Abstract
Background
The skull shape and morphometry have been used by several researchers to differentiate and classify species, breeds and also to age the specimen. This study highlights details of the gross morphometry of the skulls of two species of squirrels, Heliosciurus gambianus and Funisciurus anerythrus, using both sexes.
Results
A total of thirty-one (31) linear morphometric parameters were measured on each skull specimen, relating to individual bones. Results were presented as mean ± standard deviation with significant difference at (P < 0.05). Linear measurements were similar in both genders, although some were negligibly higher in females (51.61% in H. gambianus, 70.96% in F anerythrus); no statistically significant difference was observed (P > 0.05). In spite of the similarity in shape, size and linear morphometric values obtained in both species, Pearson’s correlation analysis of the skull height with other measured parameters gave widely differing results. Correlation data obtained may be used to understand better the pattern of the skull development in these squirrels, as well as how it differs from those obtained in other mammals. Examination of the dentition revealed a varied dental formula across the two species, relating to the presence or absence of the upper and lower premolars.
Conclusions
The results obtained in this study may find application in the fields of comparative anatomy and forensics as well as provide basis for future research in ageing, skull development and feeding patterns in these species.
Similar content being viewed by others
Background
Squirrels are warm-blooded rodents found indigenously in Africa, the Americas, Eurasia; they were introduced by humans to Australia (Boris et al., 2016). The squirrel family includes tree squirrels, ground squirrels, chipmunks, marmots (including woodchucks), flying squirrels, and prairie dogs, among other rodents. They make up a diverse group consisting of approximately 279 species, 51 genera and five subfamilies: Ratufinae, Sciurillinae, Sciurinae, Xerinae and Callosciurinae (Lurz, 2011; Thorington & Hoffmann, 2005).
Squirrels are generally small animals ranging in size from the African Pygmy squirrel at 7–10 cm in length and just 10 g in weight, to the Laotian giant flying squirrel at 1.08 m in length and the Alpine marmot which weighs from 5 to 8 kg (Thorington & Ferrell, 2006). They can survive in a wide variety of habitats, from tropical rainforest to the semi-arid desert, avoiding only the high polar regions and very dry deserts. They are herbivores, feeding predominantly on seeds and nuts but some feed on insects and even smaller invertebrates (Brown et al., 2014; Lurz, 2011). Squirrels have an excellent sense of vision, which is particularly important for their tree living lifestyle. They have an average lifespan of 5–10 years in the wild and up to 20 years in captivity (Essner, 2007; Thorington et al., 2012).
Skull size and shape have been widely used in medical research and also to study domestic animal populations and breeds (Heck et al., 2018, 2019). The skulls of most squirrels are short, with a short rostrum and an arched profile. The skull has a broad, tilted zygomatic plate that serves as the attachment point for the lateral branch of the masseter muscle. The superficial branch of masseter muscle originates on a prominent bump of bone on the side of the rostrum, called the masseteric tubercle (Hautier et al., 2010). They have small infraorbital foramen that is not enlarged to transmit muscle as it is in myomorphous (mice and rats) and hystricomorphous (cavies and guinea pigs) rodents (Cox & Jeffery, 2011).
These rodents have long jugals, well-developed postorbital processes and large bullae that are not inflated. The anterior ends of the jugals contact the frontals, and the palate is broad and relatively short, ending at the same level as the molar row. The zygomasseteric architecture of skulls is sciuromorphous (Cox et al., 2012; Hautier et al., 2010).
The Gambian sun squirrel (Heliosciurus gambianus) is a species of rodent in the family Sciuridae. It inhabits wooded savannah and other grassland with scattered trees, moving through the branches but sometimes descending to the ground (Grubb & Ekué, 2008; Kingdon, 2015). Thomas’s rope squirrel (Funisciurus anerythrus) is also a species of rodent in the family Sciuridae, weighs between 200 and 220 g, has a head and body length ranging between 16 and 23 cm and a tail length between 13 and 20 cm (Kingdon, 2015; Thorington & Hoffmann, 2005).
Morphometry is the quantitative analysis of size, shape and has always been vital to taxonomic classification (Kraatz & Sherratt, 2016; Marques et al., 2021). Its diversification could be interpreted as the consequence of the contrasting impacts of developmental constraint and adaptation to ecological factors (Meloro, 2011). Commonly, the field of morphometrics is divided into traditional or multivariate morphometrics and geometric morphometrics. The former term refers to the application of multivariate statistics to linear measurements and ratios, whereas the latter concerns the development of coordinate-based methods (Webster & Sheet, 2010).
Traditional linear measurement analysis is fundamental for the quantitative comparison of morphological variation. There are few reports on information using linear measurement to determine the skull size and shape of these squirrels. This study therefore aims to compare the gross morphometry of the skulls of Heliosciurus gambianus and Funisciurus anerythrus.
Methods
Aim
The study aim was to conduct a macroanatomical investigation of the skulls of two species of squirrels—Heliosciurus gambianus and Funisciurus anerythrus, by analysing differences and similarities in the individual bones and dentition.
Study design and setting
Twenty-nine (29) squirrels of two different species, 9 of Heliosciurus gambianus squirrel (6 males, 3 females) and 20 of Funisciurus anerythrus (8 males, 12 females), were used for this study. The squirrels were estimated to be adults, based on weight, using previously documented references (Kingdon et al., 2015). Ethical approval was obtained from the Animal Care and Use Research Ethics Committee of the University (with ethical code number NHREC/UIACUREC/05/12/2022A). The squirrel heads were mostly sourced from different research units using squirrels, to make up the number. They were trapped in the wild, using cages with food as bait, from the University of Ibadan environment. All the squirrels were handled humanely, transported in cages and identified by an animal taxonomist using the field guides outlined in previous reports (Happold, 1987; Kingdon, 2015). They were euthanised with an intramuscular injection of xylazine 6 mg/kg and ketamine HCl at 150 mg/kg, adapted from previous reports (Igado et al., 2021). The animals were eviscerated and skinned, and all muscles were removed as much as possible. Animals were tagged individually for easy identification. All animals were humanely handled according to The Guide for Care and Use of Laboratory Animals, NIH, USA (2011).
Hot water maceration The heads were decapitated at the atlanto-occipital junction and macerated individually in labelled plastic containers. The skulls were macerated using the hot water maceration method as earlier described (Igado & Ekeolu, 2014).
Morphometry Linear skull measurements were determined with the aid of a digital vernier caliper, centimetre rule, mathematical dividers and compass to the nearest 0.01 mm (Igado, 2014; Igado & Ekeolu, 2014). A total of thirty-one (31) parameters were determined on each skull. Landmarks for all parameters are highlighted in Figs. 1, 2 and 3. The parameters measured were whole skull height (WSH); whole skull length (WSL); skull height without the mandible (SWHM 1); maximum width of the skull (MWS); total length of the frontal bone (FBL); overall length of the nasal bone (NBL); length of parietal bone (FNE); length of the mandibular bone (MDL); mandibular symphysial length (MSL); length of palate (PAL): orbital height/vertical diameter (OVD); orbital width/horizontal diameter (OHD); height of the foramen magnum (FMH); width of the foramen magnum (FMW); occipital height (OCH); occipital height without foramen magnum (OCHW); maximum width of the occipital condyles (OCW); distance between the most medial points of the most rostral left and right mental foramen (RMF); length along a horizontal line, from the ventral limit of the mandibular foramen to the caudal border of the mandible (MFCB); length along a vertical line, from the ventral limit of the mental foramen to the ventral border of the mandibular foramen (MFMF); length along a vertical line, from the ventral limit of the mandibular foramen to the base of the mandible (MFMB); length along a vertical line, from the ventral limit of the mandibular foramen to the most dorsal aspect of the coronoid process (MFCP); length of the lower jaw from the most rostral point of the dental bone to the most caudal projection of the coronoid process (MDL-1); length of the lower jaw from the most rostral point of the dental bone to the most caudal projection of the mandibular condyle (condylar process) (MDL-2); length of the lower jaw from the most rostral point of the dental bone to the most caudal projection of the angular process (MDL-3); height of the mandibular body between the mid-point of premolar 1 and 2 and the mandibular base (HMP); thickness of mandible at molar 1 (TM-1); length of the mandible between the cranial and caudal angles (RAM); distance from the rostral tip of the nasal bone to the rostral tip of the incisive bone (NIL); height of the mandibular symphysis (HSPh); length of the mandibular symphysis (LSPh).
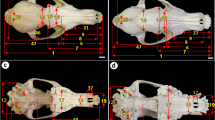
Skull of the male Gambian sun squirrel (Heliosciurus gambianus). Dorsal view a of the male showing the length of the parietal—FNE, the total length of the frontal bone—FBL, the overall length of the nasal bone—NBL, and the maximum width of the skull, MWS, from zygoma to zygoma. Panel ‘b’ is the ventral view, showing the length of the palate—PAL, black arrowhead indicates 1st premolar, and asterisk (*) indicates the bulla. Panels ‘c’ and ‘d’ are the lateral views with and without the mandible, respectively. OVD—vertical diameter of the orbit, OHD—horizontal diameter of the orbit, WSH—whole skull height, NIC—distance between nasal and incisive bones (accommodating the conchae), WSL—maximum skull length, white arrowhead indicates the auditory meatus. Scale bars—0.5 cm
Right lateral (a and b), latero-medial (c) and dorsal (d) views of the mandible of the male Gambian sun squirrel (Heliosciurus gambianus). MDL-1, 2 and 3 depict distance from the mandibular foramen (black circle) to the coronoid process, condylar process and angle of mandible, respectively; black arrow (in ‘a’)—mental foramen; white arrowhead—coronoid process; black arrowhead—condylar process; black circle depicts the position of the mandibular foramen, which is visible on the medial surface of the mandible (c); TM-1 is the thickness of the mandible at molar 1, while LSPH is the length of the mandibular symphysis. Scale bars—0.5 cm
The foramen magnum index (FMI) was calculated as (FMH/FMW) × 100 and expressed in percentage.
All pictures were obtained with a Sony® Cyber-shot digital still camera (DSC-HX400/HX400V).
Statistical analysis
Linear morphometric values obtained were presented as mean ± standard deviation. Data obtained for sexes and between species were subjected to Student's t test, while relationship between parameters was determined by Pearson’s correlation analysis. GraphPad Prism (v. 6) software package was used for this analysis, and values of P < 0.05 were considered statistically significant.
Results
General appearance
The skulls of the two species were identical in shape, differing only in size, with H. gambianus being bigger than F. anerythrus. The skulls had the typical rodent appearance with very prominent and protruding upper and lower incisors. The zygoma was wide and prominent, while the nasal, frontal and parietal bones were flattened (Figs. 1, 2). The shape of the foramen magnum in both species was consistent in all specimens examined, having the typical circular appearance, but possessing a very slight notch at the dorsal aspect (Fig. 3). No sexual dimorphism was observed in any of the features.
Skull morphometry
The values obtained from morphometry for both species are presented in Table 1 as means ± standard deviation. Values obtained were similar in both genders; therefore, no statistically significant differences were observed. Although the females had more values which were negligibly higher, this could not be regarded as of any importance (Table 1).
Correlating WSH with other parameters using Pearson’s correlation (r) showed that for the H. gambianus skulls, WSH had significant positive correlation with OVD, FMW, RMF, HMP and TM-1 (0.74, 0.76, 0.67, 0.62 and 0.60, respectively) and significant negative correlation with OCHW ( − 0.77) (Table 2).
In F. anerythrus, there was a significant positive correlation with RMF and MFCB (0.62 and 0.60, respectively). No negative correlation was recorded (Table 2).
Dentition
All squirrel teeth examined had generalized attrition, which were more pronounced on the occlusal surfaces of all the teeth. There was the presence of prominent orange-fringed stain on the labial surfaces, towards the cervical region, of all examined incisor teeth. Bone resorption was also observed in all examined skulls, especially on the upper molars (palatal surface).
Carious lesions were found on majority of the molars occlusally. There were no observable tooth fractures or dental calculus (calcified dental plaque, composed mainly of calcium phosphate).
The dental formula varied greatly: I1/0–1 C0/0 PM0−1/0–1 M4/4 and was distributed thus:
I1/1 C0/0 PM0/0 M4/4—6 H. gambianus (3 males, 3 females) and 5 F. anerythrus (2 males, 3 females).
I1/1 C0/0 PM1/0 M4/4—2 H. gambianus (2 males, 0 females) and 9 F. anerythrus (4 males, 5 females).
I1/0 C0/0 PM1/1 M4/4—5 F. anerythrus (2 males, 3 females). No H. gambianus had this dental formula.
Discussion
Morphometry is an important tool in comparing morphological variations between population and species of animals, studying evolutionary changes, including impact of mutation on shape, size and developmental changes (Coker et al., 2020; Kraatz & Sherratt, 2016). Morphological changes and phylogenetic divergence can be tested using Sciurid morphology as a model, because the characteristics of their skeleton are considered to be both conservative and inclined to convergence (Cardini, 2003).
Generally, rodents have highly specialized masticatory musculature, the morphology being classified as the sciuromorph (squirrel-like), hystricomorph (porcupine-like) and myomorph (mouse-like). These descriptions of their skulls and masseter are useful in phylogenetic relationship studies (Hautier et al., 2010). Therefore, in this study we refer to the skull parameter as being sciuromorph.
The growth of the skull and its components in rodents is influenced by sex, breed or strain and their nutritional status. Differences in the cranial parameters of squirrels could be associated with sex, climate change and feeding habit (Bamidele & Akinpelu, 2019). Sexual dimorphism is a common phenomenon in animals, including the squirrel, that has been demonstrated among species, and it is ascribed to different selection pressure which could be natural (Lukas & Clutton-Brock, 2013). In this study, the female squirrels had higher measurements of skull parameters relative to the males. However, there were similarities in some cranial parameters in both sexes. The higher skull parameters in the females in this study could be attributed to the need for fecundity and nurturing of offspring. This result of higher cranial morphometric values in females is similar to previous reports in some other mammals, e.g. the Nigerian local dog (Igado, 2017) and the goat (Olopade & Onwuka, 2008). Reports on skull work on other species of the squirrel did not highlight sexual dimorphism (Bamidele & Akinpelu, 2020a, 2020b). Also, previous reports on weight differences show that the female H. gambianus weighed more and were bigger than the male species (Coker et al., 2020). This heavier weight may probably account for the higher cranial values (although not statistically significant), but the reverse was the case in the dog, where the males, although having heavier body weights, had most cranial parameters having lower values (Igado, 2011, 2017).
The skull height naturally increases as the animal advances in age and size. The significant negative correlation (− 0.77) observed in the H. gambianus, between the skull height (WSH) and the height of the occiput (OCHW), may probably indicate a relatively smaller neurocranium as the animal progresses in age. Also, the parameters showing a strong positive correlation (OVD, FWM, RMF, HMP and TM-1) with the skull height may indicate a slight change in shape as the animal advances in age. Age-related studies may help shed light on these theories.
The foramen magnum showed a slight variation from what was reported in the Cricetomys gambianus—the African giant rat (Olude et al., 2009) where the width of the foramen magnum was higher than the height, giving an oval shape, and the foramen magnum index ranging from 80 to 82%. In the current study, FMH was closer in values to FMW, resulting in a more circular shape of the foramen magnum, and FMI ranging from 89 to 94.4%. In the two rodents (Cricetomys gambianus and the 2 squirrel species) studied, the presence of a dorsal notch on the foramen magnum was constant.
In spite of the keen similarity in shape and size of the two species examined (Fig. 4), the correlation data were different, pointing at a possible difference or variation in their skull shape development patterns including ageing and feeding patterns.
The different dental pathologies observed on this species might be due to the different diets (Koyabu et al., 2009) over the course of time. We noticed pronounced generalized attrition on the occlusal surfaces of the teeth; this is quite strange because attrition is commoner on posterior teeth rather than anterior teeth. The abrasive nature of the diet taken might account for the occlusal attrition. Bone resorption is a complication from many dental maladies such as caries, periodontal diseases and dento-alveolar abscesses. Infections can spread from the primary site into the jaw bones ultimately leading to bone pathologies. Dental caries is not common in rodents because of the inhibitive combination of oral pH absence of cariogenic microflora and low sugar diet (Sainsbury et al., 2004). Though caries has been documented to be less common among free range species, we observed occlusal caries in majority of the animals. We opined that the squirrels might have been feeding on food remnants from garbage around the university staff quarters. Lack of observable tooth fractures or dental calculus might also be ascribed to the soft diet and its composition. There were variations in the dental formula of the different species of the squirrels. All the species observed in this study had 4 sets of molars, but the number of incisor and premolar varied with none having canine. Geographic variations, climatic gradients and sexual dimorphism have been documented to account for the variations observed in the dental formula of squirrel species (Watts, 1993). However, we are surprised by the variations observed even within the species used for this study. Human and animal species have developed various adaptive and evolutionary changes in their dentition (Sachdev et al., 2020). The total number of teeth in the species was either 20 or 22 which is in agreement with literatures that also studied the dentition of squirrels but in different species (Miles & Grigson, 1990; Mitchell & Carsen, 1967; Sachdev et al., 2020; Sainsbury et al., 2004).
Conclusions
In cases of surgical intervention or application of local anaesthesia, some of the landmarks highlighted in this study are easily accessible in a restrained animal, making them ideal parameters to assess the position or extent of some nerves. Moreover, data from this study may also be useful in the fields of comparative anatomy, archaeology and forensic odontology.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- WSH:
-
Whole skull height
- WSL:
-
Whole skull length
- SWHM 1:
-
Skull height without the mandible
- MWS:
-
Maximum width of the skull
- FBL:
-
Total length of the frontal bone
- NBL:
-
Overall length of the nasal bone
- FNE:
-
Length of parietal bone
- MDL:
-
Length of the mandibular bone
- MSL:
-
Mandibular symphysial length
- PAL:
-
Length of palate
- OVD:
-
Orbital height/vertical diameter
- OHD:
-
Orbital width/horizontal diameter
- FMH:
-
Height of the foramen magnum
- FMW:
-
Width of the foramen magnum
- OCH:
-
Occipital height
- OCHW:
-
Occipital height without foramen magnum
- OCW:
-
Maximum width of the occipital condyles
- RMF:
-
Distance between the most medial points of the most rostral left and right mental foramen
- MFCB:
-
Length along a horizontal line, from the ventral limit of the mandibular foramen to the caudal border of the mandible
- MFMF:
-
Length along a vertical line, from the ventral limit of the mental foramen to the ventral border of the mandibular foramen
- MFMB:
-
Length along a vertical line, from the ventral limit of the mandibular foramen to the base of the mandible
- MFCP:
-
Length along a vertical line, from the ventral limit of the mandibular foramen to the most dorsal aspect of the coronoid process
- MDL-1:
-
Length of the lower jaw from the most rostral point of the dental bone to the most caudal projection of the coronoid process
- MDL-2:
-
Length of the lower jaw from the most rostral point of the dental bone to the most caudal projection of the mandibular condyle (condylar process)
- MDL-3:
-
Length of the lower jaw from the most rostral point of the dental bone to the most caudal projection of the angular process
- HMP:
-
Height of the mandibular body between the mid-point of premolar 1 and 2 and the mandibular base
- TM-1:
-
Thickness of mandible at molar 1
- RAM:
-
Length of the mandible between the cranial and caudal angles
- NIL:
-
Distance from the rostral tip of the nasal bone to the rostral tip of the incisive bone
- HSPh:
-
Height of the mandibular symphysis
- LSPh:
-
Length of the mandibular symphysis
References
Bamidele, A. O., & Akinpelu, A. I. (2019). Cranial and external morphology of male and female orange headed tree squirrels (Funisciurus leucogenys) in selected locations of savannah forest in Nigeria. Journal of Applied Life Sciences International, 21, 1–12.
Bamidele, A. O., & Akinpelu, A. I. (2020a). Comparison of cranial and body morphology of tree squirrels (Helioscurius rufobranchium) in selected locations of rainforest in Nigeria. The Zoologist, 17(July), 47–53. https://doi.org/10.4314/tzool.v17i1.8
Bamidele, A. O., & Akinpelu, A. I. (2020b). Comparison of cranial and external morphology of tree squirrels (Funiscurus leucogenys) in selected locations of rainforest in Nigeria. Journal of Scientific Research and Reports. https://doi.org/10.9734/jsrr/2019/v25i530198
Boris, K., Ahmad, M., Alexey, T., Jan, M., & Rainer, H. (2016). A review of bristly ground squirrels Xerini and a generic revision in the African genus Xerus. Mammalia, 80(5), 521–540.
Brown, E., Peri, A., & Santarosa, N. (2014). Sciuridae. Animal Diversity Web. https://animaldiversity.org/accounts/Sciuridae/
Cardini, A. (2003). The geometry of the marmot (Rodentia: Sciuridae) mandible: Phylogeny and patterns of morphological evolution. Systematic Biology, 52(2), 186–205.
Coker, O., Jubril, A. J., Isong, O. M., & Omonona, A. O. (2020). Genetic variations in Thomas’s rope squirrel (Funisciurus anerythrus) and Gambian sun squirrel (Heliosciurus gambianus) Ibadan, Nigeria using allozyme markers. Animal Research International, 17(2), 3747–3760.
Cox, P. G., & Jeffery, N. (2011). Reviewing the morphology of the jaw-closing musculature in squirrels, rats, and guinea pigs with contrast-enhanced microCT. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology, 294(6), 915–928.
Cox, P. G., Rayfield, E. J., Fagan, M. J., Herrel, A., Pataky, T. C., & Jeffery, N. (2012). Functional evolution of the feeding system in rodents. PLoS ONE, 7(4), e36299.
Essner, R. L. (2007). Morphology, locomotor behaviour and microhabitat use in North American squirrels. J Zool Lond., 272, 101–109.
Grubb, P., & Ekué, M. R. M. (2008). Heliosciurus gambianus. In IUCN Red List of Threatened Species.
Happold, D. C. D. (1987). The Mammals of Nigeria. Clarendon Press.
Hautier, L., Weisbecker, V., Sánchez-Villagra, M. R., Goswami, A., & Asher, R. J. (2010). Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proceedings of the National Academy of Sciences, 107(44), 18903–18908.
Heck, L., Sanchez-Villagra, M. R., & Stange, M. (2019). Why the long face? Comparative shape analysis of miniature, pony, and other horse skulls reveals changes in ontogenetic growth. Peer J, 7, e7678.
Heck, L., Wilson, L. A., Evin, A., Stange, M., & Sánchez-Villagra, M. R. (2018). Shape variation and modularity of skull and teeth in domesticated horses and wild equids. Frontiers in Zoology, 15(1), 1–17.
Igado, O. O. (2011). Neurometry and Neurocraniometry of the Nigerian local dog (Canis lupus familiaris). Journal of Veterinary Anatomy, 4(2), 99–109.
Igado, O. O. (2014). Rostrofacial indices of the nigerian local dog: Implications in veterinary oral and maxillo-facial anaesthesiology of the dolichocephalic canine breed. International Journal of Morphology, 32(2), 738–743. https://doi.org/10.4067/S0717-95022014000200059
Igado, O. O. (2017). Skull typology and morphometrics of the Nigerian local dog (Canis lupus familiaris). Nigerian Journal of Physiological Sciences, 32, 153–158.
Igado, O. O., Braimah, S. F., & Obasa, A. A. (2021). Gross morphology of the brain and spinal cord of the African pygmy hedgehog (Atelerix albiventris). Folia Veterinaria, 65(3), 15–21. https://doi.org/10.2478/fv-2021-0023
Igado, O. O., & Ekeolu, O. K. (2014). Morphometric investigation of the occipital area of the adult Nigerian Local Dogs. African Journal of Biomedical Research, 17, 125–128.
Kingdon, J. (2015). The Kingdon field guide to African mammals (second). Bloomsbury Publishing.
Koyabu, D. B., Oshida, T., Dang, N. X., Can, D. N., Kimura, J., Sasaki, M., Motokawa, M., Son, N. T., Hayashida, A., Shintaku, Y., & Endo, H. (2009). Craniodental mechanics and the feeding ecology of two sympatric callosciurine squirrels in Vietnam. Journal of Zoology, 279(4), 372–380.
Kraatz, B., & Sherratt, E. (2016). Evolutionary morphology of the rabbit skull. PeerJ, 4, e2453.
Lukas, D., & Clutton-Brock, T. H. (2013). The evolution of social monogamy in mammals. Science, 341(6145), 526–530.
Lurz, P. (2011). Squirrels and relatives III: Tree squirrels. In Grzimek’s Animal Life.
Marques, P., Zandonà, E., Mazzoni, R., & El-Sabaawi, R. (2021). Individual variation in feeding morphology, not diet, can facilitate the success of generalist species in urban ecosystems. Ecology and Evolution, 11(24), 18342–18356.
Meloro, C. (2011). Feeding habits of Plio-Pleistocene large carnivores as revealed by the mandibular geometry. Journal of Vertebrate Paleontology, 31(2), 428–446.
Miles, A. E. W., & Grigson, C. (1990). Colyer’s variation and diseases of the teeth of animal. Cambridge University Press.
Mitchell, O. G., & Carsen, R. A. (1967). Tooth eruption in the arctic ground squirrel. Journal of Mammalogy, 48(3), 472–474. https://doi.org/10.2307/1377784
Olopade, J. O., & Onwuka, S. K. (2008). A craniometric analysis of the skull of the red sokoto (Maradi) goat (Capra Hircus). European Journal of Anatomy, 12(1), 57–62.
Olude, M. A., Olopade, J. O., Fatola, I. O., & Onwuka, S. K. (2009). Some aspects of the neurocraniometry of the African giant rat (Cricetomys gambianus Waterhouse). Folia Morphologica, 68(4), 224–227.
Sachdev, S. S., D’Souza, Z. I., Chettiankandy, T. J., Sardar, M. A., Pakhmode, V., & D’Souza, T. (2020). Characteristic features and terminologies of mammalian dentition—A conspectus. Int J Forensic Odontol, 5, 23–29.
Sainsbury, A. W., Kountouri, A., DuBoulay, G., & Kertesz, P. (2004). Oral disease in free-living red squirrels (Sciurus vulgaris) in the United Kingdom. Journal of Wildlife Diseases, 40(2), 185–196.
Thorington, R. W., & Ferrell, K. (2006). Squirrels—The animal answer guide. Johns Hopkins University Press.
Thorington, R. W., & Hoffmann, R. S. (2005). Family sciuridae. In D. E. Wilson & D. Reeder (Eds.), Mammal species of the world: A taxonomic and geographic reference (3rd ed., pp. 754–818). The Johns Hopkins University Press.
Thorington, R. W., Koprowski, J. L. J., Steele, M. A., & Whatton, J. F. (2012). Squirrels of the world. Baltimore: Johns Hopkins University.
Watts, K. M. (1993). Comparison of the geographic variation in two species of ground squirrels (Spermophilus). http://athenaeum.uiw.edu/uiw_etds/298
Webster, M., & Sheet, H. D. (2010). A practical introduction to landmark-based geometric morhometrics. The Paleontological Society Papers, 16, 163–188.
Acknowledgements
The authors gratefully acknowledge the input of Dr. Latifah A. Adekunle and Late Dr. A. Jagun-Jubril, both of the Department of Veterinary Pathology, and Mr. A.W. Ramoni of the Department of Veterinary Anatomy, University of Ibadan, Nigeria.
Protocol registration
Before the commencement of the study, the protocol for the study was prepared and was registered with the Animal Care and Use Research Ethics Committee (ACUREC) of the University of Ibadan, Ibadan, Nigeria.
Funding
The study was conducted solely on personal funds of the authors.
Author information
Authors and Affiliations
Contributions
IOO & FOM helped in conceptualization, data analysis, manuscript draft and editing; IOO, FOM & AAO generated the data. All authors approved of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the ethical committee of the university—Animal Care and Use Research Ethics Committee (ACUREC).
Consent for publication
Not applicable. Animals were used throughout this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Igado, O.O., Femi-Akinlosotu, O.M. & Akibu, A.O. Macroanatomical investigations of the skulls of both genders of Heliosciurus gambianus (Gambian sun squirrel) and Funisciurus anerythrus (Thomas’s rope squirrel). JoBAZ 84, 22 (2023). https://doi.org/10.1186/s41936-023-00343-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-023-00343-9