Abstract
Background
Biological control of insect pests is an economic, eco-friendly and harmless approach to integrated pest management strategies. Bracon hebetor (Say) (Hymenoptera: Braconidae) considers a polyphagous ectoparasitoid of various pests of the order Lepidoptera. The parasitized host insects' defense mechanisms are triggered as a result of the parasitic wasps' injury and penetration. Thus, induce the host cellular and humoral immune responses through a blend of secretions injected into the host body. The present study was designed to evaluate the efficacy of B. hebetor on the immune response of the parasitized full grown larval instar of the rice moth, Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) under natural envenomation.
Results
On cellular level, the ultrastructure examination of the hemocytes displayed a considerable structural deformation in hemocyte morphology of the detected types of the hemocytes. Moreover, the effects of parasitism on both differential hemocyte counts (DHCs) and total hemocyte counts (THCs) were investigated. The number of prohemocytes (PR) (40.33 ± 5.61, 43 ± 8.33 and 26 ± 2.31) was statistically differed after (24, 48 and 72 h) of parasitism, respectively, compared to unparasitized (31.33 ± 6.49) larvae. Similar observations were recorded in plasmatocytes (PL) before and after the parasitism. However, Spherulocytes (SP) and Oenocytoids (OE) were recorded in the hemolymph with little abundance. On the other side, the quantitative analysis of total hemolymph proteins (THP) provoked a significant effect of considering parasitized and non-parasitized larvae. As the Phenoloxidase (PO) cascade plays a critical role in immune defenses, so the substantial activation of PO in the host's hemolymph following successive hours of parasitism compared to unparasitized larvae using L-DOPA as a substrate indicated the induction of larval immune system. The parasitized larvae showed a gradual increase in the PO activity (0.442 ± 0.103) after 24 h reached up to (1.482 ± 0.272) at the end of parasitism (72 h) in comparable to unparasitized larvae (0.177 ± 0.0.044).
Conclusion
The present investigations clarified the efficacy of B. hebetor parasitism on the host immune mechanism, which will enable the progress of sustainable stored product protection approaches for the control of an important pest rice moth C. cephalonica.
Similar content being viewed by others
Background
Natural enemies such as parasitic wasps represent an alternative and environmentally compatible approach that pay attention to ecologists to overcome the use of synthetic insecticide in post-harvest systems. These natural enemies are able to reproduce incessantly for as long as the hosts are available, thus confirming the sustainability of their populations for long-term regulation of pest populations (Dieckhoff et al. 2017). Parasitoid wasps can disperse rapidly and detect hosts in hidden crevices and corners. The parasitoids of insects have established different lifestyles for example, idiobiont prevent the host growth after initially immobilizing it. On the other side, the koinobiont, where the parasitoids host continues to feed in addition to growth also, remains after parasitization (Resh and Cardé 2009).
The innate immune system of insects consists of two broad categories of effectors which are constitutive and induced (Schmid-Hampel 2005). Constitutive responser generally comprises the humoral prophenoloxidase activating system and cellular responsers including coagulation, phagocytosis, nodule formation and encapsulation (Fathy 2019).
The ectoparasitoid, Bracon hebetor (Say) (Hymenoptera: Braconidae), is considered as one of the most efficient biological control agents of several lepidopterous pests. It completes its larval growth on host larvae after parasitized it.
The rice moth, Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae), is considered an economically serious stored product pest. It causes severe damage to grains and products in storage under changing environmental conditions. It can be easily maintained on a variety of rearing media, and it has a short life cycle making it an abundant host (Vincent et al. 2021). Therefore, detailed information on rice moth immune response toward the parasitoid wasp is required, as a biological control agent for developing integrated management systems.
The cellular defense reaction against foreign bodies is formed by hemocytes (Ratcliffe 1993). So, the concentration of circulating hemocytes in the hemolymph of the parasitoid indicates the activation of host's immune system or not (Brehélin 1982). Hemocytes are convenient to investigate host-parasitoid interactions.
Encapsulation and melanization of the foreign particles consider one of the most vital immune responses in insects. Phenoloxidase (PO) cascade is the main factor in the process of melanization. It is generally present as inactive proenzyme proPhenoloxidase (proPO) in the hemolymph of insects (Leonard et al. 1985). Parasitism releases constituents that are immune-competent cells to suppress immune phenoloxidase cascade (Vinson 1990). The present study was planned to evaluate the significant effect of the ectoparasitic wasp, B. hebetor on cellular and humoral immunity of C. cephalonica parasitized host larvae compared to the unparasitized ones.
Methods
Rearing of Corcyra cephalonica
The initial culture of C. cephalonica eggs used in this study was obtained from the Center of Bio-organic Agricultural Services (CBS), in Aswan, Egypt. Larvae were originated in Biological Control Department, Plant Protection Research Institute, Agricultural Research Center, Giza, Egypt. A colony from the moth was maintained and reared for several generations. The insect rearing technique was conducted according to the methodology reported by Bernardi et al. (2000), moths were reserved for 24 h to permit oviposition in container, half filled with wheat germ (97%) and yeast (3%), and an equal number of females and males were paired after adult emergence. Eggs were collected out and placed in a new container with new food. Experimental procedure was done under controlled laboratory conditions of 25 ± 1 °C, Relative Humidity 60 ± 10% and 14/10 light and dark photo-period. The fully developed larvae were subjected to parasitism.
The parasitoid wasp, Bracon hebetor
The parasitoid, B. hebetor adults were obtained from the Egyptian Desert Research Centre, Cairo, Egypt. According to the procedures described by Mansour (2012) and Manzoor et al. (2016), only full grown instar larvae of C. cephalonica were used to raise the wasp population. Pairs of parasitoids were placed in vials (2 cm × 10 cm) and provided with host larvae. The parasitoid's females usually paralyze their hosts before laying their eggs on the body of their hosts. The newly hatched parasitoid larvae were fed on the paralyzed host. Adult parasitoids were reared in glass jars (500-ml) under laboratory conditions of 28 ± 2 °C; 75 ± 5% RH and 16:8 h (L: D).
Host parasitization
Two couples of B. hebetor that had been mated for 24 h were transferred into a glass jar (10 × 20 cm). The jar was provided with 15 C. cephalonica fully developed larvae, and jars were covered with a rubber band and a piece of muslin cloth to prevent escaping the larvae. The larval parasitized by the wasp was separated individually in a Petri dishes (5 × 3.5 cm) and incubated under controlled chamber for supplementary observations. Three independent Petri dish sets each contained 5 larvae were maintained for different post-parasitization experiments.
Hemolymph collection
This technique was used for separating hemocytes from pure plasma needed for biochemical analysis, in which most works were done with pooled samples of larger volumes. According to Hoffmann (1980), the hemolymph from several larvae was pooled and immediately collected into a sterile micro-centrifuge tube containing traces of phenylthiourea and then, diluted 5 × with saline solution 0.5% previously cooled. Normal and parasitized larvae were removed from the rearing cages, weighted individually, then submerged in a hot water bath at 60 °C for 2–5 min and then, allowed to dry on a paper towel. The hemolymph samples were then centrifuged at 2000 rpm for 5 min (Human Centrifuge, TGL-16XYJ-2, 16,000 rpm, Korea). The supernatant (Plasma) fractions were removed from the hemocytes pellet, poured into another sterile tube and stored at − 18 °C until use for humoral response experiments directly or frozen until use. While, hemocytes were collected and prepared for hemocyte identification.
Hemocytes examination
The hemocytes of normal and parasitized larvae were collected as described above and prepared for Transmission Electron Microscope (TEM) in the Regional Center for Mycology and Biotechnology- AlAzhar University, Cairo. Egypt. The first step was the fixation of hemocyte samples with 2.5% glutaraldehyde, 5 mM calcium chloride, and 2% sucrose in 0.1 M cacodylate buffer (pH 7.2) for 1 h at room temperature. Then, samples were rinsed in 0.1 M cacodylate buffer (pH 7.2) containing 2% sucrose and post-fixed in 1% osmium tetroxide (OSO4), 0.8% potassium ferrocyanide and 5 mM calcium chloride in 0.1 M cacodylate buffer (pH 7.2) for 1 h at room temperature. Followed by dehydration in graded acetone and embedded in PolyBed 812 epoxy resin, ultrathin sections obtained using a Leica ultramicrotome (Nussloch, Germany) were stained with uranyl acetate and lead citrate and subsequently observed using an FEI Morgagni F268 transmission electron microscope (operating at 80 kV).
Parasitization effects on the hemocyte counts
In order to observe the effect of parasitism on the larval host hemocytes, differential and total blood cells were examined. Differential Hemocyte Counts (DHCs) was investigated, using stained blood films of healthy and parasitized larvae with light microscopy, according to Salt (1970). By inspecting around 100 cells on each slide (10 slides made from 10 specimens), distinct hemocytes were differentially enumerated. The percentages of the various types of hemocytes were determined using the following formula:
For Total Hemocyte Counts (THCs), the control and parasitized larvae were removed from the vials, according to the technique outlined by Jalali and Salehi (2008). To execute hemolymph dilution, Thoma white blood cell pipette was used to suction hemolymph up to 0.5 mark and then, filled with diluting fluid up to the 11 mark. After shaking the pipette for many min, the first three drips were discharged. The diluted hemolymph was placed into a double line hemocytometer, and hemocytes were counted using four corner squares and one central square. The formula was used to determine the quantity of floating or circulating hemocytes/mm3. The experiment was replicated three times for both differential and total hemocyte counts, and the data were analyzed to obtain mean value and standard error.
Hemolymph protein analysis
For humoral examination of the larval immune response toward the parasitism, it was necessary to evaluate either quantitative or qualitative hemolymph proteins. Using a spectrophotometer and Coomassie brilliant blue G-250 (CBB), the amount of total protein in the hemolymph was determined. The absorbency was determined at 595 nm. The method of Bradford (1976) was applied to create a standard calibration curve utilizing Bovine Serum Albumin (BSA) solutions as the reference proteins.
Electrophoretic separation of hemolymph proteins was carried out referring to the protocol stated by Laemmli (1970). Using SDS-PAGE, hemolymph proteins were separated and documented to both of normal (unparasitized) and parasitized larvae. Through serial days of application, analysis of protein bands was made using SynGene GeneTools—File version: 4.3.10.0, in the Faculty of Science's Central Laboratory, Ain Shams University, Cairo, Egypt.
Assessment of hemolymph phenoloxidase activity
A preliminary test was set up for quantify phenoloxidase activity, in which the production of dopachrome from l-dihydroxyphenylalanine (l-DOPA) spectrophotometrically at 490 nm was recorded according to Aso et al. (1985) with some modifications. l-DOPA (2 mg/ml) was dissolved in sodium phosphate buffer (SPB) (0.01 M, pH 5.9). To ensure that the linear increase in optical density was respected, aliquots (20 µl) of hemolymph were diluted (v/v) in a sodium cacodylate buffer (SCB) of 0.01 M sodium cacodylate, 0.25 M sucrose, and 0.01 M trisodium citrate before being added to 2 ml of l-DOPA solution. This process took place over the course of five min. In the control state, 20 µl SCB was employed. The amount of Phenoloxidase activity was expressed as a PO unit, where one unit is the amount of enzyme activity necessary to enhance the absorbance by 0.001 min/mg protein.
Statistical analysis
Data were compared using the one-way analysis of variance (ANOVA) in GraphPad Prism software version 8.0.1. followed by a Duncan multiple rang test, with significant differences at P < 0.005.
Results
Physical aspects of parasitism process
The recent investigation showed that B. hebetor was highly effective in controlled lab settings with significant paralysis rates. Through a brief squeeze of the head capsule with forceps, larvae were examined for paralysis; when paralyzed, they exhibited no movement (Fig. 1).
The most significant signs of the immune system activation are the melanization of the insect body. Such symptoms provoke the effective combination of both cellular and humoral responses inside the body of treated larvae. Results presented in Fig. 2 demonstrate the physical differences between larvae in their normal state and after parasitized by B. hebetor. Control larvae showed non-symptoms of melanization, while those with moderate to severe melanization exhibited minimal movement (lethargy), and gradually shrank over time (brachytosis). Melanization was seen in parasitized larvae. Morbid larvae also exhibited anorexia and brachytosis 24, 48 and 72 h after exposure to the parasitoid.
Ultrastructural examination of larval hemocytes
Five distinct hemocyte types were documented using full grown larval instar of C. cephalonica using Transmission Electron Microscope (TEM). That were represented as Prohemocytes, Plasmatocytes, Granulocytes, Spherulocytes and Oenocytoids (Fig. 3).
Electromicrographs of the ultrastructure of hemocytes of fully developed larval instar of Corcyra cephalonica in normal physiological state A, B Prohemocyte; C, D: Plasmatocytes; E, F: Granulocytes; G: Spherulocytes; H: Oenocytoids with Nucleus (N); mitochondria (MI); Rough endoplasmic reticulum (RER); Electron Dense Granules (d). Scale bar represents 500 nm (A, C, D, E, F, G, H) and 100 nm (B)
Following successive hours of parasitization, an obvious alternation and destructive effects in the ultrastructure of different hemocyte types were noticed and represented in (Fig. 4). Prohemocytes grew larger or assumed an elongated shape, cytoplasmic granules that were larger with badly deformed nucleus, and the cell membrane was lysed as shown in (Fig. 4A). Intense vacuoles were present in the cytoplasm of plasmatocytes, furthermore, containing material in various phases of degradation and ruptured plasma membrane, which suggested the onset of necrosis (Fig. 4B–C). Granulocytes showed clear cytoplasmic vacuolization with nearly damaged cell membrane and Fig. 4D. Spherulocytes had larger cytoplasmic granules; moreover, the nucleus was significantly deformed, the cell membrane was entirely lysed, and there were several patches on the nucleus and apparent vacuoles in the cytoplasm Fig. 4E. Oenocytoids were difficult to identify under TEM due to their rarity among the hemocyte population, although cells with a distinctive uniformly dense cytoplasm and a dearth of organelles, consistent with their description, were seen. Oenocytoids had several patches and either eccentric or centrally placed nuclei Fig. 4F.
Electromicrographs of the ultrastructure of hemocytes of fully developed larval instar of Corcyra cephalonica in irregular physiological conditions. A Prohemocyte; B, C: Plasmatocytes; D: Granulocytes; E: Spherulocytes; F: Oenocytoids. with Nucleus (N) and Mitochondria (MI), Electron Dense Granules (d), Vacuole (v); Cytoplasmic projections (Cp); Cell Membrane degenerated (Cd); heterogeneous granules (h). Scale bar represents 500 nm (A, B, C, D, F) and 100 nm (E)
Larval hemocyte counts
The immune system of C. cephalonica was clearly affected by the application of B. hebetor. Both differential and total hemocyte counts (DHC and THC) were subjected to considerable fluctuations.
Data obtained in Table 1 presented that the DHCs of both parasitized and non-parasitized larvae contain prohemocytes and plasmatocytes as the most prominent types. The number of (PR) was statistically differed after successive hours of parasitism 24, 48 and 72 h, respectively (40.33 ± 5.61, 43 ± 8.33 and 26 ± 2.31) compared to unparasitized (31.33 ± 6.49) larvae. Similar observations were recorded in PL before and after the parasitism. The GR also considered among the most common hemocyte type circulating in the hemolymph, while their relative percentages persisted lower than PR and PL (Table 1). The residual hemocyte (SP and OE) was documented in the hemolymph samples, but their abundance was little (Table 1). A significant investigation was also recorded; all the hemocyte types were gradually decreased in their abundance through the application period than the unparasitized larvae, except plasmatocytes which recorded their maximum values after 72 h of biological control application (Table 1).
The consequence of B. hebetor natural envenomation on the full grown larvae of C. cephalonica reflected an obvious effect in the cellular immune response of the parasitized larvae through the fluctuations that were recorded in the total hemocyte count in (Table 2). Non-significant difference (P > 0.05) was recorded between unparasitized and parasitized larvae after 24 h. While after consecutive hours (48 and 72 h) of response, there was a significant difference in the THC (P < 0.05), considering parasitized and non-parasitized larvae.
Analysis of hemolymph protein
The effectiveness of biological control progressions depends on the host-parasitoid systems' physiological and biochemical alternations. The period after parasitism (24, 48, and 72 h) revealed a slight reduction in total protein, the difference in total hemolymph protein between parasitized and unparasitized hosts was statistically significant as presented in (Table 2).
After the spectrophotometric analysis of total plasma protein further documentation was made by SDS-PAGE to evaluate the qualitative changes that occurred in the larval hemolymph following natural envenomation as shown in Fig. 5 and analyzed in Fig. 6. Lane (1) represented the molecular weight marker that was separated into 13 bands with MW ranging from (245–7.37 KDa) (Figs. 5 and 6A). The hemolymph proteins of unparasitized larvae (Normal) were documented in Lane (2); separated into 16 bands with MW (436.73–7.37 kDa) (Figs. 5 and 6B). Protein bands of parasitized host's hemolymph were shown in Lanes (3, 4, and 5) at 24, 48, and 72 h of application (Fig. 5). Lane 3 marked 15 protein bands of the larval hemolymph after 24 h of parasitism with the same MW range of unparasitized larvae (Figs. 5 and 6C). While, Lane 4 showed 18 protein bands, following 48 h of parasitism with MW (422.38–7.46 kDa) (Figs. 5 and 6D). At end of tracking, the effect of parasitism on the host's hemolymph Lane 5 indicated slight decline in the protein banding pattern reached 14 bands with MW (436.73–7.37 kDa). A common band was documented in the hemolymph of parasitized larvae at 48 and 72 h with Mw (49.51KDa).
Electrophoretically separated hemolymph Protein bands of full grown larval instar Corcyra cephalonica of Unparasitized and Parasitized larvae by natural envenomation of Bracon hebetor at Consecutive period of parasitism. Separated using SDS- PAGE (10%). MW: Wide range molecular weight marker (245–11 kDa). Equivalent protein amounts of 20 μg/lane were loaded. lane1: Marker of protein; lane 2: (N) Normal hemolymph of Corcyra cephalonica larvae; lane 3: hemolymph after 24 h post-parasitism; lane 4: hemolymph after 48 h post-parasitism; lane 5: hemolymph after 72 h post-parasitism
Changes in the Retardation factor (RF) of separated hemolymph protein bands of full grown larval instar Corcyra cephalonica of both Unparasitized and Bracon hebetor Parasitized larvae through natural envenomation of at Successive period of parasitism. A lane 1 (MW Marker of protein); B lane 2 (Unparasitized hemolymph of C. cephalonica larvae); C lane 3 (hemolymph after 24h post parasitism); D lane 4 (hemolymph after 48h post parasitism); E lane 5 (hemolymph after 72h post parasitism)
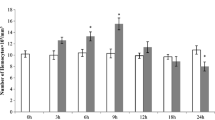
Evaluation of hemolymph phenoloxidase (PO) activity
The activity of PO in the full-grown larval instar hemolymph of unparasitized as well as naturally envenomated larvae of C. cephalonica by the parasitic wasp, B. hebetor is documented in Table 2. The Initial PO activity levels were (0.177 ± 0.0.044) for unparasitized larvae. The parasitized larvae showed a gradual increase in the PO activity starting from 24 h after stinging (0.442 ± 0.103). The maximum activity reached up to (1.482 ± 0.272) at the end of a successive period of parasitism (72 h). Data revealed significant gradual induction of Phenoloxidase activity as a result of parasitism (Table 2).
Discussion
The effects of endo-parasitism by parasitic wasps on physiological changes and host immunological defense that takes place during parasitism in insect hosts for koinobiont endoparasitoids have been the subject of numerous studies. However, little is known about idiobiont ectoparasitoids and how these parasitoids affect the physiology of their target hosts. Also, the insect's immune system condition during parasitization is less well understood. The present study sought to understand some physiological changes in the host C. cephalonica full-grown larvae as well as the cellular and humoral immune responses.
In this study, moribund larvae were identified by their melanization, paralysis and reduced survival periods. The symptoms of the illness are probably brought on by a reaction in the hemolymph and collateral damage from insect immune responses toward the parasitic wasp's natural envenomation. The paralysis of the parasitized larvae is arbitrated by three partially characterized proteins as a result of natural envenomation of the parasite's venom that presynaptically blocks glutaminergic transmission (Labrosse et al. 2005).
In this investigation, melanization which indicates the activation of the PPO cascade was the first apparent sign of an immune response against a parasitoid activity. The start of the humoral response was at the dorsal vessel, where melanin can be seen around it through the cuticle. This was probably caused by hemolymph movement accumulating melanized (darkening of cuticle) particles around the dorsal vessel, which sessile hemocytatic cells then phagocytosed. The same explanation for the results seen was made clear by Sigle and Hillyer (2016). Since the degree of melanization grew over time, it is possible to use it as a visual signal to evaluate immune responses. Also, may be a consequence of increasing hemolymph secretions due to the immune induction in the hemocoel and increasing activation of the PPO cascade. Our explanation is consistent with (Chen and Keddie 2021), who offered elucidations regarding the impact of intrahemocylic injection of pathogenesis in Galleria mellonella (L.). Such an explanation is supported by our previously stated results of biochemical analysis and cellular investigations against the parasitic effects.
The venomous substances that the parasitoid's female injects into the host to paralyze it, while it lays eggs often can lower the host's immunity (Shelby et al 2000).
Obtained results showed that the host larvae's differential hemocyte counts were reduced after one day and two days' intervals of envenomation, and their hemocytes profiles considerably changed over the course of the experiment in response to parasitization. As a result of parasitization, it was found that there was a decrease in the number of PR, PL, GR, SP, and OE. Given that these cells do not play specialized roles in the cellular immune response and are, therefore, more susceptible to pathogenic infection, it is hypothesized that limited imaging of these cell types reflects the reduction in their concentration (Cunha et al. 2009). In contrast to Silva et al. (2002), who showed that at the end of the third instar but not at the beginning, parasitized larvae by Anastrepha obliqua had more hemocytes than unparasitized ones based on circulating hemocyte's count. DHC asserts that the increased amount of prohemocytes is the cause of the higher number of hemocytes. They attributed this increase to the fact that an increase in the number of hemocytes in an insect's hemolymph is a typical reaction to parasitism (Eslin and Prévost 1998).
Numerous proteins found in insect hemolymph are involved in a variety of immunological, physiological, and developmental processes. Some of these proteins have been extracted, and it has been demonstrated that they have dual functions in hemolymph (Rosell and Coons 1991). According to earlier research, ectoparasitism and endoparasitism results in a decrease in the total amount of hemolymph protein (Coudron et al. 1997). It is well established that ichneumon and braconid endoparasitoids elicit a variety of physiological changes in their insect hosts, including changes to their endocrine and developmental programming (Lawrence 1993). There are qualitative and quantitative alterations in the plasma protein composition of hosts when endoparasitoids parasitize them, notably lepidopteran species (Rahbé et al. 2002).
The present study demonstrated that parasitism had a number of consequences on the host hemolymph. These might have something to do with the host's immunosuppression, parasitoid development, and nourishment. Following parasitization, there will likely be a drop in some hemolymph proteins in host larvae; on the other side, an increase in other proteins may be caused by the breakdown of fat body and tissue proteins or may be influenced by parasitoid venom to regulate the host, such explanations are similar to that of Altuntaş et al. (2010). Additionally the noticeable appearance of new protein bands for the secerned protein profile maybe as defensive proteins, which were synthesized as the host's immune reactive proteins or may be related to ectoparasitoid venom that contains a various range of proteins and peptides (Nakamatsu and Tanaka 2003). In other studies, venom proteins are reported to have an immunosuppressive role or even induce host castration (Asgari et al. 2003). Similarly, in the present study, elevation in the quantity of protein may have been a result of the effects of paralytic toxins in the venom. The recorded common protein band with molecular weight (49.51 KDa) at 48, 72 h post exposure to parasitoid was suggested to be phenoloxidase (PO) that was triggered and released as a result of parasitization and induction of the host's immune response toward the envenomation. Such elucidation is in accordance with those of many researchers (Radwan et al. 2022) who recorded the appearance of new protein band refereed as PO after induction the immune system of their studied lepidopterous pests by different immune activating factors.
Phenoloxidase (PO) is only one enzyme in a multi-enzyme cascade also called tyrosinase, which possesses both monophenol monooxygenase activity. The present study revealed rare enzyme activity in unparasitized larvae since the enzyme is in the pro-form, lysis of hemocytes alone would not result in a direct rise in PO activity. Following exposure to the parasitic effect, a significant increase in PO activity was recoded and such elevation was attributed to the activation of host immune system. The activation of enzymes upstream such as prophenoloxidase activating proteinases or venom components themselves may cause PPO to convert into its active form PO (Hartzer et al. 2005).
The present study demonstrated that parasitism had many consequences on the host hemolymph. These might have something to do with the host's immunosuppression, parasitoid development, and nourishment. Following parasitism, there will likely be a drop in some hemolymph proteins in host larvae, but an increase in other proteins may be caused by the breakdown of fat body and tissue proteins or may be influenced by parasitoid venom to regulate the host. Also, showed that parasitism significantly affects the host's cellular and humoral immune response, which ultimately results in the pest insect's death.
Conclusion
An evidence that both the adult wasp and the parasitoid larva can trigger humoral immune responses in the host larvae was provided, despite the fact that an attack by the idiobiont ectoparasitoid, B. hebetor, leads in the lethal envenomization of C. cephalonica larvae. So, the natural enemy B. hebetor, a biological control agent that has also advantageous for the environment, can thus be utilized effectively by integrated pest management programs.
Availability of data and materials
All data and materials are available in this manuscript.
References
Altuntaş H, Kilic AY, Sivas H (2010) The effects of parasitism by the ectoparasitoid Bracon hebetor Say (Hymenoptera: Braconidae) on host hemolymph proteins in the Mediterranean flour moth Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Turk J Zool 34(3):409–416
Asgari S, Zhang G, Zareie R, Schmidt O (2003) A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem Mol Biol 33(10):1017–1024
Aso Y, Kramer KJ, Hopkins TL, Lookhart GL (1985) Characterization of haemolymph protyrosinase and a cuticular activator from Manduca sexta (L.). Insect Biochem 15(1):9–17
Bernardi EB, Haddad MD, Parra JR (2000) Comparison of artificial diets for rearing Corcyra cephalonica (Stainton, 1865) (Lep., Pyralidae) for Trichogramma mass production. Revista Brasil Biol 60:45–52
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Brehélin M (1982) Comparative study of structure and function of blood cells from two Drosophila species. Cell Tissue Res 221(3):607–615
Chen RY, Keddie BA (2021) Galleria mellonella (Lepidoptera: Pyralidae) hemocytes release extracellular traps that confer protection against bacterial infection in the hemocoel. J Insect Sci 21(6):17
Coudron TA, Brandt SL, Raqib A (1997) Comparison of the response of Heliothis virescens to parasitism by Euplectrus comstockii and Euplectrus plathypenae. Comp Biochem Physiol B Biochem Mol Biol 116(2):197–202
Cunha FM, Wanderley-Teixeira V, Teixeira ÁA, Albuquerque AC, Alves LC, Lima EA (2009) Caracterização dos hemócitos de operários de Nasutitermes coxipoensis (Holmgren) (Isoptera: Termitidae) e avaliação hemocitária após parasitismo por Metarhizium anisopliae. Neotrop Entomol 38:293–297
Dieckhoff C, Tatman KM, Hoelmer KA (2017) Natural biological control of Halyomorpha halys by native egg parasitoids: a multi-year survey in northern Delaware. J Pest Sci 90:1143–1158
Eslin P, Prévost G (1998) Hemocyte load and immune resistance to Asobara tabida are correlated in species of the Drosophila melanogaster subgroup. J Insect Physiol 44(9):807–816
Fathy H (2019) Comparative studies on the immune responses of two lepidopteran hosts and their effects on the biology of the parasitoid wasp, Bracon hebetor (Hymenoptera: Braconidae). In: Ph.D Thesis, Fac of Sci, Banha Univ
Hartzer KL, Zhu KY, Baker JE (2005) Phenoloxidase in larvae of Plodia interpunctella (Lepidoptera: Pyralidae): molecular cloning of the proenzyme cDNA and enzyme activity in larvae paralyzed and parasitized by Habrobracon hebetor (Hymenoptera: Braconidae). Arch Insect Biochem Physiol 59(2):67–79
Hoffmann D (1980) Induction of antibacterial activity in the blood of the migratory locust Locusta migratoria L. J Insect Physiol 26(8):539–549
Jalali J, Salehi R (2008) The hemocyte types, differential and total count in Papilio demoleus L. (Lepidoptera: Papilionidae) during post-embryonic development. Mun Ent Zool 3:199–216
Labrosse C, Eslin P, Doury G, Drezen JM, Poirié M (2005) Haemocyte changes in D. melanogaster in response to long gland components of the parasitoid wasp Leptopilina boulardi: a Rho-GAP protein as an important factor. J Insect Physiol 51(2):161–170
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lawrence PO (1993) Hormonal interactions between insect endoparasites and their host insects. Parasites and pathogens of insects.
Leonard WJ, Depper JM, Krönke M, Robb RJ, Waldmann TA, Greene WC (1985) The human receptor for T-cell growth factor. Evidence for variable post-translational processing, phosphorylation, sulfation, and the ability of precursor forms of the receptor to bind T-cell growth factor. J Biol Chem 260(3):1872–1880
Mansour NA (2012) Biocontrol studies on using Bracon sp. (Hymenoptera: Braconidae) to control lepidopterous pests infesting olive trees. In: PhD Thesis, Fac Sci, Al-Azhar Univ, Egypt.
Manzoor A, UlAbdin Z, Webb BA, Arif MJ, Jamil A (2016) De novo sequencing and transcriptome analysis of female venom glands of ectoparasitoid Bracon hebetor (Say.) (Hymenoptera: Braconidae). Comp Biochem Physiol D Genom Proteom 20:101–110
Nakamatsu Y, Tanaka T (2003) Venom of ectoparasitoid, Euplectrus sp. near plathypenae (Hymenoptera: Eulophidae) regulates the physiological state of Pseudaletia separata (Lepidoptera: Noctuidae) host as a food resource. J Insect Physiol 49(2):149–159
Radwan MH, Alaidaroos BA, Jastaniah SD, Abu el-naga MN, El-Gohary EG, Barakat EM, ElShafie AM, Abdou MA, Mostafa NG, El-Saadony MT, Momen SA (2022) Evaluation of antibacterial activity induced by Staphylococcus aureus and Ent A in the hemolymph of Spodoptera littoralis. Saudi J Biol Sci 29(4):2892–2903
Rahbé Y, Digilio MC, Febvay G, Guillaud J, Fanti P, Pennacchio F (2002) Metabolic and symbiotic interactions in amino acid pools of the pea aphid, Acyrthosiphon pisum, parasitized by the braconid Aphidius ervi. J Insect Physiol 48(5):507–516
Ratcliffe NA (1993) Cellular defense responses of insects: unresolved problems. Parasit Pathog Insects 1:267–304
Resh VH, Cardé RT (2009) editors. Encyclopedia of insects. Academic press.
Rosell R, Coons LB (1991) Purification and partial characterization of vitellin from the eggs of the hard tick. Dermacentor Variabilis Insect Biochem 21(8):871–885
Schmid-Hempel P (2005) Natural insect host-parasite systems show immune priming and specificity: puzzles to be solved. BioEssays 7(10):1026–1034
Shelby KS, Adeyeye OA, Okot-Kotber BM, Webb BA (2000) Parasitism-linked block of host plasma melanization. J Invertebr Pathol 75(3):218–225
Sigle LT, Hillyer JF (2016) Mosquito hemocytes preferentially aggregate and phagocytose pathogens in the periostial regions of the heart that experience the most hemolymph flow. Dev Comp Immunol 55:90–101
Silva JE, Boleli IC, Simões ZL (2002) Hemocyte types and total and differential counts in unparasitized and parasitized Anastrepha obliqua (Diptera, Tephritidae) larvae. Braz J Biol 62:689–699
Vincent A, Singh D, Mathew IL (2021) Corcyra cephalonica: a serious pest of stored products or a factitious host of biocontrol agents? J Stored Prod Res 94:101876
Vinson SB (1990) How parasitoids deal with the immune system of their host: an overview. Arch Insect Biochem Physiol 13(1–2):3–27
Acknowledgements
The authors would like to thank Dr. Ibrahim El-Sayed Shehata, Pests and Plant Protection Department, National Research Centre, Cairo, Egypt, for his help in conducting the hemolymph sections in the manuscript. Many thanks to Dr. Marwa Mahmoud Department of Medical Malacology, Theodor Bilharz Research Institute, Giza and Dr. Hend Omar, Biological Control Research Department, Plant Protection Research Institute, Agricultural Research Center, for their keen interest, support and help.
Funding
This work was not supported by any funding body, but personally financed.
Author information
Authors and Affiliations
Contributions
AY, NZ, EM, HAA conceived and designed the research. SHM and RF conducted the experiments. AY, SHM and RF analyzed the data and wrote the manuscript. AY, NZ, EM, HAA made critical reviewed and approved the final version. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fathy, R., Zohdy, N., Abd-El-Samie, E.M. et al. Effects of parasitism by the braconid wasp, Bracon hebetor (Hymenoptera: Braconidae), on the host hemolymph and phenoloxidase activation of the rice moth, Corcyra cephalonica larvae (Lepidoptera: Pyralidae). Egypt J Biol Pest Control 33, 30 (2023). https://doi.org/10.1186/s41938-023-00678-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-023-00678-z