Abstract
Background
The predatory mite, Blattisocius mali Oudemans (Mesostigmata: Blattisociidae), feeds on various species of storage mites such as Tyrophagous putresentiae (Schrank). In this study, life table parameters and consumption rates of B. mali were determined when fed on the eggs and larvae of T. putrescentiae as the prey under laboratory conditions.
Results
Based on the age-stage two-sex life table analysis, the total pre-ovipositional period (TPOP) of the predator was considerably lower by feeding on host larvae than that on eggs. Also, mean fecundity (eggs/female) of the B. mali was 2.60-fold higher on host larvae than its eggs. Moreover, the intrinsic rate of increase (r), as the most important parameter was 0.272 and 0.357 day−1, respectively, by host mite eggs and larvae as food. This showed the significantly greater influence of T. putrescentiae larvae than eggs on mass production of the predatory mite. The same trend was observed in finite rate of increase (λ), net reproductive rate (R0), gross reproductive rate. But mean generation times (T) were statistically the same by feeding both host stages. Maximum longevity of male and female individuals was, respectively, 21 and 22 days when eggs were fed. By feeding on larvae as prey, maximum longevity was lowered to 20 days in both predator sexes. However, the net predation rate (C0) and transformation rate from prey population to predator offspring (QP) were significantly higher by feeding on host eggs (97.420 and 3.049) than its larvae (41.3936 and 0.5575), respectively.
Conclusion
From practical biocontrol view, both eggs and larvae of T. putrescentiae can be consumed and therefore controlled by the predatory mite, B. mali. From mass production view, larvae of T. putrescentiae produced a better fitness in the predatory mite than its eggs and can be used in augmentation programs of B. mali.
Similar content being viewed by others
Background
The storage mite, Tyrophagus putrescentiae (Acaridae), is one of the important pest mites. It has distributed worldwide in stored foods. T. putrescentiae constitutes a serious problem during storage of ornamental plant materials (Muller and Hollinger 1980). It can cause bud necrosis in storage bulbs. Bud necrosis is one of the most important diseases of tulip bulbs. High infestations of T. putrescentiae reduce the quality of a product, accelerating spoilage (Kucerova and Horak 2004). Due to allergenic potential, this mite has received more attention as an important factor of allergic asthma and allergic diseases and rhinitis in humans (Yu et al. 2014).
The long-term and extensive application of pesticides by growers has become prevalent practice and because of associated problems of excessive use of chemicals like pesticide residues, insecticide resistance and other various environmental hazards, there is much desire for reducing pesticide applications and using environmentally safe measures including biological control (Naher et al. 2005).
Predatory mites are among the most effective biological control agents of pest mites and insects (Gerson et al. 2003). Fifteen species of the genus Blattisocius (Acari: Blattisociidae) have been classified that are often found in storage facilities (Britto et al. 2012). It has been reported that some of Blattisocius species have an ability to be biological control agents. More than other species, Blattisocius dentriticus Berlese, B. keegani Fox and B. tarsalis Berlese have been studied (Thomas et al. 2011). Pollen, fungi, nematodes and small arthropods are among Blattisocius spp. hosts (Lindquist et al. 2009).
There is no previous research to illustrate the potential of T. putrescentiae eggs and larvae separately on B. mali’s mass production by estimating the predator biological parameters, nor any data on its predation rate. Life tables characteristics developmental time, survival rate and reproduction of a population, can be invest to show the effects of diverse biotic and abiotic agents on pest or natural enemy populations. But, evaluation of predation rate along with the life table examinations can prepare more useful information about the biocontrol agent efficacy, because having a higher intrinsic rate of increase, is not necessarily a sign of predator greater efficiency (Tuan et al. 2015). Knowledge of the predator–prey relationships is needed to conduct a successful augmentative biological control program and predator release into a crop production system. There is no data in this area on the predation capacity of B. mali on T. putrescentiae. Therefore, the objectives of this research were to evaluate a thorough understanding of the effects of two different stages (eggs and larvae) of T. putrescentiae separately (as diet) on the fertility life table parameters and predation rate of B. mali. This information is essential to understand and predict the performance of this natural enemy in greenhouse environments and mushroom cultivation salons.
Methods
Preparation of mite colonies
A stock colony of B. mali was afforded at the Ecology and Biocontrol Laboratory of the Research Institute of New Biological Technologies, University of Zanjan, Iran. The predator colony was reared in glass Petri dishes (9 cm in diameters), placed in glass containers. (45 × 45 × 12 cm). Rearing units set on the plastic plates that were covered by lonolite.
To prevent mites from escaping and retaining moisture, inside enclosure of the glass was filled with water. Predatory mites were reared on egg and larval stages of T. putrescentiae, individually, for three generations before using in the experiments.
Tyrophagus putrescentiae colonies were initiated from a stored date facility in Marand, Azarbayejan-sharghi, Iran. Mites were reared separately on Baker's yeast, Saccharomyces cerevisiae (Saccharomycetales: Saccharomycetaceae) in a jar (12 × 17 cm) (Asgari et al. 2020). The colonies were kept in a growth chamber at 25 ± 1 °C and 75 ± 5% RH.
Experiments
Life table and predation rate experiments were started by cohort of 70 predator eggs. The experimental units consisted of 14 plastic Petri dishes (6 cm in diameter). At the bottom of each dish, five plastic cylindrical cells (6 × 9 mm) were fixed by thermal adhesive. Remaining parts were filled with water to enclose the cells and prevent mites from escaping. Eggs and larvae of T. putrescentiae were separately supplied to the predatory mite in each experiment.
Until emergence of adult mites, survival and the stage developments of immature B. mali were recorded, daily. After maturity, males were transferred to the female cells for mating. The fecundity and longevity of females were recorded daily until the death of last individual. Moreover, consumption rates of all immature and adult stages were recorded. Deposited eggs and extra food were removed daily and replaced with new food. Experiments were carried out at 25 ± 1 °C, 75 ± 5% RH and a photoperiod of 16L: 8D h.
Data analysis
Life table data were analysed based on age-stage, two-sex life table theory using the TWOSEX-MSChart program (Chi 2019). Predation rate data were analysed by two-sex consumption rate analysis method. In this method, longevity and predation rate for male and female mites at different stages of life were calculated separately (Chi and Yang 2003). Means and standard errors of the population parameters were calculated by bootstrap method (Yu et al. 2013). Graphs were drawn using Sigmaplot 11.
The age-specific survival rate (lx) and fecundity (mx), as well as the age-stage specific reproductive value (vxj), survival rate (sxj) and fecundity (fxj) were calculated as described in Chi and Su (2006). The net reproductive rate (R0), finite rate of increase (λ) and mean generation time (T) were calculated according to:
The intrinsic rate of population increase (r) was estimated by using the method described by Goodman (1982):
where Cxj, is the mean number of preys consumed by predator in age x and stage j, so the daily consumption of all individuals, including males, females, and those dying before the adult stage was recorded to estimate the age-specific consumption rate Cxj (Chi and Yang 2003).
The age-specific predation rate (kx) is the mean number of preys consumed by predator at age x and was calculated as:
The net predation rate (C0), based on Chi and Yung (2003), demonstrated the mean number of preys consumed by an average individual predator during its whole life and is estimated as:
The ratio of the net predation rate to the net reproductive rate gives the transformation rate from pray population to predator offspring (Qp) (Chi and Yung 2003). It was calculated as:
The difference between treatments at 5% significance level was evaluated based on the bootstrap method and p value amounts (Chi 2019; Wei et al. 2020).
Results
Life tables of B. mali on two different diets
Most of eggs used at the initial cohort hatched in egg treatment, because in this experiment, 56 out of 61 eggs hatched. The developmental time for pre-adult stages and longevity of adult males and females are presented in Table 1. Stage differentiation is essential for quantifying the control efficacy of a predator population. Mean developmental time of all pre-adult stages of B. mali was 8.07 and 6.03 days on prey eggs and larvae with significant differences.
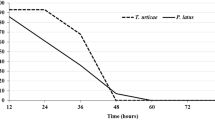
All active pre-adult stages of the predatory mite showed significant differences in their developmental times between two diets. Predator larvae finished their development in shorter time on the host eggs than on its larvae. However, other protonymphal and deutonymphal stages were passed in longer time on host eggs compared with its larvae. Moreover, total pre-adult period (6.03 days) was significantly shorter on T. putrescentiae larvae in comparison with its eggs (8.07 days). There were non-statistical differences between longevities of female predatory mites when reared on host eggs or larvae. The same result was observed in B. mali males (Table 1). Maximum adult longevity was 22 and 21 days for females and males, respectively, on host eggs, but they both lived utmost 20 days on larval diet. The age-stage specific survival rate (sxj) of the different stages of B. mali in two treatments are shown in Fig. 1. Parameter sxj is the probability that a newborn will survive to age x and stage j. Due to using of the age-stage two-sex life table, that calculates variation in developmental rate, overlaps in the survival rate of different stages of the mite are seen in the curve. According to Fig. 1, mortality was high in the pre-adult stages of the predatory mite on host egg diet. The highest survival rate of male and female predators was, respectively, 0.33 and 0.43 in days 9–11. The highest survival rate of male and female mites fed on larval diet, was longer and equal to 0.31 on days 6–10 and 0.38 on days 6–16, respectively (Fig. 1).
The ovipositional period (the mean number of days that a mite has laid eggs) was 12.96 days on host egg diet and 14.77 days on host larval diet (Table 2). The mean APOP (adult pre-ovipositional period of female adult) and TPOP (total pre-ovipositional period of female counted from birth) were 0.12 and 8.23 days, respectively, on egg diet. These parameters were 0.09 and 6.14 days on larval diet, respectively. In other words, the mated females spent 0.12 and 0.09 a day before starting oviposition on T. putrescentiae eggs and larvae, respectively.
The age-specific survival rate (lx), female age-stage specific fecundity (fx), the age-specific fecundity (mx) and age-specific maternity (lxmx) of B. mali on two different diets, are plotted in Fig. 2. Oviposition began on the 7th day and peaked on the 11th day. On the larvae diet, however, it started on the 4th day and reached to maximum on the 14th day. The mean fecundities were 76.23 and 198.68 eggs/female and the maximum fecundities were 103 and 264 eggs/female, respectively, on host eggs and larvae diets.
Life expectancy (exj) shows the time that individuals of age x and stage j are expected to live (Fig. 3). The highest life expectancies were 16.46 and 17.55 days, respectively, for male and female mites on the 7th day, when the predator fed on egg diet. On host larval feeding they were equal to 14.35 and 17.55 days on the 5th day.
Figure 4 shows the reproductive values (vxj) of B. mali fed on eggs and larvae of T. putrescentiae, which is the share of an individual of age x and stage j in the next population. Reproductive value of female adults started on the 7th and 5th days, respectively, on host eggs and larvae. In addition, the peak values of age-stage reproductive value were 30.55 on 9th day on egg diet and 55.52 on 13th day on larval diet. The female predator mites started oviposition from the 7th and 5th day, respectively, on egg and larval diets.
The intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0) and mean generation time (T) were obtained for treatment of eggs and larvae (0.272, 0.357 day), (1.313, 1.429 day−1), (32.491, 75.362 individual) and (12.773, 12.1 day), respectively (Table 3). Gross reproduction rate (GRR) was not examined, because it ignored the survival rate.
Predation rate of B. mali on two different diets
The age-stage predation rate (Cxj) showed that the mean number of eggs consumed by a predator of age x and stage j (Fig. 5). The highest peak of female predation rates was recorded on day 11th on host eggs and days 14 and 15 on host larvae (Fig. 6). Predation rate of the females decreased after oviposition period.
The consumption rates of different life stages of B. mali fed on eggs and larvae of T. putrescentiae are shown in Table 4. The mean consumption (predation) rate of a stage was calculated based on all individuals entered that stage. Among the immature stages, deutonymphs had the highest mean consumption rates on both diets. Moreover, males had the lower mean consumption rates compared to females (Table 4). Higher consumption rates of B. mali pre-adults, females and males were recorded on T. putrescentiae eggs than on its larvae (Table 4). The stage-specific net predation rates of B. mali on two host diets were presented in Table 5. Predation rates were increased by stage improvements and female adults preyed more host eggs (50.443) and larvae (28.506) than male adults, respectively (31.345, 10.684). The sum of all stage-specific net consumption rates was 97.42 and 41.39 hosts per predator, respectively on T. putrescentiae eggs and larvae.
As shown in Table 5, net predation rate (C0), finite predation rate (ω) and stable predation rate (Ψ) were comparatively higher on host eggs than on its larvae. Moreover, predator mites required 3.049 prey eggs to produce one offspring (QP); however, they produced an offspring per 0.5575 host larva.
Discussion
This study assessed the life tables and predation parameters of the predatory mite, B. mali, when fed on eggs and larvae of the stored mite T. putrescentiae. Life table is a useful tool for studying the dynamics of insect populations (Harcourt 1969) and a primary estimation, not only for demography, but also for general biology. It is the basis, because in this type of research, developmental time and the survival rate of each stage of life, adult longevity and daily fecundity rate of females are recorded separately for each individual in the statistical population (Chi 1988). Our results showed that B. mali could be recommended as a candidate biocontrol agent of T. putrescentiae eggs and larvae. No studies have estimated predation rate of B. mali before; however, some have described biological characteristics and fertility life table parameters of B. mali and some other predator species (Pirayeshfar et al. 2022).
In a study conducted by Da Silva et al. (2016), they estimated some biological characteristics of B. dentriticus when fed on T. putrescentiae. Fecundity rate of this predatory mite was reported as 10.46 eggs/female. But, in the results of the present study, B. mali fecundity was 76.23 and 198.68 eggs/female, respectively, on host eggs and larvae diets. Higher fecundity of predator indicated the suitability of pre-adult stages of T. putrescentiae as host. Prey larvae were more profitable, as B. mali fecundity was 2.60-fold higher than host eggs. However, in studies on B. dentriticus, host stages were not considered separately (da Silva et al. 2016). Pirayeshfar et al. (2021) reported that the fecundity of B. mali fed on mixed life stages of T. putrescentiae was 22.5. Possibly, because of the existence of the mature stages in diet and non-uniformity of the host, the predatory mite has spent more energy coping and hunting the host and less food was earned. Afterwards, due to less nutrition, fecundity was decreased.
Furthermore, fecundity of the first, 8th and 16th generations of B. mali fed on frozen mixed life stages of T. putrescentiae, was obtained 42.19, 45.73 and 41.89, respectively. Combination of frozen mixed life stages of T. putrescentiae with Cattail pollen and olive pollen improved the fecundity to 62.23 and 69.38 in the first generation, but decreased it in the 8th and 16th generations (Pirayeshfar et al. 2022). As can be seen from above data, fecundity of B. mali was higher in this research. This showed that separation of host stages as diet had better effect on predator fecundity. Probably predator acted more specifically on a single stage and adapted to its possible defense more efficiently.
Total pre-ovipositional period (TPOP) of B. mali decreased by feeding on larvae of T. putrescentiae than on host eggs. Decreased TPOP in line with increased fecundity are important characteristics for mass production of the predatory mite. Longer TPOPs of B. mali was recorded in other experiments by using mixed life stages and frozen stages of T. putrescentiae (Pirayeshfar et al. 2022).
Total pre-ovipositional period (TPOP) and fecundity of Cheyletus malaccensis Oudemans were 20.9 days and 32.7 eggs/female, respectively, by feeding on T. putrescentiae (Granich et al. 2016). This indicates that C. malaccensis had lower fecundity and a longer pre-ovipositional period than B. mali on the same feeding host. Also, the longevities of male and female adults of C. malaccensis were 21.9 and 21.5 days, respectively, which was shorter than those in B. mali by feeding on the same host eggs (23.35 and 24.27 days) and larvae (19.17 and 22.27 days), respectively. Higher fecundity and lower TPOP of B. mali compared to C. malaccensis was probably due to the better preference to T. putrescentiae. These characteristics may have a positive effect on population of B. mali and improve its mass rearing process in predator production programs or enhance pest control efficiency in pest management programs.
Mean ovipositional days for B. mali were recorded as 12.96 and 14.77 days, respectively, on prey eggs and larvae. Long ovipositional period does not necessarily equal a high fecundity. On host larvae as diet, predator had shorter lifespan than on egg diet. However, ovipositional days were longer. Therefore, predator mites matured earlier and had shorter total pre-ovipositional period and their fecundity was higher.
The intrinsic rate of increase (r) is an important indicator in assessing natural enemies among the parameters of the life table and shows the reproductive power, development and survival of the population (van Maanen et al. 2010). This parameter obtained 0.272 day−1 and 0.357 day−1 for B. mali on eggs and larvae of T. putrescentiae, respectively. This parameter was reported 0.14 day−1 for B. dentriticus fed on T. putrescentiae (Da Silva et al. 2016) Other demographic parameters such as finite rate (λ), net reproductive rate (R0) and mean generation time (T) for B. dentriticus were reported 1.15 day−1, 7.53 individual and 14.3 day in foregoing study, respectively (Da Silva et al. 2016). Whereas these parameters are obtained in the present study on two diets (egg and larvae) of T. putrescentiae, (1.313, 1.429 day−1), (32.491, 75.362 individual) and (12.773, 12.1 day), respectively. Pirayeshfar et al. (2021) estimated the r value for B. mali equal to 0.316 on mixed life stages of T. putrescentiae. Based on present results, r value was lower than that on the same host eggs in our experiment; however, it was higher than that on host larvae.
Moreover, r value of the predatory mites, Gaeolaelaps aculeifer Raumilben and C. malaccensis were, respectively, 0.12 and 0.09 day−1, when fed on T. putrescentiae. (Granich et al. 2016). In comparison, B. mali had a higher r by feeding on eggs and larvae of T. putrescentiae. As a result, it seemed that the host more efficiently than B. dentriticus and G. aculeifer for population growth and probably pest control.
Due to the change in the predator's demographic structure and the existence of non-predatory stages in the population such as eggs, the integration of life table information and age-stage specific predation rate had a special importance in studying the prey-predator model (Chi and Yang 2003).
In the present study, the number of effective predators (those that didn’t die prior to the first predatory stage) was 54 and 42 on host mite eggs and larvae, respectively. Also, mean consumption rates by effective predators (P-bar) were 110.06 and 57.17 preys. Therefore, the total consumed T. putrescentiae eggs and larvae were, respectively (110.06 × 54 = 5943.24) and (57.17 × 42 = 2401.14).
The start of feeding by B. mali on prey eggs and larvae were recorded on 3rd and 4th days, respectively. Moreover, feeding peaks were observed in ages 12 and 15 days, respectively. Predation rate was decreased with the increase in the predator mortalities and both kx and qx reached zero in age 30 (days) on prey eggs and age 26 (days) on its larvae.
The mean consumption rate of B. keegani per female was 35.9 (Rezk 2000), while it was 118.35 and 75.15 in B. mali, respectively, fed on eggs and larvae of T. putrescentiae. The results showed a high predation capacity of B. mali. Moreover, higher potential of B. mali to consume prey eggs compared to its larvae was because of immobility of prey eggs and defense capacity of its larvae, that makes eggs more available and feasible prey than its larvae. Handling of larvae was more difficult than non-defensive eggs.
Conclusions
Obtained results showed that when the predatory mite B. mali was fed on T. putrescentiae larvae as prey, its females were matured earlier, had higher fecundity and longer ovipositional period than the experiment conducted with prey eggs as diet. Accordingly, the predator showed a higher intrinsic rate of increase (r) on the prey larvae than on its eggs. This is important from mass production aspect that the larval prey represented the higher survival and reproduction in the predator. However, net consumption rate of B. mali was lower by feeding on prey larvae than its eggs, as a result of defensive behavior in prey larvae. This can be important from biocontrol aspect. As its application on prey eggs can control the T. putrescentiae damages to stored products before feeding. More research is needed to clarify the better establishment of B. mali and more effective control of prey populations.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Asgari F, Sarraf Moayeri HR, Kavousi A, Enkegaard A, Chi H (2020) Demography and mass rearing of Amblyseius swirskii (Acari: Phytoseiidae) fed on two species of stored-product mites and their mixture. J Econ Entomol 113:2604–2612
Britto EP, Lopes PC, De Moraes GJ (2012) Blattisocius (Acari: Blattisociidae) species from Brazil, with description of a new species, redescription of Blattisocius keegani and a key for the separation of the world species of the genus. Zootaxa 3479:33–51
Chen GM, Chi H, Wang RC, Wang YP, Xu YY, Li XD, Zheng FQ (2018) Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J Econ Entomol 111:2143–2152
Chi H (1990) Timing of control based on the stage structure of pest populations: a simulation approach. J Econ Entomol 83:1143–1150
Chi H (2019) TIMING-MSChart: a computer program for the population projection based on age-stage, two-sex life table. http://140.120.197.173/Ecology/Download/Timing-MSChart.rar
Chi H, Su HY (2006) Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ Entomol 35:10–21
Chi H, Yang TC (2003) Two-Sex Life Table and Predation Rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) Fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ Entomol 32:327–333
da Silva GL, Radaelli TFDS, Esswein IZ, Ferla NJ, da Silva OS (2016) Comparison of biological development of Blattisocius dentriticus (Blattisocidae) fed on Tyrophagus putrescentiae (Acaridae) and Megninia ginglymura (Analgidae). Int J Acarol 42(8):405–411
Gerson U, Smiley RL, Ochoa R (2003) Mites (Acari) for pest control. Blackwell Science, Oxford
Goodman D (1982) Optimal life histories, optimal notation, and the value of reproductive value. Am Nat 119:803–823
Granich J, Horn TB, Körbes JH, Toldi M, Da Silva GL, Ferla NJ (2016) Development of Cheyletus malaccensis (Acari: Cheyletidae) feeding on mite species found in commercial poultry systems: Megninia ginglymura (Acari: Analgidae) and Tyrophagus putrescentiae (Acari: Acaridae). Syst Appl Acarol 21:1604–1614
Kucerova Z, Horak P (2004) Arthropod infestation in samples of stored seeds in the Czech Republic. Czech J Genet Plant Breed 40:11–16
Lindquist EE, Krantz GW, Walter DE (2009) Order Mesostigmata. In: Krantz GW, Walter DE (eds) A manual of acarology. Texas Tech University Press, Lubbock, pp 124–232
Muller PJ, Hollinger TC (1980) Damage by Rhizoglyphus mites in some ornamental bulbous crops. Acta Hortic 109:449–456
Naher N, Islam W, Haque MM (2005) Predation of three predators on two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). J Life Sci 1:1–4
Pirayeshfar F, Safavi SA, Moayeri HRS, Messelink GJ (2021) Active and frozen host mite Tyrophagus putrescentiae (Acari: Acaridae) influence the mass production of the predatory mite Blattisocius mali (Acari: Blattisociidae): life table analysis. Syst Appl Acarol 26(11):2096–2108
Pirayeshfar F, Moayeri HRS, Da Silva GLA, Ueckermann E (2022) Comparison of biological characteristics of the predatory mite Blattisocius mali (Acari: Blattisocidae) reared on frozen eggs of Tyrophagus putrescentiae (Acari: Acaridae) alone and in combination with cattail and olive pollens. Syst Appl Acarol 27(3):399–409
Thomas HQ, Zalom FG, Nicola NL (2011) Laboratory studies of Blattisocius keegani (Fox) (Acari: Ascidae) reared on eggs of navel orange worm: potential for biological control. Bull Entomol Res 101:499–504
Tuan SJ, Yeh CC, Atlihan R, Chi H (2015) Linking life table and predation rate for biological control: a comparative study of Eocanthecona furcellata (Hemiptera: Pentatomidae) fed on Spodoptera litura (Lepidoptera: Noctuidae) and Plutella xylostella (Lepidoptera: Plutellidae). J Econ Entomol 109:13–24
Van Maanen R, Vila E, Sabelis MW, Janssen A (2010) Biological control of broad mites (Polyphagotarsonemus latus) with the generalist predator Amblyseius swirskii. Exp Appl Acarol 2:29–34
Wei MF, Chi H, Guo YF, Li XW, Zhao LL, Ma RY (2020) Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri and P. communis (Rosales: Rosaceae) pears with estimations of confidence intervals of specific life table statistics. J Econom Entomol. https://doi.org/10.1093/jee/toaa149
Yu LY, Chen ZZ, Zheng FQ, Shi AJ, Guo TT, Yeh BH, Chi H, Xu YY (2013) Demographic analysis, a comparison of the jackknife and bootstrap methods, and predation projection: a case study of Chrysopa pallens (Neuroptera: Chrysopidae). J Econ Entomol 106:1–9
Yu SJ, Liao EC, Tsai JJ (2014) House dust mite allergy: environment evaluation and disease prevention. Asia Pac Allergy 4:241–252
Acknowledgements
This research was supported financially by Urmia University as a part of the first author Ph. D. thesis that is acknowledged hereby.
Funding
This research was supported financially by Urmia University as a part of the first author Ph. D. thesis.
Author information
Authors and Affiliations
Contributions
FA conducted assays and statistical analyses, designed the figures and tables, and wrote the manuscript draft. SAS supervised all experimental set-ups and assisted in statistical analyses and writing/improving the manuscript. HSM afforded experimental conditions and facilities and advised in experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors would state that they only worked with small arthropods (insects and mites) and the international guiding principles are not applicable for these organisms.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asgari, F., Safavi, S.A. & Moayeri, H.R.S. Life table parameters of the predatory mite, Blattisocius mali Oudemans (Mesostigmata: Blattisociidae), fed on eggs and larvae of the stored product mite, Tyrophagus putrescentiae (Schrank). Egypt J Biol Pest Control 32, 118 (2022). https://doi.org/10.1186/s41938-022-00616-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00616-5