Abstract
Cotton (Gossypium hirsutum L.) is an important fiber crop cultivated in > 50 countries of the world. The cotton leaf roller Syllepte derogata (Fabricius, 1775) (Lepidoptera: Crambidae) is considered as an invasive and widespread species in many geographic regions of the world and pose significant damages to cotton crop. This study reported the first record of S. derogata and its biological characteristics in Azerbaijan. The results indicated that S. derogata was only observed on cotton crop from the Absheron region of the country. The larvae only attacked on cotton leaves and did not damage the reproductive organs (i.e., bolls). S. derogata completed one generation in 31–42 days in the Absheron region. Larvicidal efficacy of Bacillus thuringiensis var. thuringiensis (Btt) against S. derogata was also tested under laboratory and field conditions. Three different Btt concentrations (0.3, 0.4, 0.5 g/l) were tested against 2nd and 4th larval instars of S. derogata. The 0.5 g/l concentration of Btt caused 72.88% larval mortality under field conditions. However, under laboratory conditions, 0.3, 0.4, and 0.5 g/l concentrations caused 50, 66.67, and 100% mortality of the 2nd instar larvae on the 7th day, respectively. The 0.5 g/l suspension of Btt proved the most effective against S. derogata; therefore, it can be recommended for the management of S. derogata in the infested region of the country. This study further warns that S. derogata could spread to the other cotton-producing regions of the country; thus, an effective early warning and rapid response system must be developed for the pest in the country.
Similar content being viewed by others
Background
Cotton (Gossypium hirsutum L.) is currently the leading plant fabric worldwide, grown commercially in the temperate and tropical regions of more than 50 countries. Numerous insect pests attack cotton crop reducing seed-cotton yield and fiber quality. The species capable of causing monetary damages include Anthonomus grandis (Boheman, 1843), Pectinophora gossypiella (Saunders, 1844), Syllepte derogata (Fabricius, 1775), Pseudatomoscelis seriatus (Reuter, 1876), Aphis gossypii (Glover, 1877), Adelphocoris rapidus (Say, 1832), Nezara viridula (Linnaeus, 1758), Lygus lineolaris (Palisot de Beauvois, 1818), Polyphagotarsonemus latus (Banks, 1904), Dysdercus superstitiosus (Fabricius, 1775), and Spodoptera littoralis (Boisduval, 1833) (Pierre et al., 2013).
Syllepte derogata (Fabricius, 1775) (Lepidoptera: Crambidae) was recorded in Azerbaijan 2017–2018. Several studies have reported various natural enemies (parasitoids and pathogens) of S. derogata. These include larval and pupal parasitoid species and larval pathogens, B. thuringiensis var. kurstaki (Rajesh et al., 2013).
The high toxicity of pesticides and associated health and environmental risks have made microorganisms a viable option for pest control. The Gram-positive bacterium Bacillus thuringiensis (Berliner 1909) [Bt] represents ~ 95% of microorganisms used for pest control in agriculture (Lambert et al. 1992). The Bt is considered safe for human health; thus, it is used in the production of biopesticides and insect-resistant plants (Siegel, 2001 and Van Frankenhuyzen, 2009). Bacillus thuringiensis var. thuringiensis (Btt) has shown pathogenic effects against insect species belonging to Lepidoptera. The Btt is safe for human, warm-blooded animals, fish, hydrobionts, bees, and entomophagous when applied at proper concentration (Anonymous 2019).
The sporulation of Bt produces large parasporal proteinaceous crystalline inclusions (Cry toxins) and kills susceptible insects that eat them (Heckel, 2020). The most widely used Bt toxins are 3-domain Cry toxins. Examples include Cry1Ac active against certain Lepidoptera, Cry2Ab targeting some Lepidoptera but also active on Diptera, and the coleopteran-active Cry3Aa (Heckel 2020). Several steps are involved in the mode of action of 3-domain Cry toxins after their ingestion by insects (Ferré & Van Rie 2002 and Bravo et al. 2007). The protein either associated with spore or produced by plant dissolves insect midgut releasing protoxin. The protoxin is cleaved down to active toxin by an insect’s digestive system. The active toxin binds to membrane-bound proteins on the surface of the midgut epithelial cells. Oligomers are eventually formed by the monomers of toxin, either in solution or inserted into the lipid bilayer. Small pores are created in the membrane by membrane-spanning alpha-helix hairpins of the oligomers. These pores enable cations to flow into the cell, and water follows, possibly through aquaporins, causing the cells to swell and lyse. This is the so-called “colloid-osmotic lysis” mechanism (Knowles & Ellar 1987), which causes insect death.
In the literature, there is no study relating to biology and control of S. derogata in Azerbaijan. With the first record of it, its biology was studied and larvicidal efficacy of Btt against the pest was also tested under laboratory and field conditions.
Materials and methods
Identification of the pest species
The insect samples collected from cotton fields were sent to Prof. Dr. Levent Ünlü, an expert working on the lepidopteran pests in Selçuk University, Konya, Turkey. The morphological examination of the insect revealed that the insect was Syllepte derogate (Fabricius, 1775) (Lepidoptera: Crambidae).
Sampling and determination of biological characteristics
S. derogata larvae were collected from cotton fields located in Absheron Peninsula (N 40° 27′ 26″ E 49° 44′ 18″) of Azerbaijan during 2017–2018 to study biological characteristics of the pest. The general entomological methodology was followed for sample collection and preparation (Fasulati 1971). The larvae were collected together with nourishing leaves (without opening the leaf curls) from different parts of the field (~ 150 larvae). The zigzag sample collection procedure was followed and 5–10 plants were randomly selected for sample collection at each point in the field. The collected samples were kept in transparent plastic jars with thin cotton cloth used as cover and brought to the laboratory. In the laboratory, collected larvae were placed in transparent glass containers of different sizes, depending on the number and age (i.e., same-aged larvae were kept in same jar/container). A soft white paper was placed at the bottom of the container for easier moisture absorption and larval excrement cleaning. The containers were labeled and covered by a thin cotton cloth for allow airflow. Fresh cotton leaves were placed in the containers daily. The larval excrement and the dried leaves were cleaned every other day. After emergence, adult moths were transferred to another container for mating and oviposition. The pest was reared at 27 ± 2 °C and 65 ± 5% RH conditions. During this process, photographs were taken, and the biological characters of the pest were recorded. Simultaneously, observations were conducted until the end of plant vegetation in the cotton fields.
Biopesticide
BitoxybacillinTM, a commercially available preparation of Btt crystals (titer, 106 spores/ml) was used in a larvicidal efficacy study. Bacterial spores, proteinaceous crystals (delta endotoxin), and thermostable b-exotoxin of Btt culture are active basis of Bitoxybacillin. Excipients ensure safety, wettability, spread ability, and stability of the insecticide (Anonymous 2020). BitoxybacillinTM is s pale-beige to brown powder, with 1500 UA/mg biological activity and 0.6–0.8% exotoxin content.
Efficacy of Btt against Syllepte derogate larvae
Laboratory studies
Three different concentration of Btt (0.3, 0.4, 0.5 g/l) were tested against 2nd and 4th larval instars of S. derogata. The experiment had 3 replications for each instar and concentration. Different larval instars (~ 300 for each instar) were collected from cotton fields and brought to the laboratory. Selected 2nd and 4th larval instars were placed in Petri dishes (10 per dish), and each dish was considered as a replication. The Btt concentrations were applied with the help of hand sprayer. The sprayed larvae were fed on cotton leaves until termination of experiment. The larvae were counted on the 3rd and 7th day after spray, and mortality was calculated, using Abbott’s formula (Abbott 1925). Only distilled water was sprayed in control treatment and larvae were fed on untreated cotton leaves. Filter paper was placed at the bottom of the Petri dishes to regulate humidity.
Field studies
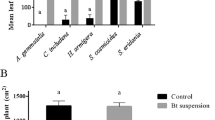
Under field condition, 0.5 g/l concentration of Btt was tested against naturally occurring larvae in the field by 3 replications, each had 100 cotton plants. Twenty different points were selected from 1 hectare and 5 plants were randomly selected at each point. The larvae present on the selected plants were counted before spraying. The cotton plants in control treatment were sprayed by distilled water only. The larval counts were done 3rd and 7th days after spray and mortality rate was calculated and corrected, using the Henderson and Tilton (1955) formula.
Statistical analysis
All data relating to larvicidal efficacy of Btt in laboratory and field conditions were subjected to the Abbott’s correction. In this way, percent mortality was corrected using Abbott’s formula (Abbott 1925; Eq. 1).
NT = Insect population after treatment on 3rd and 7th days
NC = Insect population in control on 3rd and 7th days
Mortality data was tested by Shapiro-Wilk normality test, which indicated a non-normal distribution. The data were converted by the Arcsin transformation for statistical analysis. The converted dataset was analyzed using Fisher’s analysis of variance (ANOVA) technique (Steel et al. 1997). The data variance was visually inspected by plotting the residuals to confirm homogeneity of variance before statistical analysis. One-way ANOVA was used to infer the differences among the results of concentrations of Btt. Tukey’s honestly significant difference (HSD) test at 99% probability was used as post hoc test to separate the means of treatments, where ANOVA indicated significance, at P = 0.01. All statistical analyses were performed on JMP statistical software (SAS Institute, 8th version). Comparison of the results of the 3rd and 7th days for each Btt concentration and the control treatment was done by independent t test.
Results and discussion
Biological characteristics of Syllepte derogata
This study reports the first record of S. derogata on cotton crop in Azerbaijan. Under laboratory conditions, the pest laid eggs on the lowest surface of the cotton leaves. Female laid 200–300 eggs singly on the under surface of cotton leaves (Atwal and Dhaliwal, 2013). The eggs are minute, flat oval or scale-like and pale white in color, turn to brown at the time of hatching (Shakya and Saxena 2016). Adult moths lived for about a week depending on the ecological conditions. They are yellowish-white with black and brown spots on the caput and thorax and wings with series of dark brown wavy lines. The adults direct their antennas to back in a calm position. Body length is 12.5 mm and wing span is 25 mm. Head and thorax region have dotted black marking (Sontakke et al. 2015) (Fig. 1a).
The 1st instar larvae hatched from the eggs and fed the lowest epidermis of leaves. Larvae of the pest are green in early stages and their head is dark brown in color. The body is covered by small hairs (Fig. 1b). However, the caterpillar turned dark pink before pupating. The length of mature larvae is about 1.5 cm (Fig. 1c). Larvae pupated within 15–20 days. In parallel with the present study, Dhawal et al. (1979) reported that S. derogata larval period lasted 19.25 days under laboratory conditions in Pakistan. Pupation period, lasted for 8–10 days, occurred either inside of the rolled leaves of plant (Fig. 1d), or under the plant debris on the soil. The total life cycle of a generation was completed within 31–42 days in cotton field in Absheron. Similarly, a study conducted in Punjab, India, reported that the pest’s pupal period and total life cycle of a generation were 7.62 and 35.47 days, respectively (Dhawal et al. 1979), whereas Dhindsa et al. (1980) reported that the development of the immature stages averaged 32.97 days at 25 °C and 60% RH.
S. derogata hibernates in larval stage inside leaf fold on the soil. It was noted that the pest population and biological parameters were significantly influenced by cotton cultivar, abiotic factors, and anthropogenic agricultural activities. Likewise, significant differences were reported among different cotton cultivars for resistance against the pest. Similarly, a correlation was reported between hair density of cotton leaf and pest’s ability of rolling leaf (Di et al. 2008).
The surveys carried out in the Absheron region of Azerbaijan revealed that S. derogata was observed only on cotton plant, although many studies have reported that the pest is a polyphagous and damages many hosts (Rajesh et al. 2013 and Mariselvi & Manimegalai 2016). The pest folds up the leaves of host plants like tube by web meshing like thread shape and uses it either as shelter or food. Therefore, S. derogata is also named as cotton leaf folder. This type of nutrition protects the pest from the effects of external factors, natural enemies, and contact insecticides. Since mouth apparatus is underdeveloped, young larvae grow on only epithelium of leaves (Fig. 2).
The oldest larvae open holes in different sizes by eating both parenchyma and epithelium of leaves and reduce photosynthesis areas. It should be noted that the larvae preferred soft fresh leaves for feeding and did not feed on other organs. The severely damaged leaves turn yellow and dry. The harmful feeding activity of larvae reduces photosynthesis area and reduces quantity and quality of minerals and water uptake. If the abovementioned process continues, the plant dries completely. Mathew (1980) reported that some of the 1-year-old Balsa trees (Ochroma pyramidale Cav.) were totally defoliated, while others showed varying degrees of leaf damage in India. The infestation of other pests results in secondary bacterial, viral, and fungal diseases. It is interesting that these symptoms did not observe on cotton plants infected by S. derogata. The pest was more commonly observed on the field edges.
Effects of Btt against Syllepte derogate larvae
No work has been done to control S. derogata under climatic conditions of Azerbaijan; therefore, this is the first study performed on the management of the pest. The data obtained from laboratory studies and calculated by Abbott’s formula is given in Table 1.
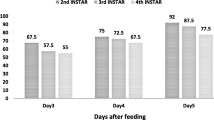
The highest mortality rate of both larval instars was noted with 0.5 g/l Btt concentration. Larvicidal efficacy for 0.5 g/l concentration on 2nd instar larvae was 93.33% on the 3rd day and 100% on 7th day, while it was 30 and 36.66% for 4th instar larvae on 3rd and 7th days, respectively. The lowest mortality rate of both larval instars was observed in control treatment (Table 1). Different Btt concentrations applied to both instars significantly differed for larvicidal efficacy (P < 0.01). The highest larvicidal efficacy was noted with 0.5 g/l concentration on both larval instars (Fig. 3).
These experiments revealed that young instars were more susceptible to Btt than older ones. These results agree with previous study indicating that Btt is more effective against young larvae (Tsatsia & Jackson 2017) (Fig. 4). Sufyan et al. (2019) reported that larvicidal efficacy of Bt on 2nd and 4th larval instars of maize stem borer was 44.20 and 37.46%, respectively. Similarly, Cantwell and Cantelo (2017) reported that the effectiveness of the 1:1500 dilution Btt had tremendous mortality rate (99.9 and 98.6%) against larvae and adults of Colorado Potato Beetle [Leptinotarsa decemlineata (Say, 1824) (Coleoptera: Chrysomelidae)] compared to untreated plots.
The data obtained from the field studies corrected by Henderson, and Tilton, (1955) formula is given in Table 2.
The larvicidal efficacy 0.5 g/l concentration of Btt was 57.41 and 72.88% on 3rd and 7th days, respectively. The Btt had lower efficacy in the open field environments than laboratory conditions. However, Btt can reduce the number of pests to the extent of economic threshold if applied before the leaves roll, given the fact that the pest lives in a secret life inside leaf curl. Likewise, Badiyala (2011) reported that Btt sprays were effective against S. derogata. Researchers also recommended that Btt can be combined with endosulfan (at half of recommended dosages), which will help in reducing the pesticide pressure on the environment.
Conclusion
This study is the first report of S. derogata on cotton crop in Azerbaijan. The total life cycle of a generation was completed within 31–42 days on cotton. Activity of Btt against S. derogata larvae was confirmed and recommended as a potential safe tool for use in integrated management.
Availability of data and materials
All data are available at the end of the article and the materials used in this work are of high quality and grade.
Abbreviations
- Btt :
-
Bacillus thuringiensis var. thuringiensis
- L.:
-
Linnaeus
- i.e.:
-
id est (that is)
- RH:
-
Relative humidity
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol. 18:265–267
Anonymous, 2019. Biological library: cotton leaf-roller Syllepte derogata (Fabricius, 1775).
Atwal, A. & Dhaliwal, G. S. (2013). Agricultural pests of South Asia and their management. In: Pest of Fibre Crops, Kalyani Publishers, New Delhi, pp. 319
Badiyala, A. (2011) Seasonal incidence and management of cotton leafroller Sylepta derogata (Fabricius) infesting okra in Himachal Pradesh, India. SAARC Journal of Agriculture. DOI: https://doi.org/10.20546/ijcmas.2018.710.003
Bravo A, Gill SS, Soberon M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49(4):423–435
Cantwell, G. E., & Cantelo, W. W. (2017) Control of the Colorado potato beetle (Coleoptera: Chrysomelidae) on tomatoes with Bacillus thuringiensis var. thuringiensis. The Great Lakes Entomologist, 17(3), 6.
Dhindsa MS, Dhindsa MK, Sekhon BS (1980) Studies on the population growth of cotton leaf roller, Sylepta derogata Fabr. (Pyralididae: Lepidoptera). Sci Culture 46(6):236–238
Di JC, Xu NY, Chen XS, Wu QJ, Xiao SH, Liu JG, Yin JM (2008) Study on the resistance of different type of upland cotton varieties to cotton leaf roller (Sylepta derogata) [J]. Cotton Sci 5
Fasulati KK (1971) Field study of insects of invertebrates. Moscow, Higher school
Ferré J, Van Rie J (2002) Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Ann Rev Entomol 47(1):501–533
Heckel DG (2020) How do toxins from Bacillus thuringiensis kill insects? An evolutionary perspective. Archives of Insect Biochemistry and Physiology 104(2):e21673
Henderson CF, Tilton EW (1955) Tests with acaricides against the brow wheat mite. J Econ Entomol. 48:157–161
Knowles BH, Ellar DJ (1987) Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis δ-endotoxins with different insect specificity. Biochimica et Biophysica Acta (BBA)-General Subjects 924(3):509–518
Lambert B, Höfte H, Annys K, Jansens S, Soetaert P, Peferoen M (1992) Novel Bacillus thuringiensis insecticidal crystal protein with a silent activity against coleopteran larvae. Appl. Environ. Microbiol. 58(8):2536–2542
Mariselvi, S. & Manimegalai, K. (2016) Biochemical studies of cotton pest Sylepta derogata by Econeem, Acorus calamus and Piper longum extracts. Int J Sci Res Publications, 6(1): 388-393.
Mathew G (1980) Occurrence of Sylepta derogata Fb. (Lepidoptera, Pyraustidae) as a pest of balsa (Ochroma pyramidale) in Kerala. Entomon 5(1):71–72
Pierre, J.S., Alain, R., Samuel, V., Gustave, B., Moïse, O.A., et al. (2013) Threshold-based interventions for cotton pest control in West Africa: what’s up 10 years later? Crop protection, v. 43, 157-165.
Rajesh K, Vishal M, Neeraj K, Ramamurthy V (2013) Taxonomic aid to major crambid vegetable pests from North India (Lepidoptera: Crambidae). Munis Entomol Zool 8(2):858–875 https://www.munisentzool.org/yayin/vol8/issue2/vol8issue2-8595850.pdf
Shakya S, Saxena A (2016) Studies on pests of cotton plants and their control measures in Vindhya Region. Int J Adv Sci Res 1(7):06–09
Siegel JP (2001) The mammalian safety of Bacillus thuringiensis-based insecticides. J Invertebrate Pathol 77(1):13–21
Sontakke PP, Radhika NS, Pattapu S (2015) Biological attributes of cotton leaf roller on okra. Ann Plant Soil Res 17(2):219–220
Steel, R. G. D., Torrie, J. H. & Dickey, D. A. (1997) Principles and procedures of statistics: a biological approach. McGraw-Hill, 666 pp.
Sufyan M, Abbasi A, Wakil W, Gogi MD, Arshad M, Nawaz A, Shabbir Z (2019) Efficacy of Beauveria bassiana and Bacillus thuringiensis against maize stem borer Chilo Partellus (Swinhoe) (Lepidoptera: Pyralidae). Gesunde Pflanzen 71(3):197–204
Tsatsia, H. & Jackson, G. (2017). Pacific Pests and Pathogens - Fact Sheets. http://www.pestnet.org/fact_sheets/bele_abelmoschus_leaf_roller_087.htm
Van Frankenhuyzen K (2009) Insecticidal activity of Bacillus thuringiensis crystal proteins. J Invertebrate Pathol 101(1):1–16
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
GG and ZM designed the study and carried out the experiments. MM analyzed the data. GG, MM, and ZM wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gahramanova, G., Mamay, M. & Mammadov, Z. Biological characteristics and efficacy of Bacillus thuringiensis var. thuringiensis against the cotton leaf roller, Syllepte derogata (Fabricius, 1775) (Lepidoptera: Crambidae). Egypt J Biol Pest Control 30, 85 (2020). https://doi.org/10.1186/s41938-020-00289-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00289-y