regions, including China, Japan, Iran, and other places (Moghaddam et al. 2015). The insect is omnivorous, with host plants belonging to 66 families and 128 genera. It prefers to infest Magnoliaceae plants and harms Musa sp., Citrus spp., Pyrus communis, Ficus spp., and many more (Claude et al. 2023). The insects float and spread over short distances with the wind, usually landing on a plant and using that plant as its host. The most suitable host of the insect is parasol leaf tree, Macaranga tanarius (L.) Muell. Arg., followed by Magnolia denudate Desr., P. guajava Linn. and Ficus microcarpa Linn. (Liu and Shi 2020a, b). The most severe impact of the insect occurs in areas with low wind speeds, such as inside trees, leading to stunted plant growth, as well as the shedding flowers and fruits, even the occurrence of sooty blotch, and plant death in severe cases (Zhang et al. 2020a, b). The insect mainly harms the back of the leaf and can secrete a large amount of wax covering the body surface, resulting in unsatisfactory control effects of various insecticides. The long-term use of chemical pesticides improves environmental pollution and insect resistance to insecticides (Zhong et al. 2022). Therefore, biological control can not only protect the environment but also reduce pesticide residues. Natural enemy insects serve as the common biological control method to control the population of I. aegyptiaca, such as Rodolia pumila Weise, Rodolia. rufopilosa Muls, Rodolia cardinalis MuIsant, and Mallada basalis Walker. However, there are great challenges in practical applications in most cases, including the injured plants in the field with a large tree height, a large amount of natural enemies are needed, with high control cost (Jiang et al. 2013).
Entomogenous fungi are important biological pesticides, serving as one of the important methods of comprehensive management dominated by biological control, an important factor in the natural regulation of pest populations, and an important material for the biological control of pests (Li 2012). Entomogenous fungi are highly safe to humans, the environment, and nontarget organisms, suitable for mass production, and easily spread among pests, playing a key role in population control and biological control of agricultural and forestry pests (Wang et al. 2021). Entomogenous fungi are widely used as biocides include Beauveria bassiana, Metarhizium anisopliae, Verticillium lecanii. Among these fungi, Isaria javanica IJID003 and Purpureocillium lilacinum 12ID-1 have a high toxicity to I. aegyptiaca (Deng et al. 2020), which can be used for biological control of I. aegyptiaca.
Entomogenous fungi kill pests by invading the body wall and releasing toxins in the host, showing great utilization and development value (Wang et al. 2010). At present, there is relatively a little research on the collection, development, and application of the entomogenous fungal resources of I. aegyptiaca at home and abroad. Therefore, exploring the resource-saving, environmentally friendly, economically and socially sustainable development of biocontrol fungi plays a more important role in the practical production and biological control of I. aegyptiaca. In this study, a strain of entomogenous fungi with high pathogenicity to I. aegyptiaca was isolated from dead insects. The strain was identified from the perspective of morphology and meristem biology. The biological characteristics were investigated, and pathogenicity tests and field control tests were carried out. Technical support was provided for the development and application of the biocontrol of I. aegyptiaca by entomogenous fungi.
Methods
Test materials
Test insects
Adults and nymphs of I. aegyptiaca were collected from the M. tanarius tree of South China Agricultural University. Second and third nymphal instars or female adults of the same size and health status were selected and continued to be cultured in the insect room (25 °C), as the test insect source in the laboratory.
Tested medicaments
10% acetamiprid microemulsion, Guangzhou Nongtai Biotechnology Co., Ltd.; 1.3% matrine water agent, Hengyuan Weiye Biological Technology Co., Ltd.
Test method
Collection of dead insects and isolation of entomopathogenic fungi
The dead insects of I. aegyptiaca were collected from the M. tanarius tree of the South China Agricultural University in Tianhe District, Guangzhou City, Guangdong Province, and brought back to the laboratory for the separation of entomogenous fungi.
The obtained I. aegyptiaca dead insects were immersed in 75% alcohol for 10 s. under sterile conditions, followed by 1% sodium hypochlorite solution for 30 s. The samples were finally rinsed six times with sterile water. Insect corpses were crushed and inoculated onto a PDA medium for cultivation in an incubator. The culture conditions were set at 25 °C and a photoperiod of 24 h/day. After the colony was grown, the mycelium block was punched with a 5-mm-diameter puncher at the edge of the growing colony, and the mycelium block was re-inoculated onto the new PDA medium for further cultivation and purification. The purified strain was cultured on the slope of the PDA medium and stored in a refrigerator at 4 °C. The isolated target strain was numbered as ZHKUAP1.
Morphological and molecular identification
The target strain ZHKUAP1 was stored in the refrigerator at 4 °C until removed, isolated, purified, and cultured. The mycelial block was punched with a 5 mm diameter puncher and inoculated on a PDA medium for co-cultivation at 25 ± 1 °C for 5 days (Senthil Kumar et al. 2021). When spores grew on the surface of the colony, the colony morphology was observed, and the mycelium, sporulation structure, and micromorphology of conidia were observed by electron microscopy.
The Chelex-100 method (Chen et al. 2011) was used to extract the DNA samples of the strains. A total of 10 μl of Chelex-100 chelating resin was placed into a 2.5-μl finger tube, and a few hyphae were placed in each tube. Subsequently, the samples were subjected to 9000 r/min shock centrifugation for 5 min and 95 °C water bath heating for 5 min twice, and the supernatant was taken for standby. 1 μl of the centrifuged DNA supernatant was taken, and 1 μl each of ITS1(5ʹ-TCCGTAGGTGAACCTGCGG-3ʹ) and ITS4(5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) primers were added. 12.5 μl of Tap PC mix polymerase and 9.5 μl of dd H2O were used as the reaction system DNA for PCR amplification in the PCR tube. The reaction conditions of amplification were set as 30 cycles of predenaturation at 94 °C for 3 min, 94 °C for 30 s, 55 °C for 30 s., and 72 °C for 40 s., 72 °C extension for 10 min, 1% agarose gel amplification for 15 min, and 120 V electrophoresis detection. The PCR products obtained were sent to Guangzhou Tianyihuiyuan Gene Technology Co., Ltd. for sequencing (He et al. 2022). Finally, the generated ITS-rDNA sequence was determined. The BLAST gene program of the NCBI website (\https:blast.ncbi.nlm.nih.gov/Blast.cgi) was used for comparison. Strains with similar ITS sequences were identified using the adjacency method and the maximum likelihood method in MEGA 11 software. After running 1000 bootstrap validations, the phylogenetic trees of strains and their relatives were constructed. Combined with the morphological and biological characteristics of the strain, its position in the system classification was finally determined.
Tie-back verification and re-isolation of ZHKUAP1
The pure culture of pathogenic fungus isolated in 2.1 was cultured on a PDA plate for 7 days. The spore suspension with a concentration of 1 × 107 cfu/ml was prepared using 0.02% Tween water. Fresh M. tanarius leaves were cut into 3–4 cm squares. After disinfection with 75% alcohol, the cells were washed with sterile water, dried in the air, and placed in a sterile six-well plate. Healthy female adults of I. aegyptiaca were fully immersed in spore suspension for approximately 10 s. After drying, they were placed on the leaves of a six-well plate, and ten insects were placed on each six-well plate. Insects immersed in 0.02% Tween water were used as a blank control group. Each group had three replicates. The above insects were reared at 25 °C, photoperiod (24 L: 0 D), and relative humidity of 80% for 5 days. On the second day after inoculation, the infection symptoms of the insects were recorded for 5 days, and pictures were taken and archived. After the insect body grows spores, a small number of spores were picked from the surface of the insect body and placed on PDA plates. The spores were cultured in a constant temperature incubator at 25 ± 1 °C for 5 days. Until spore powder appeared on the surface of the colony, colony characteristics were observed by microscopy. After purification, the pure colonies were compared to the morphological structure of the test strains. Pathogenic strains that comply with Koch's rule for I. aegyptiaca were screened.
Biological characteristics of ZHKUAP1 strain
The effects of different media, temperatures, pH values, carbon sources, nitrogen sources, lethal temperatures, light, and other conditions on the growth and sporulation of ZHKUAP1 mycelium were tested, and each treatment was repeated three times. After the optimal medium was screened out, the other determinations were based on the optimal medium. In addition to temperature tests, the other biological characteristics were cultured in a constant temperature incubator at 25 ± 1 °C for 7 days. The colony diameter was measured by the cross method. Then, the spores were washed off the surface of the plate with 0.02% Twain water. The number of spores was measured by a blood cell counting plate under a biological microscope, and spore production was calculated.
Optimum medium
After the fungus cake was made with a 5-mm puncher, ZHKUAP1 was cultured in PDA, PDAA, SMAY, SDAY, and Czapek media to select the optimum medium and each treatment was repeated three times. After 7 days, the colony diameter and the number of spores in each treatment were recorded.
Optimum temperature
The 5 mm mycelium block was inoculated into the center of the optimum medium and then cultured at 5, 10, 15, 20, 25, 30 and 35 °C for 7 days, and each treatment was repeated three times.
pH
With 0.1 mol/l HCL or 0.1 mol/l NaOH as the regulating solution, the pH of the optimal medium was adjusted to 9 needed pH values (3, 4, 5, 6, 7, 8, 9, 10, and 11) and each treatment was repeated 3 times.
Carbon source
Czapek's medium was used as the basic medium. The sucrose in the Czapek medium was replaced with the same amount of starch, lactose, glucose, and maltose as the carbon source, and a medium without a carbon source was used as the control. Each treatment was repeated three times.
Nitrogen source
Czapek's medium was used as the basic medium. The potassium nitrate in the Czapek medium was replaced with the same amount of ammonium sulfate, beef extract, yeast, and peptone as the nitrogen source, and a nitrogen-free medium was used as the control group. Each treatment was repeated three times.
Lethal temperature
Five mm diameter mycelium block was put into a 5-ml centrifuge tube and then placed in a water bath for 10 min at 40, 45, 50, 55, 60, 65, 70 and 75 °C. After rapid cooling, the mycelium block was inoculated on the PPDA plate for cultivation, and each treatment was repeated 3 times.
Light
The culture was carried out under the conditions of 24 h full light, 24 h full dark, and alternating light and dark (12 h light and 12 h dark), and each treatment was repeated three times.
Pathogenicity of ZHKUAP1 to I. aegyptiaca experiment
Five fully inflated dark green leaves outside the heart leaves of the unsprayed M. tanarius tree were collected. The leaves were cut into 3–4 cm2, disinfected with 75% alcohol for 10 s, cleaned with sterile water for 30 s, then allowed to dry. The disinfected, cleaned, and dried leaf blocks were placed into sterile six-well plates for use. The isolated and purified strain ZHKUAP1 was prepared into spore suspensions with concentrations of 1 × 106,5 × 106, 1 × 107, 5 × 107, and 1 × 108 cfu/ml (Liang et al. 2022). Then, the second and third nymphal instars and female adults were immersed in 5 concentrations of spore suspension for 10 s. The control group was immersed in sterile water for 10 s. The immersed insects were placed on filter paper to absorb the moisture on the surface of the insect body. After that, the insects were moved to a six-well plate with the leaves of M. tanarius. Each six-well plate had a total of 20 heads, and each treatment had 3 replicates. The insects were placed in the above six-well plates for 10 days under a photoperiod of 16 L: 8 D at 25 °C. The leaves of M. tanarius were changed once every 3 days. If the surface of the insect was obviously covered with mycelium, it was recorded as dead. Infection symptoms and the number of dead insects were observed and recorded on the 1st, 3rd, 5th, 7th, and 10th days after infection. The cumulative corrected mortality, regression equation, median lethal time (LT50), and correlation coefficient (R) were calculated.
Bio control of I. aegyptiaca by ZHKUAP1 under field conditions
A field experiment was conducted at South China Agricultural University. Spore culture and spore suspension preparation were carried out according to the 2.5 method. The conidial suspension of ZHKUAP1 at a concentration of 1 × 107 cfu/ml, 2 ml of 1000 times matrine, and 2 ml of 3000 times acetamiprid were used as treatments group, while, water was used as blank control, with three replicates. Infected M. tanarius trees were selected, and a small sprayer was used to spray the liquid on the leaves of the branches of the M. tanarius tree. Then, the branches that were about the same level of harm were tagged. The number of adults and nymphs on branch leaves and branches was recorded one by one (Cheng et al. 2013a, b). On the 1st, 3rd, and 10th days of treatment, the numbers of living insects of I. aegyptiaca on the tree was counted and statistically analyzed. The insect decline % was calculated, and the insect decline % was corrected.
Processing data and statistical analysis methods
In the experiment, Excel 2021 and DPS 7.05 statistical software were used to process and statistical analyzed the collected data of the experiments. SPSS 26.0 was used to calculate the regression equation, median lethal time (LT50), median lethal concentration (LC50), and correlation coefficient (R). The main calculation formulas are as follows:
Cumulative corrected mortality % = (treatment cumulative mortality%-control cumulative mortality %)/(1-control cumulative mortality %) × 100.
Insect decline rate % = (number of live insects before spraying-number of live insects after spraying)/number of live insects before spraying × 100.
Corrected insect mortality % = (treatment area insect decline rate %-control area insect decline rate %)/(1-control area insect decline rate %) × 100.
Results
Isolation, purification and morphological identification of ZHKUAP1 strain
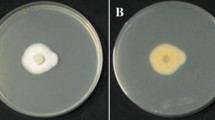
A total of 180 A. parasiticus colonies of three different colors were isolated and cultured from the dead insects, including 33 white colonies, 4 black colonies, and 143 green colonies. The isolated colonies of fungus were purified and examined under a microscope. According to the morphology of hyphae and spores on the surface of naturally infected dead insects and the symptoms of dead insects (Fig. 1), the color of the pathogen colony was determined to be green, which was attributed to the similarity of the green colonies growing on the culture dish. As shown in the microscopic morphology of each colony, each green colony exhibited a similar microstructure, which was further named ZHKUAP1 and purified cultured.
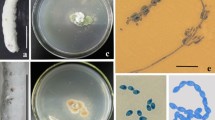
ZHKUAP1 grew normally in PDA medium, exhibiting a color of dark green after 5 days of cultivation. The colonies had concentric flat and round wheel patterns, with a diameter of 4.33–5.09 cm with a texture of villous hairs (Fig. 2A). The back of the colony is gray‒green (Fig. 2B). The mycelium was well-developed, with a diaphragm (Fig. 2C). The sporulation structure is yellow‒green, with a yellow conidia head, no septum, smooth surface, nearly round, and a conidial diameter of 3.5–5.5 μm (Fig. 2D). The conidial pedicel was composed of a top capsule, a bottle pedicel, a pedicel base, and a conidial chain (Fig. 2E). The above morphological characteristics were similar to those of Aspergillus fungi, and the strain was preliminarily identified as an Aspergillus fungus.
Molecular identification of the ZHKUAP1 strain
The strain ZHKUAP1 was amplified using universal primers (ITS1/ITS4) to obtain a product of approximately 750 bp (Fig. 3). The sequencing results showed that the rDNA ITS sequence of the tested strain was 700 bp in length. The obtained sequence (accession number: OR287098) was imported into the NCBI database for BLAST gene homology comparison, suggesting that the sequence similarity between this strain and A. parasiticus (accession numbers: KU182391.1 and AY373859.1) in the database reached 99%. The highly similar sequences were downloaded into MEGA 11 to construct a phylogenetic tree. The strain and A. parasiticus belongs to the same branch in the phylogenetic tree (Fig. 4). Combined with the colony morphological characteristics and molecular identification results of the strain, ZHKUAP1 was identified as A. parasiticus.
Pathogenicity symptoms of ZHKUAP1 on insect stages
The results of the back-splicing verification test are shown in Fig. 5. The symptoms of female adults inoculated with were consistent with those of natural infection. Before inoculation, the insect body of I. aegyptiaca was healthy, brightly colored, and covered with wax on its surface (Fig. 5A). One day after inoculation, the color of the insect body became darker, the wax on the surface of the insect body gradually degraded, and a small amount of white hyphae and green spores appeared around the insect body (Fig. 5B). Two days after inoculation, the color of the insect body gradually became darker, the wax on the body surface was almost invisible, the mycelium covered a large area of the insect body, and the green spores increased (Fig. 5C). Four days after inoculation, the insects were completely covered with conidia (Fig. 5D).
Analysis of biological characteristics of the pathogenic fungus
Effects of different media on the growth and sporulation of ZHKUAP1
Strain ZHKUAP1 was cultured on PDA, PPDA, SMAY, SDAY, and Czapek for 7 days. The strain grew and produced spores normally on each medium, as shown in (Fig. 6a). ZHKUAP1 grew best on the PPDA medium, with a colony diameter of 5.8 cm. ZHKUAP1 grew poorly on the Czapek medium, and the colony diameter was only 3.65 cm. The medium with the largest sporulation was PPDA medium, and the spore yield was 9.16 × 107 cfu/ml after 7 days of culture. Sporulation was very low on the Czapek medium, with a spore production of 5.78 × 106 cfu/ml.
Effects of different temperatures on the growth and sporulation of ZHKUAP1
The effects of different temperatures on the growth and sporulation of ZHKUAP1 are shown in Fig. 6b. The mycelium of strain ZHKUAP1 grew at 10–35 °C and stopped growing at 5 °C. The optimal temperature for mycelial growth was 30 °C, with a colony diameter of 6.77 cm and the largest sporulation of 17.15 × 107 cfu/ml. The strain did not produce spores below 10 °C.
Effects of different pH values on mycelial growth and sporulation of ZHKUAP1
The mycelium grew in the pH range of 5.0–11.0 (Fig. 7a). When the pH was set to 7, the mycelium grew best with a colony diameter of 6.18 cm when pH was less than 5, the strain stopped growing. pH 7 was the optimum pH for the mycelial growth of strain ZHKUAP1, showing the largest spore production of 7.65 × 107 cfu/ml. In addition, the spore production was poor at pH 11, about 5.60 × 107 cfu/ml.
Effects of different carbon sources on mycelial growth and sporulation of ZHKUAP1
The strain ZHKUAP1 was cultured on Czapek's medium with five different carbon sources, acting as a test carbon source culture medium. Figure (7-b) shows that five carbon sources promoted mycelial growth and sporulation. Among them, the mycelial growth was the best on the medium with glucose as the carbon source, with a colony diameter of 5.15 cm, which was higher than that of the other carbon sources. Therefore, glucose was determined to be the optimal carbon source for the mycelial growth of strain ZHKUAP1. The strain produced spores under various carbon source media. The medium with soluble starch as the carbon source had the highest sporulation, reaching 6.16 × 107 cfu/ml.
Effects of different nitrogen sources on mycelial growth and sporulation of ZHKUAP1
Strain ZHKUAP1 grew on five nitrogen source media tested on the basis of Czapek (Fig. 8a). The mycelium grew fastest on the medium with peptone as the nitrogen source, and the colony diameter was 7.12 cm. The mycelium growth of ammonium sulfate as a nitrogen source culture medium was the worst, with a colony diameter of only 2.32 cm, even worse than that of nitrogen deficient treatment, which indicated that ammonium sulfate inhibited the growth of the strain. The strain had the highest sporulation under the condition of beef extract as a nitrogen source, which was 5.47 × 107 cfu/ml. Spore production was very small under the conditions of ammonium sulfate, potassium nitrate, and nitrogen deficiency as nitrogen sources Peptone was the optimum nitrogen source for mycelial growth, and beef extract was the most suitable nitrogen source for sporulation.
Effect of lethal temperature on mycelial growth and sporulation
After 10 min of incubation in a high-temperature water bath at 40–65 °C, the mycelia of the strains grew and produced spores normally (Fig. 8b). After 10 min of treatment at 70 °C or above, mycelia stopped growing and sporulating. Therefore, the lethal temperature of ZHKUAP1 mycelium was 70 °C.
Effects of different light conditions on mycelial growth and sporulation of ZHKUAP1
Different light conditions affected the mycelial growth and sporulation of strain ZHKUAP1 (Fig. 8c). Mycelial growth was the fastest under dark conditions, and the colony diameter was 5.78 cm. Following full light and 12 h light/dark conditions, the colony diameters were 5.67 cm and 5.23 cm, respectively. The sporulation was the largest under full light conditions at 7.17 × 107 cfu/ml and the lowest in the dark.
Indoor pathogenicity test results of insect pathogens
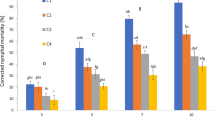
Different concentrations of ZHKUAP1 spore suspension were employed to treat second and third nymphal instars and female adults of I. aegyptiaca. As listed in Tables 1, 2, and 3, mortality of I. aegyptiaca occurred in each concentration treatment, indicating that ZHKUAP1 had a good pathogenicity. With the extension of time, the mortality rate of experimental insects with young age was high under the same concentration of spore suspension. When the spore concentration was 1 × 108 cfu/ml, the corrected mortalities of second and third nymphal instars and female adults on the tenth day were 88.33, 80 and 65%, respectively. The LT50 was 0.66, 0.72 and 2.95 days. After inoculation of 10 days, LC50 decreased with insect age, the LC50 of second and third nymphal instars and female adults were 4.07 × 104, 5.91 × 105 and 8.06 × 105 cfu/ml. The tolerance of female adults to the strain ZHKUAP1 was significantly higher than that of second and third nymphal instars.
Field control effects of pathogenic fungus strains on I. aegyptiaca
A field experiment was conducted on I. aegyptiaca using a ZHKUAP1 conidial suspension with a concentration of 1 × 107 cfu/ml and 2 commonly used insecticides. As shown in (Table 4), acetamiprid 3000 times liquid had the best control effect on I. aegyptiaca, and the insect decline rate was 97.51% after 10 days. The insect decline rate of strain ZHKUAP1 was low, with an insect decline rate of 79.82% after 10 days of treatment.
Discussion
There are many species of entomogenous fungi and abundant resources, such as Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces lilacinus (Beatrice et al. 2023). Aspergillus has a relatively high pathogenicity. At present Aspergillus as a biocontrol fungus mainly includes A. terreus, A. aculeatus, A. niger, and A. nomius. The addition of Aspergillus aculeatus to the organic manure of laying hens enhanced the disease resistance of crops and improved soil structure. Aspergillus nomius possessed high pathogenicity to Solenopsis invicta Buren (Xiao et al. 2022). Aspergillus oryzae effectively controlled locust plague (Zhang et al. 2020a, b). Aspergillus flavus had a good pathogenicity and control effects on Bactrocera dorsalis (Yin et al. 2022). Zhao Pengfei (Zhao et al. 2019). The optimum conditions for the growth of A. parasiticus Q527 were PDA medium, pH of 8, lethal temperature of 65%, lactose carbon source for the growth of mycelium, and soluble starch carbon source for its sporulation, and peptone nitrogen source for its mycelial growth and sporulation. The laboratory toxicity test showed a strong lethality to Heortia vitessoides. Dai Yuanfeng (Dai et al. 2017) found that A. parasiticus SF-2 had the best mycelial growth and sporulation at approximately 32 °C and a pH of 4, showing a good infection effect on Ceratovacuna lanigera, Icerya urchase Maskell, and Unaspis yanonensis.
In this study, the strain ZHKUAP1 was identified as A. parasiticus by molecular identification. The strain grew best on the PPDA medium at 30 °C and pH of 6–7, using glucose and peptone as the most suitable carbon and nitrogen sources for mycelial growth and soluble starch and beef extract as the best carbon and nitrogen sources for sporulation. The lethal temperature was 70 °C, and light conditions had no effect on the growth of ZHKUAP1. Different from the results of researchers (Zhao et al. 2019; Dai et al. 2017), the cause analysis was due to the difference in geographical location and host range. The laboratory virulence of strain ZHKUAP1 was determined, and the fungus had high pathogenicity to I. aegyptiaca at all ages. As the concentration of conidial suspension increased, the pathogenicity increased for the low age of the test insects. The conidia suspension of the 1 × 107 cfu/ml strain ZHKUAP1 was prepared in the field experiment. After 10 days of experiment, the correction insect decline rate of I. aegyptiaca by ZHKUAP1, which indicated that ZHKUAP1 had a good activity and biocontrol potential against I. aegyptiaca. Field trials may be affected by uncontrollable factors such as temperature, humidity, ultraviolet intensity, and dosage form (Chai et al. 2011).
Conclusions
This study shows that A. parasiticus has great potential for the prevention and control of I. aegyptiaca, but its development as a biological pesticide needs further safety evaluation test research to ensure its reliability and effectiveness in application. It provides more resource reference for the prevention and control of I. aegyptiaca.
Availability of data and materials
Not applicable.
Abbreviations
- I. aegyptiaca :
-
Icerya aegyptiaca
- A. parasiticus :
-
Aspergillus parasiticus
- M. tanarius :
-
Macaranga tanarius
- A. terreus :
-
Aspergillus Terreus
- A. aculeatus :
-
Aspergillus aculeatus
- A. niger :
-
Aspergillus niger
- A. nomius :
-
Aspergillus nomius
References
Beatrice NA, Michael TH, Bishwo M (2023) Virulence of Beauveria sp. and Metarhizium sp. fungi towards fall armyworm (Spodoptera frugiperda). Arch Microbiol 205(10):328
Chai FH, Guo Q, Jiang L (2011) Study on the spatial distribution pattern of Icerya aegyptiaca on garden plants. Environ Entomol 33(4):548–551
Chen JL, Huang XL, Wu AD (2011) A rapid and efficient method for extracting DNA from pathogenic fungi as PCR template. J Fungi 30(1):147–149
Cheng DM, Zhang ZX, Huang YJ (2013a) Indoor toxicity and field efficacy of two biopesticides against Icerya aegyptiaca. Northwest Agric J 22(10):200–203
Cheng DM, Zhang ZX, Huang YJ (2013b) Indoor toxicity and field efficacy of several insecticides against Icerya aegyptiaca. Guangdong Agric Sci 40(16):76–77+ 86
Claude B, Paula B, Elisavet C et al (2023) Pest categorisation of Icerya aegyptiaca. EFSA J Eur Food Saf Auth 21(1):e07739–e07739
Dai Y, Zhang C, Yu H (2017) A preliminary study on the infection effect and biological characteristics of Aspergillus parasiticus SF-2 on Ceratovacuna lanigera. J Yunnan Agric Univ (Nat Sci) 32(6):1006–1011
Deng JR, Sun LH, Bi KK et al (2020) Screening, identification and pathogenicity determination of entomopathogenic fungi from Icerya aegyptiaca. J Plant Prot 47(1):53–64
He JL, He JC, Hua HW et al (2022) Isolation, identification and activity test of parasitic fungi of Spodoptera frugiperda. Jiangsu Agric Sci 50(8):32–36
Jiang L, Han SC, Li ZG et al (2013) Life table of experimental population and field population survey of Mallada basalis (Walker). For Dis Insect Pests China 32(4):13–15+ 23
Li CW (2012) Studies on the biological characteristics of Icerya aegyptiaca blow weed and the predation behaviour of moving red Ladybug D1. Guangzhou: South China Agricultural University, 2012
Liang CP, Liang JR, He JL et al (2022) Biological characteristics of Metarhizium anisopliae and its infection on Spodoptera frugiperda. J Jiangxi Agric Univ 44(2):386–392
Liu Y, Shi J (2020a) Predicting the potential global geographical distribution of two icerya species under climate change. Forests 11(6):684
Liu Y, Shi J (2020b) Prediction of the suitable area of the Icerya aegyptiaca in China under the background of climate change. Plant Prot 46(1):108–117
Moghaddam M, Esfandiari M, Khosravi M (2015) First record of Icerya aegyptiaca (Hemiptera: Coccoidea: Monophlebidae) from Iran. In: 1st Iranian international congress of entomology, Tehran, 2015
Senthil-Kumar CM et al (2021) Characterization and biocontrol potential of a naturally occurring isolate of Metarhizium pingshaense infecting Conogethes punctiferalis. Microbiol Res 243:126645
Unruh CM, Gullan PJ (2008) Identification guide to species in the scale insect tribe Iceryini (Coccoidea: Monophlebidae). Zootaxa 1803:1–106
Wang LD, You MS, Huang J et al (2010) Entomogenous fungal diversity and its role in biological control of pests. J Jiangxi Agric Univ 32(5):920–927
Wang J, Zhang K, Zhang X et al (2021) Isolation, identification and diversity analysis of entomogenous fungi in Qinling-Daba Mountains. J Northwest A & F Univ (Natural Science Edition) 49(6):122–129
Xiao Y, Zhang JH, Chen JX et al (2022) Isolation, identification and pathogenicity of pathogenic fungi from red imported fire ant. Environ Entomol 44(6):1502–1509
Yin Z, Zhang J, Diao LG et al (2022) Preliminary determination of pathogenicity of Aspergillus flavus to Bactrocera dorsalis pupae. Plant Quar. 36(3):17–22
Zhang PF, Yuan SK, Zhang L (2020a) Entomopathogenic microorganisms and their application in locust plague control. J Environ Entomol 42(3):529–544
Zhang JY, Bi KK, Wu C et al (2020b) Study on the occurrence regularity of Ficus plant diseases and insect pests in Guangzhou. Garden 09:8–14
Zhao PF, Chang MS, Luo J et al (2019) The infection effect and biological characteristics of a aspergillus parasiticus Q527 onheortia vitessoides. Guangdong Agric Sci 46(2):106–112+ 173
Zhou Y, Wu J, Lin S et al (2022) The synergistic effects of rosehip oil and matrine against Icerya aegyptiaca (Douglas) (Hemiptera: Coccoidea) and the underlying mechanisms. Pest Manag Sci 78(8):3424–3432
Acknowledgements
Thank Professor Zhang Zhixiang 's team of National Key Laboratory of Green Pesticides, South China Agricultural University for providing a platform. Thanks for Liu Yu 's help.
Funding
Supported by the Guangdong Provincial Innovation Team for General Key Technologies in Modern Agricultural Industry, Project No. 2023KJ133; The Foundation of Guangdong Provincial Department of Education (2022ZDJS020); and the Key-Area Research and Development Program of Guangdong Province (No.2020B020224002).
Author information
Authors and Affiliations
Contributions
YHW: Investigation, Resources, Data curation, Writing–original draft, Writing—review & editing, Visualization. JCH: Methodology, Software. CPL: Formal analysis, Writing-review & editing. DMC: Methodology, supervision, writing–review & editing, conceptualization, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare no conflict of interest and no competing interests exist regarding the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, Y., He, J., Liang, C. et al. Isolation, identification, biological characteristics, and pathogenicity of an entomogenous fungus against the Egyptian mealybug, Icerya aegyptiaca (J.) (Hemiptera: Monophlebidae). Egypt J Biol Pest Control 34, 39 (2024). https://doi.org/10.1186/s41938-024-00802-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-024-00802-7