Abstract
Background
Anthropogenic activities is a major factor that determines the condition and sustainability of an environment. The nature and type of activities will determine the health, ecosystem services and general well-being of the environment. These activities have increased as a result of industrialization and urbanization, notwithstanding pressure on fish species for balanced nutrition and economic returns. The paucity of documented information about the limnological parameters and finfish assemblage in Osun River nessiciated this study.
Results
Mean temperature (18.7 ± 2.69 °C) and dissolved oxygen (3.2 ± 0.54 mg/L) values were below the recommended limit for fish survival, pH (7.1 ± 0.25) and ammonia (1.2 ± 0.18 mg/L) were within and above the recommended limits. The river had a total of 4544 finfish belonging to 11 families and 19 species. Chrysichthys nigrodigitatus, sampling zone A and Cichlid family had the highest abundance in the river with 21.00%, 24.20% and 32.50% respectively.Fin fish abundance had a negative relationship between dissolved oxygen (r = − 0.032), ammonia (r = − 0.668), pH (r = − 0.819); temperature and DO with ammonia(r = − 0.668, r = − 0.590) and pH (r = − 0.043, r = − 0.011) respectively. Dissolved oxygen (b = − 137.1), ammonia (b = − 522) and pH (b = − 93.4) had a negative effect on finfish assemblage in the river. Significant correlated values (p < 0.05) were observed between fin fish and temperature (p = 0.008), ammonia (p = 0.017), pH (p = 0.001). dissolved oxygen (b = − 2.14), ammonia (b = − 2.72) and pH (b = − 1.97) had a negative association with fin fish abundance while significance (p < 0.05) were observed with dissolved oxygen (p = 0.048).
Conclusion
It is worthy to note that human activities principally dictates the quality and quantity of limnological parameters which directly affect finfish assemblage; therefore proper environmental management is essential for sustenance of finfish in the river as elaborated by the United Nations Sustainable Development Goals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
The environmental conditions of an area govern the fish abundance, biological conditions, spawning seasons, water level fluctuations, and flooding which can affect breeding activities of fish (Admassu et al., 2015). Rivers have contributed significantly to fish production with little attention to its quality and this has become increasingly important due to the clamor for a cheap source of protein which fish proffer. Fish provides up to 20% of animal protein intake in 127 developing countries (FAO, 2005), it is a cheap source of various vitamins, iron, calcium, and omega 3 fatty acids and contributes 0.48% to the agricultural Gross Domestic Product (GDP) in Nigeria (FAO, 2016). There has been an increase in demand for fish in Nigeria because it provides about 41% animal protein to the inhabitants (FDF, 2010) and the successful management of this resource will require a periodic assessment of water quality (UNEP, 2012). Nigeria is rich in diverse fish species and their presence can be affected by factors such as climatic conditions, anthropogenic activities, and habitat degradation (Badejo & Oriyomi, 2015; UNEP, 2012). These factors can affect the well being of the fish by limiting the availability of food and water quality degradation (Adedokun et al., 2013; Kareem et al., 2015).
Rapid industrialization and urbanization is one of the major activities that threaten the health of natural ecosystems and it is important to note the effects of these on the water quality as it affects the productive capacity of the river and fish assemblage. In the time past, nature’s own waste treatment systems took care of decomposition of wastes generated on land; the case is not the same today because the wastes generated are far beyond what nature can degrade. This is due to the different types of wastes especially non-biodegradable which have adverse effects on the aquatic ecosystem and the services provided by it (Makori et al., 2017). These in turn cause serious environmental problems which trickles to the aquatic systems which is mostly seen and serves as a sink for all activities on land, therefore aquatic resources are threatened (Adedokun et al., 2015; FAO, 2019; Iyiola et al., 2023). This scenario is not good for the environment and advocates are kicking against such activities; a majpr one is the United Nations 17 Sustainable Development Goals (17 SDGs). These goals were geared towards year 2030 and tagged Agenda 2030, such that the environment, humans and life in general can be maintained and ecosystem services can be in continuoum. Goals 14 and 15 which emphasizes on preservation of life under water and life on land respectively are the major focal point of this study (United Nations, 2015). Ait-Kadi (2016) reported that the success of the united Nations Goals depends on water and it is essential to develop integrated water manageemt systems. The clean use of resources and ecosystem services in ways to promote sustainability of aquatic and terrestrial life must be advocated (Giggs et al., 2013). This will go a long way in provision of food and improved nutrition for humans that depend on them as elaborated by Goal 2 (Zero humger) and Goal 3 (Good health and well being).
Osun River is a major river in the Ogun-Osun river basin located in South-western Nigeria. Due to its stretch and size, it is estimated that it will house diverse fin fish species. The status of fin fish species in various reservoirs flowing into the Osun River has been reported; Taiwo, (2010) on Owalla reservoir, Badejo and Oriyomi, (2015), Komolafe et al., (2016) on Eko Ende reservoir, Ipinmoroti et al., (2018) on Asejire reservoir, and Iyiola and Iyantan (2021) on Erinle reservoir. An assessment of the fish species in the river was reported by Iyiola and Jenyo-Oni (2018) but there is limited documented information on the influence limnological factors have on finfish assemblage in the Osun River. This study will, therefore, provide baseline information on the status of the finfish species and the effects of water quality for sustainable management of the river.
Methods
Study area
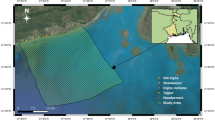
Osun River located between coordinates 7° 23′ 0ʺ–7° 35′ 0ʺ North and 4°5′ 0ʺ–4° 5′ 15ʺ East (Fig. 1) and is fed by various rivers and reservoirs. The river is encompassed with various activities such as agricultural practices, industrial, domestic, and effluent discharge. The upper course of the river is majorly surrounded by agricultural activities while the Nigerian Bottling Company plant is located at the lower end of the river at Asejire. Sampling points were identified based on the information on drainage, soil type, land usage, and geological formation of the river catchment, and seven sampling points (A–G) were purposively selected based on accessibility for the study. The sampling points were identified using an handheld Etrex 2000 Global Positioning System (GPS) manufactured by Garmin. The description and characteristics of each of the sampling points and coordinates are presented in Table 1. Sampling was done fortnightly for twelve months (November 2017–October 2018) and covered both dry and wet seasons.

Source: Iyiola and Jenyo-Oni, (2018)
Map of Osun River Catchment showing the sampling points (A–G).
Limnological parameters
Water samples were collected fortnightly from the river for twelve months between the hours of 7.30 am and 8.00 am in 50 ml sterile bottles and measured in situ for Temperature, Dissolved Oxygen (DO), pH and Ammonia. Temperature was measured using a mercury-in-glass thermometer calibrated in degrees Celcius (°C). DO was measured using Dissolved Oxygen Meter (Model: DO-5509) manufactured by LUTRON, UK. pH and Ammonia were measured in situ using the API Freshwater Master Test Kit manufactured by MARS Fishcare, USA following the protocols described for each parameter. DO and Ammonia were recorded in mg/L.
Finfish assemblage
Fish were sampled fortnightly from the sampling points using monofilament gill nets (10 m × 2 m) with mesh sizes 25 mm, 38 mm, 76 mm, 101 mm, 126 mm, and 177 mm. These nets were set for 12 h i.e. set at 7.00 pm and retrieved at 7.00 am the next day as described by Iyiola and Jenyo-Oni, (2018). A large number of the fish species were dead due to the long duration and were identified to species level using a combination of monographs by Olaosebikan and Raji (2013) and Froese and Pauly (2019). The abundance by family and species were recorded and relative abundance determined.
Statistical analysis
Tukeys mean comparism was used to separate the monthly means of the limnological parameters measured. Pearson correlation analysis was used to determine the relationship between the mean of limnological parameters and between fish abundance and limnological parameters measured across the months. Regression analysis (Eq. 1) was used to estimate the degree of association between finfish assemblage (Dependent variable) and limnological parameters (Independent variable). All statistical analyses were at p < 0.05 significance level and graphical illustrations were done using Minitab 19.0 and SPSS 23.0 statistical software.
Multiple Linear regression model:
where Y = Finfish assemblage (Dependent variable), a = intercept, b1–b4—regression coefficients, X1–X4 (Independent variables—Temperature, DO, Ammonia, pH).
Results
Limnological parameters
The monthly mean limnological parameters measured during the study is presented in Table 2. Monthly mean temperature was measured in November (22.60 ± 0.20 °C) while the least was measured in May (18.30 ± 0.18 °C) and an overall mean of 18.70 ± 2.69 °C was measured throughout the study. Mean monthly dissolved oxygen was highest in November (4.28 ± 0.05 mg/L) and the least measured in March (2.60 ± 0.16 mg/L) with an overall mean value of 3.20 ± 0.54 mg/L was measured in the study. Mean monthly ammonia levels was highest in July (0.30 ± 0.09 mg/L) were not detected in November and January. An overall mean of 1.20 ± 0.18 mg/L was measured throughout the study with mean monthly values in July, August and October were significantly different (p < 0.05) from other months of study. The highest mean pH value was measured in January (7.40 ± 0.10) and the least was in August (6.80 ± 0.06) with an overall mean value of 7.10 ± 0.25 throughout the study.
Correlation between limnological parameters
The correlation coefficient between limnological parameters across the sampling months during the study is presented in Table 3. A negative significant value (p < 0.05) was observed between mean temperature and mean ammonia values (r = − 0.668, p = 0.01) and mean temperature and mean pH values (r = − 0.59, p = 0.04). Negative relationship were observed between mean dissolved oxygen and mean ammonia values (r = − 0.04, p = 0.89) and mean dissolved oxygen and mean pH values (r = 0.01, p = 0.97). The relationship between mean ammonia and mean pH va;ues (p = 0.00) was significant (p < 0.05).
Finfish assemblage
The relative abundance by species, across the sampling zones and family of fin fish identified during the study period are presented in Tables 4, 5 and 6 respectively. A total of 4544 individuals comprising of 19 species belonging to 11 families were identified. The fin fish abundance fluctruated across the months and the highest relative abundance was in February (13.20%) while the least abundance was in July and August with 2.00% respectively. Chrysichthys nigrodigitatus was the most abundant fish species (21.00%) while the least was Clarias macromystax with 0.2%. Across the sampling zones, it was observed that fin fish abundance reduced from zone A to Zone G with the highest relative abundance in zone A (24.20%) and the least in zone G (6.20%).
Cichlids were observed to record the highest relative abundance (32.50%) and had the highest number of fish species (5) namely Oreochromis niloticus (14.10%), Sarotherondon galileaus galileaus (6.80%), Coptodon zilli (10.40%), Red Tilapia (0.60%) and C. marie (0.60%). O. niloticus was the most abundant cichlid while red tilapia and C. marie were the least. Alestidae recorded the presence of three species (29.80%), Schilbeidae and Mormyridae recorded the presence of two species with 6.80% and 2.20% respectively. Lastly, Distichontidae (1.80%), Latidae (0.80%), Claroteidae (21.00%), Mochokidae (3.40%), Kneriidae (0.30%) and Clariidae (0.20%) had just one species each identified.
Correlation coefficient between limnological parameters and fish assemblage
The correlation coefficient between limnological parameters and fin fish assemblage diting the study is presented in Table 7. A negative significant relationship (p < 0.05) was observed between fin fish assemblage with mean ammonia (r = − 0.668, p = 0.017) and pH (r = − 0.819, p = 0.001) values. Mean temperature value had a negative significant relationship (p < 0.05) with mean pH value (r = − 0.590, p = 0.044). A negative relationship was observed between fin fish assemblage and mean dissolved oxygen value (r = − 0.032), mean temperature with mean ammonia value (r = − 0.668), mean dissolved oxygen value with mean ammonia value (r = − 0.043) and mean pH value (r = − 0.011). A significant relationship (p < 0.05) was observed between mean ammonia and mean pH values (p = 0.005).
Regression parameters between fin fish assemblage and limnological parameters
The regression parameters between fin fish assemblage and limniligical parameters during the study is presented in Table 8. The multiple regression model was observed to be significant (p < 0.05) as well as the relationship between fin fish assemblage and mean dissolved oxygen values (b = − 2.14), ammonia (b = − 2.72) and mean pH values (b = − 1.97). The regression model for the relationship was:
Fish abundance = 3.63 + 1.75 Temperature − 2.14 DO − 2.72 Ammonia − 1.97 pH.
Discussion
Limnological parameters
Water is important for life and it is therefore essential to periodically assess its quality to maintain a healthy aquatic system (Bhalerao, 2012). The quality of water can also dictate the abundance and composition of resources in them (USEPA, 2019a). The mean limnological parameters measured across the sampling months are presented in Table 1. The highest mean monthly temperature was measured in January (24.00 ± 0.22 °C) and the least was in July (15.10 ± 0.14 °C) with an overall mean monthly temperature of 18.70 ± 2.69 °C. Generally, it was observed that most of the mean parameters measured across the months were were below the recommended limit of 20–32 °C for the survival of freshwater organisms as stated by USEPA (2019b) except for November (22.60 ± 0.20 °C) and January (24.00 ± 0.22 °C) which had mean values within the recommended limit. Both months were in the dry season and their mean values may be due to the environmental conditions of the dry season because water temperature can be influenced by rainfall and air temperature (Adedeji, 2011). During the wet season (April to October), the mean temperature across the months was below the recommended levels as similarly reported by Kolawole and Aramowo (2011) and Komolafe et al., (2014).
DO is vital for the survival of fish species and the overall mean values measured from the river (3.20 ± 0.54 mg/L) were below the recommended level of 4 mg/L as stated by USEPA (2019a). The values measured fluctuated throughout the study period with the highest monthly value in November (4.28 ± 0.05 mg/L) and least in March (2.60 ± 0.16 mg/L) and were above and below the recommended level respectively. The low DO concentration can be attributed to the presence of various pollutants from anthropogenic activities around the river catchment area, from suspended materials that can be brought in by rains and organic decomposition which can inhibit oxygen in the river (Komolafe et al., 2014).
Ammonia values were Not Detected (ND) in November and January with the highest monthly value measured in July (0.30 ± 0.09 mg/L) and the overall monthly mean was 1.20 ± 0.18 mg/L which was higher than the recommended level of 0.1 mg/L as reported by Andem et al., (2012). This peak value measured in July may be attributed to the excessive wastes from land which is washed by the continuous torrential rains which preceeds the August break as stated by NiMET (2019). Most of the mean monthly values were below the recommended level and values in July, August and October were significantly different (p < 0.05) from other months with p values of 0.00, 0.04 and 0.01 respeectively. High ammonia levels can cause various gill damages, slow growth in fish, and a reduction in resistance to diseases (Jenyo-Oni et al., 2014). Sunday and Jenyo-Oni (2018) reported similar elevated levels of ammonia and attributed it to organic waste decomposition. The highest mean monthly pH value was measured in January (7.40 ± 0.10) and least in August (6.80 ± 0.06) and the overall monthly mean was 7.10 ± 0.25. Slight differences were observed in mean monthly pH levels all of which were within the recommended levels of 6.5–9 (USEPA, 2019b). The overall mean monthly value measured (7.10 ± 0.25) was within the recommended range. Similar results of the mean pH range were reported by Olanrewaju et al., (2017) and Sunday and Jenyo-Oni (2018).
Correlation coefficient between limnological parameters
From the analysis, a negative relationship was observed between ammonia and temperature (r = − 0.668) which was significant significant (r = 0.017); pH and temperature (r = − 0.590) which was significant (p = 0.044), ammonia and DO (r = − 0.043) and pH and DO (r = − 0.011). All these negative relationship implied that the increase in one parameter resulted to a decrease in the other parameter. A significant relationship was observed between pH and ammonia (p = 0.005).
Fin fish assemblage
Seasons play a principal factor in the distribution and abundance of fish species (Negi and Mamgain, 2013) notwithstanding factors such as habitat degradation, pollution, and overfishing which are consequences of various anthropogenic activities (Jenyo-Oni and Oladele, 2016). The dry season had the highest fish species with peak abundance in March (14.7%) as corroborated by reports of Komolafe et al., (2014) and Badejo and Oriyomi, (2015). During this period, the volume of water was reduced because there is little or no rain (NIMET, 2019) thereby breeding activities ceased and fish are out in the open waters in search of food. C. nigrodigitatus was most abundant in the river and similar results were reported on the dominance of C. nigrodigitatus in Nigerian waters and the dominance of Cichlids as the case of Osun River (32.5%) have been reported (Iyiola and Jenyo-Oni, 2018; Taiwo, 2010). The wet season is peculiar with an abundance of average rainfall and low mean temperatures (NiMET, 2019) and these two factors are principal in the stimulation of fish species for breeding activities (Negi and Mamgain, 2013). During this period, some fish species were observed to be absent and generally, the abundance of fish species was low. A possible biological explanation for this was the breeding activities peculiar to the wet season in which most of the fish species had migrated to shallow areas of the river for spawning activities thereby causing a decline in the fish population (Agumassie, 2019).
It was observed that fish abundance was highest in zone A; which was expected due to the obstruction created by the dam wall. This enabled aggregation of fish species due the huge volume of water created by the obstruction, as well as the accumulation of various food materials which flowed by gravity from upstream (zone G) to downstream (zone A) (Table 5). Similarly, these results have been reported by various authors in man-made reservoirs and attributed the increased aggregates of fish species within the dam wall region. Badejo and Oriyomi, (2015); Ipinmoroti et al., (2018), Taiwo et al., (2018) to mention a few reported these cases in Erinle, Asejire, and Opa reservoirs respectively. Another possible reason for increased fish abundance can be attributed to the movement of fish with water current (Fish, 2010), in which fish flows with currents from upstream to downstream thereby accumulating in this area due to the dam wall. Lima et al., (2016) and Mostafavi et al. (2020) reported similar results in neotropical freshwaters and Karaj River; Iran respectively.
Correlation coefficient between limnological parameters and fin fish assemblage
From the analysis, a negative relationship was observed between finfish assemblage and DO (r = − 0.032), significant with ammonia (r = − 0.668, p = 0.017) and pH (r = − 0.819, p = 0.001). These implied that an increase in ammonia, DO and pH resulted to a corresponding decrease in fish abundance. This was expected because ammonia buildup is toxic to fish survival and inhibits DO concentration as observed from the analysis (Makori et al., 2017). Temperature had a negative relationship with ammonia (r = − 0.668) and pH (r = − 0.590) and DO with ammonia (r = − 0.043) and pH (r = − 0.011) Significant differences was observed between temperature and fish abuncance (p = 0.008) and pH and ammonia (p = 0.005).
Regression parameters between fin fish assemblage and limnological parameters
From the analysis, the degree of association between finfish assemblage and the limnological parameters measured was observed to be very dynamic. The regression model was observed to be significant and the mean temperature measured (1.75 X1) had a positive effect on the finfish assemblage in the river (Y). This implied that an increase in the mean concentration of temperature in the river increased the finfish assemblage. The effects of DO (− 2.14 X2), Ammonia (− 2.72 X3) and pH (− 1.97 X4) on the finfish assemblage was negative and DO was at a significant level (p = 0.048) which implied that as DO, ammonia and pH increased by one unit, finfish assemblage reduced by 2.14, 2.72 and 1.97 units respectively. The negative relationship with DO was expected because of the increased concentration of ammonia which reduces the dissolved oxygen concentration in water (Olanrewaji et al., 2017).
Conclusion
It was observed from the study that Osun River is very rich in fin fish composition and diversity although it fluctuated across the months due to activities associated with particular periods of the year. The limnological parameters measured were observed to below the recommended limits for mean temperature and dissolved oxygen; above and within the recommended limits for ammonia and pH respectively. The elevated levels of ammonia were expected and was due to the presence of the intense cage culture activity at a region of the river. Temperature recorded a negative significant difference with ammonia and pH; likewise ammonia with pH. Chrysichthys nigrodigitatus was the most abundant fin fish species and cichlids the most abundant family and temperature, ammonia and pH having significant differences with fin fish assemblage in the river. An indirect relationship was observed between fin fish assemblage and dissolved, ammonia and pH with significance with dissolved oxygen. It is imperative that the quality of limnological paramenets in water can influence the finfish composition in a river and its severity is a function of the human activities around the river catchment. Strict attention should also be given to the various anthropogenic activities along the water bodies because in the long run; all their wastes flush into the river system and degrades the water quality. Sustenance of fin fish species is directly affected thereby affecting its sustainability; with this scenario being advocated against by the United Nations Sustainable Development Goals. It emphasizes good nutrition (Goal 2), preservation of life under water (Goal 14) and life on land (Goal 15). Management strategies such as mesh size regulations, enforcement of open and closed seasons and constant monitoring of river can be proposed, which will enable sustainability of fin fish specie sin the river.
Availability of data and materials
Data are available on request.
Abbreviations
- DO:
-
Dissolved oxygen
- FAO:
-
Food and Agricultural Organization
- FDF:
-
Federal department of fisheries
- GDP:
-
Gross domestic product
- GPS:
-
Global positioning system
- SEM:
-
Standard error of mean
- UK:
-
United Kingdom
- UNEP:
-
United Nations Environmental Programme
References
Adedeji, A. A. (2011). The water quality, zooplankton, and macrobenthic invertebrate faunae in relation to aquacultural practice and management of fishponds in Ife North Area, Osun State, Nigeria. Ph.D. thesis, Obafemi Awolowo University, Ile Ife, (p. 284).
Adedokun, M. A., Fawole, O. O., & Ayandiran, T. A. (2013). Allometry and condition factors of African pike Hepsetus odoe Actinopterygii in a Lake. African Journal of Agricultural Research, 8, 3281–3284.
Admassu, D., Aberal, L., & Tadesse, Z. (2015). Fecundity and breeding season of African catfish C. gariepinus (Burchell), in Lake Babogaya. Global Journal of Agriculture and Agricultural Sciences, 3(8), 295–305.
Ait-Kadi, M. (2016). Water for development and development for water: Realizing the sustainable development goals (SDGs) vision. Aquatic Procedia, 6, 106–110.
Andem, A. B., Udofia, U., Okorafor, K. A., Okete, J. A., & Ugwumba, A. A. A. (2012). A study of some physical and chemical characteristics of Ona River, Apata, South-West, Oyo State, Nigeria. European Journal of Zoology Research, 1(2), 37–46.
Badejo, O. A., & Oriyomi, O. (2015). Seasonal variation, abundance and condition factor of fish species in erinle reservoir. American Scientific Research Journal for Engineering, Technology, and Sciences (ASRJETS), 12(1), 136–142.
Bhalerao, S. N. (2012). Study of fish diversity and water quality of Kasar Sai Dam, Hinjewadi Pune, MS India. International Research Journal of Biological Sciences, 1(4), 51–55.
FAO. (2005). Increasing the contribution of small-scale fisheries to poverty alleviation and food security. FAO Technical Guidelines for Responsible Fisheries (pp. 79). Rome: FAO.
FAO. (2016). Food and Agricultural Organization of the United Nations. FAO Yearbook: Fisheries and Aquaculture Statistics, (pp. 105).
FAO. (2019). Ecosystem approach to fisheries management training course (Inland fisheries)—volume 1: Handbook for trainees. FAO.
FDF. (2010). Fisheries and Aquaculture Statistics of Nigeria. Abuja: Federal Department of Fisheries (pp. 45)
Fish, F. E. (2010). Swimming Strategies for Energy Economy. In P. Domenici, & B.G. Kapoor (Eds.), Fish Locomotion (1st ed., pp. 33). CRC Press ebook ISBN 9780429063916.
Froese, R., & D. Pauly. (2019). Editors FishBase. World Wide Web electronic publication. http://www.fishbase.org, version (04/2019).
Griggs, D., Stafford-Smith, M., & Gaffney, O. (2013). Sustainable development goals for people and planet. Nature, 495, 305–307. https://doi.org/10.1038/495305a
Ipinmoroti, M. O., Iyiola, A. O., Akanmu, O. A., Orisasona, O., & Fawole, N. (2018). Diversity and distribution of fish species in Lake Asejire, Southwest Nigeria. In A. Kallel, M. Ksibi, H. Ben Dhia, & N. Khélifi (Eds.), Recent advances in environmental science from the euro-mediterranean and surrounding sections (pp. 1447–1448). Springer.
Iyiola, A. O., & Jenyo-Oni, A. (2018). Abundance and diversity of fish species in Osun River Catchment, Nigeria. In O. A. Fagbenro, E. K. Ajani, I. Magawata, B. A. Shinkafi, S. O. Ayoola & O. Orisasona (Eds.), Proceedings of the 1st Annual Conference of Association of Nigerian Fisheries Scientists (ANIFS) (pp. 251–255). University of Ibadan.
Iyiola, A. O., & Iyantan, E. A. (2021). Fish assemblage and diversity in Erinle Reservoir, Osun State, Nigeria. Nigerian Journal of Fisheries, 18(1), 2204–2209.
Iyiola, A. O., Akinsorotan, O. A., Ojeleye, A. E., & Fajimolu, A. O. (2023). An overview of environmental resources in Africa: Emerging issues and sustainable exploitation. In S. C. Izah & M. C. Ogwu (Eds.), Sustainable utilization and conservation of Africa’s biological resources and environment. Sustainable development and biodiversity. (Vol. 888). Springer.
Jenyo-Oni, A., Hassan, A. A., & Dauda, A. B. (2014). Investigation of biological conditions of fish species in lower Ogun River wetlands, Lagos, Nigeria. International Journal of Biology and Chemical Sciences, 8(2), 705–710.
Kareem, O. K., Olanrewaju, A. N., & Orisasona, O. (2015). Length-weight relationship and condition factor of Chrysichythys nigrodigitatus and Schilbe mystus in Erelu Lake, Oyo State, Nigeria. Journal of Fisheries and Livestock Production, 3, 150. https://doi.org/10.4172/2332-2608.1000150
Komolafe, O. O., & Arawomo, G. A. O. (2011). Observations on the composition, physiological condition and fisheries in Erinle Lake, Osun State, Nigeria. West African Journal of Applied Ecology, 18, 71–78.
Komolafe, O. O., Arawomo, G. A. O., Idowu, E. O., & Adedeji, A. A. (2014). Status and the economic impact of the fisheries of Osinmo reservoir, Ejigbo, Nigeria. Ife Journal of Science, 16(2), 309–317.
Komolafe, O. O., Olofinmehinti, M. O., Adedeji, A. A., & Adewole, H. A. (2016). The distribution, composition and abundance of fish species in tow abandoned gold mine reservoirs, Igun, Osun State, Nigeria. Ife Journal of Science, 18(1), 095–102.
Lima, A. C., Agostinho, C. S., Soares, V. M., & Monaghan, K. A. (2016). Alternative ways to measure impacts of dam closure to the structure of fish communities of a neotropical river. Ecohydrology, 9(5), 860–870. https://doi.org/10.1002/eco.1688
Makori, A. J., Abuom, P. O., Kapiyo, R., et al. (2017). Effects of water physico-chemical parameters on tilapia (Oreochromis niloticus) growth in earthen ponds in Teso North Sub-County, Busia County. Fish Aquatic Sci, 20, 30. https://doi.org/10.1186/s41240-017-0075-7
Mostafavi, S. M., Rahmani, M. R., Kaboli, M., & Abdoli, A. (2020). Determinants of fish distribution pattern and habitat preference in protected Karaj River. Iran Iranian Journal of Fisheries Sciences, 20(2), 410–429. https://doi.org/10.22092/ijfs.2021.123838
Negi, R. K., & Mamgain, S. (2013). Species diversity, abundance and distribution of fish community and conservation status of Tons River of Uttarakhand State, India. Journal of Fisheries and Aquatic Science, 8, 617–626. https://doi.org/10.3923/jfas.2013.617.626
Nigerian Metrological Agency (NiMET). (2019). Food Security Cluster: Nigerian Metrological Agency (NiMET) 2019. Seasonal Rainfall Prediction (SRP). Retrieved from https://fscluster.org/nigeria/document/nigerian-meteorological-agency-nimet.
Olanrewaju, A. N., Ajani, E. K., & Kareem, O. K. (2017). Physico-chemical status of Eleyele Reservoir, Ibadan, Nigeria. Journal of Aquaculture, Research and Development, 8, 512. https://doi.org/10.4172/2155-9546.1000512
Olaosebikan, B. D., & Raji, A. (2013). Field guide to Nigerian Freshwater Fishes (Revised Edition), Remi Thomas Press.
Sunday, Y. A., & Jenyo-Oni, A. (2018). Spatial and Temporal variation of limnological features of Eleyele Lake, Ibadan, Nigeria. In O. A. Fagbenro, E. K. Ajani, I. Magawata, B. A. Shinkafi, S. O. Ayoola, & O. Orisasona (Eds.), Proceedings of the 1st Annual Conference of Association of Nigerian Fisheries Scientists (ANIFS) (pp. 263–270). University of Ibadan: Proceedings Book.
Taiwo, Y. F. (2010). Fish diversity in two reservoirs in South Western Nigeria. In Proceedings of the Fisheries Society of Nigeria (FISON) ASCON, Badagry 25–29th October, 2010. FNS-FB-0007.
Taiwo, Y. F., Adedeji, A. A., & Adesakin, T. A. (2018). The ecological balance of the fish community in an aging reservoir. Natural Resources, 9, 327–335. https://doi.org/10.4236/nr.2018.99020
UNEP. (2012). The UN-water Status Report on the application of integrated approaches to Water Resources Management. DEP/1532/NA. Nairobi, Kenya: UNEP, Division of Communications and Public Information.
United Nations. (2015). Resolution adopted by the General Assembly on 25 September 2015. A/RES/70/1. Seventieth session. United Nations.
United States Environmental Protection Agency. (2019a). Aquatic life and Water Quality resources. Retrieved from https://www.epa.gov/wqc/aquatic-life-water-quality-resources.
United States Environmental Protection Agency (2019b). National Recommended Water quality criteria-Aquatic life Criteria Table. Retrieved from https://www.epa.gov/wqc/national-recommended-water-quality-criteria.
Acknowledgements
Not applicable.
Funding
There was no funding for this research.
Author information
Authors and Affiliations
Contributions
AI performed the practical section, collected the samples, and analyzed the data. AI and AJ conceived the idea, helped in designing the study, supervised the preparation of the experiment, and helped in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable for this kind of research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iyiola, A., Jenyo-Oni, A. The influence of temporal variation of some limnological parameters on finfish assemblage in Osun River, Nigeria.. JoBAZ 84, 21 (2023). https://doi.org/10.1186/s41936-023-00342-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-023-00342-w