Abstract
Background
The present study investigated the effect of dietary Parkia biglobosa pulp (PBP) on the growth performance and blood profile of mixed sex Clarias gariepinus. The PBP meal was supplemented with the basal diets at 0.0, 0.5, 1.0, 1.5 or 2.0% and fed to C. gariepinus fingerlings for 12 weeks.
Results
Supplementing the diets of C. gariepinus with 1.0–2.0% PBP enhanced (P < 0.05) the weight gain, specific growth rate, protein efficiency ratio and reduced (P < 0.05) feed conversion ratio with the highest performance at 2.0% inclusion level, compared to the control diet. The red blood cell counts of the fish fed PBP-supplemented diets did not differ (P > 0.05) from those fed the control diet while the lymphocyte counts was boosted (P < 0.05) with dietary PBP. Dietary supplementation with 1.0–2.0% PBP enhanced (P < 0.05) synthesis of total protein and albumin in the experimental fish while all fish fed PBP-supplemented diets had lower (P < 0.05) levels of aspartate aminotransferase and alanine aminotransferase.
Conclusions
The present study showed that 1.0–2.0% PBP enhanced growth and nutrient utilization, lymphocytes, serum albumin and total protein and reduced serum liver enzymes, indicating the growth-promoting, immunostimulating and hepatoprotective effects of PBP in C. gariepinus Therefore, supplementation of PBP meal as feed additive in the basal diet of Clarias gariepinus at 2.0% is recommended.
Similar content being viewed by others
Background
Aquaculture is growing globally as a result of intensification of the sector; fed aquaculture production has outpaced the non-fed subsector from 2010 (FAO, 2018, 2020). Fish farming accounts for about 46% of the world fish production and 52% of the fish for human consumption in 2018; providing fish for more than 3.3 billion people globally with 20% of their average per capita consumption of animal proteins, and could be up to 50% or more in some developing countries. Nigeria has intensified production of African catfish and contributes significantly to fish production in African region as the second major producer of fish (FAO, 2020). African catfish is a fed species, with high protein requirements for optimum production. The continuous rise in the cost of feed ingredients is a major concern in the production of the fish like any other fed fish species, as well as losses arising from diseases. Chemotherapeutic agents are often used for prophylactic, therapeutic measures and to promote production. However, the use of these synthetic drugs remains in fish tissues as residues and produce deleterious effects when consumed (Reverter et al., 2017; Santos & Ramus, 2018). Hence research into suitable alternatives to the synthetic drugs for sustainable food fish production is crucial.
African catfish (Clarias gariepinus Burchell 1822), a tropical freshwater farmed fish species, is the most cultured species in Nigeria, widely cultured intensively in other African countries, as well as in many parts of Europe and Asia due to its fast growth resulting from high feed conversion efficiency, high consumer acceptance, adaptability to a wide range of environmental conditions, and high market value (Adeniyi, 2020; Al-Dohail et al., 2009; Strauch et al., 2018). The C. gariepinus is a fed aquaculture species, requiring high protein ration, which makes cost of feeding high, hence utilization of organic additives with growth-enhancing properties will make its production more profitable to farmers.
Phytobiotics is one of the suitable alternatives to the synthetic drugs in aquaculture. The herbal products are readily available, less expensive and more biodegradable, when compared to the synthetic drugs (Adeniyi et al., 2018a; Reverter et al., 2014). Phytobiotics contains some useful metabolites such as flavonoids, alkaloids, terpenoids, tannins, saponins etc. (Adeniyi et al., 2017a; Reverter et al., 2017) and possess a wide variety of properties such as antioxidant, antimicrobial, appetite-enhancement, digestion-enhancement, growth-promotion, and immune-stimulation (Abdel-Tawwab et al., 2018; Adeniyi et al., 2017a, 2017b, 2018a, 2018b, 2022; Adeshina et al., 2021; Bahrami et al., 2015; Farsani et al., 2019; Fawole et al., 2020; Jiang et al., 2016; Saleh et al., 2014).
The African locust bean (Parkia biglobosa Jacq.) is a perennial tree in the family Leguminoseae (Campbell-Platt, 1980). The fruits of the tree consist of brown pods, containing yellow pulp which envelope the dark brown seeds. The P. biglobosa pulp is wash out to get the target seeds and disposed into the environment, causing both aquatic and water pollution. Previous studies focused on the seed as the economic part of the tree for the production of cooking condiments or spices through fermentation (Audu et al., 2008; Babalola et al., 2019; Biobaku et al., 2017; Odebunmi et al., 2010; Odunfa, 1986; Oladunmoye, 2007; Oso et al., 2011; Steinkraus, 1996). The P. biglobosa pulp is slightly acidic and contains fiber (11.75–18.94%), phenols (204.60 mg/100 g), and vitamins (Gernmah et al., 2007). There is scanty information on the utilization of P. biglobosa pulp in aquaculture. Therefore, the current study aimed to investigate the effect of dietary P. biglobosa pulp (PBP) on the performance of Clarias gariepinus.
Methods
Plant source
Dry fruits of P. biglobosa were obtained from the Teaching and Research Farm of the College of Agriculture, Kwara State University, Malete, Nigeria. The freshly plucked pods were opened; pulp content was scraped, shade-dried for 14 days and blend into fine powder with kitchen blender to obtain PBP meal.
Plant extraction
The aqueous (ordinary distilled water at room temperature of 25 °C; warm distilled water at 50 °C; hot distilled water at 80 °C), ethanol and methanol extractions of PBP meal was done at 1:10 (weight of PBP/volume of solvent). The distilled water, ethanol and methanol were thoroughly mixed PBP meal and left for 48 days, during which it was placed in a mechanical shaker at room temperature for 18 h and thereafter centrifuged (SE-CF-TDZ-WS, Labkits, U-Therm International (Hong Kong) Limited) at 4000g for 30 min at room temperature. The supernatants were collected as crude extracts and concentrated under vacuum using a rotary evaporator (IKA® RV10 digital, Artisan Technology Group, Champaign, USA), freeze-dried and stored in a freezer until used for phytochemical and antimicrobial screening (Adeniyi et al., 2017a).
Phytochemical and antimicrobial screening
The PBP extracts were screened qualitatively for the presence of tannins, saponins, flavonoids, steroids, terpenoids and reducing sugars following the methods described by Sofowora (1993) and Trease and Evans (1989). The in vitro antibacterial activity of PBP extracts against Aeromonas hydrophila and Pseudomonas putida using the agar well diffusion method (CLSI, 2012) was also investigated. Briefly, the A. hydrophila and P. putida were sub-cultured from the preserved slants for 24 h prior to use. The 24-h old test organisms were standardized to the 0.5 McFarland standards (106 CFU/mL) as described by CLSI (2012). Plates containing Mueller Hinton agar (Oxoid Limited, Hampshire, United Kingdom) were prepared under aseptic conditions. Wells were bored into the agar using sterilized 6 mm cork borer, after which one hundred microliters (100 μL) of each of the extracts at 10 mg/mL were introduced into the wells while synthetic antibiotics (oxytetracylcine and erythromycin) were used as controls. The plates were incubated at 37 °C and observed for zones of inhibition (mm) after 24 h (CLSI, 2012; Adeniyi et al., 2017a; Adeniyi, 2020).
Preparation of experimental diets
Five nitrogenous (≈ 40%) and isocaloric (≈ 1620 kj/100 g) were formulated to include PBP meal at 0.0 (control), 0.5, 1.0, 1.5 or 2.0% (Table 1). The PBP meal was used for this study based on the results of the preliminary study of the extracts and the ease of preparation of the meal by farmers. The main ingredients (fish meal, soybean meal and groundnut cake as protein source and corn meal as energy source) were milled and well homogenized with the micronutrients, test additives and starch (as binder). The homogenized diets were pelletized (Shuangying SYSLJ-1, Henan, China) using 2 mm die. The pellets were shade-dried and bagged with the assigned label and stored in cool dry environment during the experimental period. Only the scientist who mixed the ingredients was aware of the treatment allocation.
Fish and experimental protocols
Mixed sex Clarias gariepinus fingerlings inspected to be in good health condition were purchased from a reputable local fish hatchery in Ilorin and transported early in the morning in well aerated container to Fisheries unit of the Teaching and Research Farm, Kwara State University, Malete, Nigeria. The fish were acclimated for 2 weeks, during which they were fed with a commercial diet (Skrettings, 1.8 mm) in an indoor tank. Thereafter, 225 fish (3.67 ± 0.06 g initial weight) were used for the study consisting of five treatments. The experimental tanks were labeled, supplied with bore-hole water, regularly cleaned and water was completely renewed at 48-h intervals throughout the experimental period to ensure good water quality. The fish were randomly distributed into 15 experimental tanks (65 × 40 × 30 cm3) containing 60 L of water at the rate of 15 fish/tank in triplicates. The fish were hand-fed fed their respective diets to apparent satiation twice daily (at 8:30–9:30 and 4:30–5:30 h) for 12 weeks. The quantities of feeds offered to the fish were weighed to determine the feed intake; the numbers of dead fish in each tank during the feeding trial were also recorded. The water temperature was monitored before morning feed ration daily while pH, and dissolved oxygen (DO) were determined weekly using mercury-in-glass thermometer, Hanna pH meter (pHep, HI98107, USA), and AMSTAT dissolved oxygen meter (DO-Temp., AMT07, C. V. Java Multi Mandiri, Indonesia), respectively. The range of water temperature, pH and DO were 24.5–26.2 °C, 6.8–7.2, 4.7–5.2 mg/L, respectively. At the end of the 12-week feeding trial, fish (n = 15) in each tank were weighed and sampled to analyze various parameters obtained in this study. The fish were carefully handled through the experimental activities to prevent stress. After the analyses, the remaining fish were in healthy conditions and were stocked into production tanks, where they were fed and reared to table size for consumption.
Assessment of fish growth, nutrient utilization and survival
After the 12-week feeding trial the following parameters were calculated as shown in the formulae (Al-Dohail et al., 2009; Adeniyi et al., 2018a, 2021) below:
-
Weight gain (WG, g) = Final weight (FW) − Initial weight (IW)
-
Weight gain (%) = 100 (WG/IW)
-
Specific growth rate (%/day) = 100 (Ln FW − Ln IW)/Duration of experiment (days)
-
Feed conversion ratio = Feed intake/WG
-
Protein efficiency ratio = WG/Protein intake
-
Nitrogen metabolism = Duration of experiment (days) × (0.549) × (IW + FW)/2
-
Fish Survival (%) = 100 × (Number of survived fish/Initial number of fish stocked)
-
Fish productivity index = (Weight gain × Fish survival)/(Feed conversion ratio × 10).
Proximate analysis of experimental diets and fish
The test feed additive, diets and whole-body of fish (6 fish from each replicate) fed with the experimental diets were analyzed for proximate composition (AOAC, 2005). Concisely, the samples were oven-dried (Mini.50/SS, Genlab, England) at 105 °C to constant weight to determine the moisture/dry matter. The crude protein was determined by estimating the nitrogen content using automated digestor (8 Holes, Foss Tecator digestor, Denmark), Kjeltec auto distillation unit (Kjeltec 8200, Denmark) followed by titration and the crude protein was calculated by multiplying the nitrogen content by 6.25. The ether extracts of the samples were determined using petroleum ether extraction in a Soxhlet apparatus (Lab-Line Instruments, Inc., Melrose Park, Illinois, USA) and the fat-free samples were digested in acid and base, and oven-dried to estimate the crude fiber. To determine the ash content, the samples were combusted in an electric muffle furnace (Shanghai Changji Geological instrument equipment Co. Ltd, China) at 550 °C for 6 h. The gross energy in the tested diets was calculated (NRC, 1993).
Blood collection and analysis
After the 12 weeks feeding trial, blood was collected from 6 fish sampled from each replicate through the caudal vein into both heparinized (to prevent coagulation of blood) and non-heparinized (to allow blood clotting) bottles for haematology and serum biochemical analyses, respectively. Briefly, for haematological analysis: blood cell counts were determined following the description of Dacie and Lewis (1991) using Neubauer blight-line haematocytometer (Marienfeld, Agoda Company Pte. Ltd., Germany). To estimate the white blood cell differential cells (lymphocytes, neutrophils, monocytes, eosinophil and basophils), slides containing blood smears were prepared and viewed using microscope (Olympus, USA) and blood cell differential counter (Durga, Miniscence, Inc., USA). The differential cells were expressed in percentage (Harikrishnan et al., 2010). The mean corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular haemoglobin concentration were calculated (Al-Dohail et al., 2009) as follows:
-
Mean corpuscular volume (fL) = 10 × (hematocrits/Red blood cell count)
-
Mean corpuscular haemoglobin (pg) = 10 × (Haemoglobin/Red blood cell count)
-
Means corpuscular haemoglobin concentration (g/L) = 100 × (Haemoglobin/hematocrits)
The second set of blood in non-heparinized bottles were centrifuged (SE-CF-TDZ-WS, Labkits, U-Therm International (Hong Kong) Limited) at 4000g for 10 min at room temperature to obtain the sera samples which were used to determine serum total protein, albumin and globulin, albumin globulin ratio, total blood cholesterol, total bilirubin, creatinine, urea nitrogen, alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase using Randox kit (Randox Laboratories Ltd, United Kingdom), following the standard procedure of the manufacturer.
Statistical analysis
All data were tested for normality of distribution using the Kolmogorov–Smirnov test while the homogeneity of variances among the treatments was tested using Levene test, prior to statistical analysis. The data obtained were analyzed using descriptive statistics and one-way analysis of variance while Duncan multiple range test was used to compare differences among means at P < 0.05, using statistical package for social sciences (SPSS Statistics for Windows, IBM Corp., Version 23, Armonk, NY) software.
Results
Phytochemical components and antibacterial activity
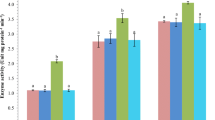
The qualitative phytochemical components of the aqueous (ordinary, warm and hot distilled water), ethanol and methanol extracts of PBP is presented in Table 2. The results of the phytochemical screening showed presence of tannin, saponin, flavonoid, steroid, terpenoids and reducing sugars in the aqueous, ethanol and methanol extracts of PBP. The results of the antibacterial screening revealed that PBP extracts exhibited antibacterial activity against A. hydrophila (Fig. 1) and P. putida (Fig. 2), although the zones of inhibition were lower (P < 0.05), when compared to the synthetic antibiotic. There were no significant (P > 0.05) differences among the zones of inhibition of the five extracts of PBP.
The zones of inhibition of Parkia biglobosa pulp (PBP) extracts and synthetic antibiotics against Aeromonas hydrophila. Data are represented as means of 3 replicates. Different letters on bars indicate significant differences among treatments (P ˂ 0.05). ODM Ordinary distilled water, WDW warm distilled water, HDW hot distilled water, ETH ethanol, MET methanol, OXY oxytetracycline, ERY erythromycin
The zones of inhibition of Parkia biglobosa pulp (PBP) extracts and synthetic antibiotics against Pseudomonas putida. Data are represented as means of 3 replicates. Different letters on bars indicate significant differences among treatments (P ˂ 0.05). ODM Ordinary distilled water, WDW warm distilled water, HDW hot distilled water, ETH ethanol, MET methanol, OXY oxytetracycline, ERY erythromycin
Fish growth performance, nutrient utilization and survival
The growth performance, nutrient utilization and survival of C. gariepinus fed PBP-supplemented diets are shown in Table 3. The final weight and weight gain were enhanced (P < 0.05) in fish fed 1.0–2.0% PBP, compared to those fed the control diet. The relative growth rate and specific growth rate also increased (P < 0.05) with dietary increase in the inclusion levels of PBP and were significantly higher (P < 0.05) than the value obtained in the fish fed the control diets. Supplementing the diets of C. gariepinus with PBP meal reduced (P < 0.05) the feed conversion ratio while the protein efficiency ratio increased (P < 0.05), compared to the control diet. The lowest feed conversion ratio and highest protein efficiency ratio were obtained at 2.0% inclusion level, and the values varied (P < 0.05) from the lower inclusion levels. The nitrogen metabolism and fish survival also increased (P < 0.05) in groups of fish fed 1.0–2.0% PBP. The overall fish productivity index of C. gariepinus increased (P < 0.05) with dietary 1.0–2.0% PBP, when compared to the control diet.
Fish whole-body proximate composition
The proximate analysis of C. gariepinus fed with the experimental diets (Table 4) showed that the moisture contents reduced significantly (P < 0.05) with dietary PBP, while on the other hand, the crude protein, lipid, and ash increased (P < 0.05). The crude protein tends to increase (P < 0.05) with increasing levels of PBP in the diets of C. gariepinus. Significantly higher (P < 0.05) crude protein and ash were obtained in fish fed 2.0% PBP-supplemented diets, when compared to other treatments.
Haematological and serum biochemistry profiles
The haematological profile of C. gariepinus fed PBP-supplemented diets is presented in Table 5. Significantly higher (P < 0.05) hematocrit, haemoglobin and white blood cells were obtained in the fish fed 1.0% PBP, compared to those fed other diets. The red blood cell counts of the fish fed PBP-supplemented diets did not differ (P > 0.05) from those fed the control diet, although the highest value was obtained in fish fed 1.5% PBP meal. The white blood cell counts of the PBP-fed fishes did not differ (P > 0.05) from the control, except the group fed with 1.0% PBP that was higher (P < 0.05). Higher (P < 0.05) platelet and neutrophil counts were obtained in fish fed control diet while the lymphocytes counts were enhanced (P < 0.05) in those fed PBP-supplemented diets. The highest monocytes (P < 0.05) was obtained in fish fed 1.0% PBP, while eosinophil and basophil counts were not altered (P > 0.05) with PBP supplementation, when compared with control treatment. The mean corpuscular haemoglobin and mean corpuscular volume were higher (P < 0.05) in fish fed 1.0% PBP, compared to those fed other diets. On the other hand, mean corpuscular haemoglobin concentration was not significantly affected (P > 0.05).
The results on the serum analysis (Table 6) showed that 1.0–2.0% PBP supplementation enhanced (P < 0.05) synthesis of total protein and albumin in C. gariepinus while higher (P < 0.05) globulin was obtained in fish fed 1.0% PBP. The albumin-globulin ratio of the group of fish fed 1.0% PBP did not differ (P > 0.05) from the control group. All fish fed PBP-supplemented diets had lower (P < 0.05) levels of aspartate aminotransferase and alanine aminotransferase while alkaline phosphatase was reduced (P < 0.05) at 0.5–1.5% PBP levels. Lower (P < 0.05) blood urea nitrogen was obtained in fish fed diets supplemented with PBP, compared to those fed the control diet. The level of creatinine and total bilirubin did not differ (P > 0.05) in fish fed PBP, compared to the control diet while the cholesterol increased (P < 0.05) with increase in dietary levels of PBP.
Discussion
The present study has shown that PBP contains tannin, saponin, flavonoid, steroid, terpenoids and reducing sugars, which could have been responsible for the antibacterial activities of the extracts observed. The results on the phytochemical components and antibacterial activity coincide with the report of Ajaiyeoba (2002) on Parkia leaf. Ibraheem et al. (2019) also reported similar observation on the antibacterial activity of Parkia pulp against Pseudomonas species, which could be the scientific basis for wide utilization of PBP and other parts of Parkia species traditionally to treat different types of ailments, including diarrhea, dysentery, measles, ulcers, and skin diseases (Saleh et al., 2021) and its application in the present study.
Dietary supplementation with 1.0–2.0% PBP enhanced growth performance (final weight, weight gain, relative growth rate and specific growth rate) and nutrient utilization (feed conversion ratio, protein efficiency ratio, nitrogen metabolism) and the productivity of African catfish. Several herbal supplements have been tested and proved to possess similar growth-promoting effects, including Gossypium herbacium leaves (Adeniyi & Lawal, 2017), Curcuma longa leaves (Adeshina et al., 2017), Ocimum gratissimum leaves (Abdel-Tawwab et al., 2018), Tamarindus indica leaves and pulp (Adeniyi et al., 2018a), Psidium guajava leaves (Setufe et al., 2018), Cymbopogon citratus leaves (Adeniyi, 2020) in the production of African catfish, C. gariepinus. The growth-promoting potentials of Allium sativum (Saleh et al., 2014), Rehmannia glutinosa (Wang et al., 2014), curcumin from Curcuma longa root (Jiang et al., 2016), Coriandrum sativum (Farsani et al., 2019), Zingiber officinale (Mohammadi et al., 2020), Rosmarinus officinalis leaves (Naiel et al., 2020), Tridax procumbens leaves (Adeshina et al., 2021) were also reported in other fish species.
The observed growth-promotion in the present study may be associated with high phenolic compounds (Gernmah et al., 2007) and other phytochemicals in PBP reported in the current study. Herbal polyphenolic compounds are said to undergo biotransformation by gut microbiota to produce bioavailable compounds with antimicrobial, anti-inflammatory, anti-oxidative, and digestive properties (Espin et al., 2017; Karl et al., 2018; Scalbert & Williamson, 2000), contributing to higher productivity of healthy animal products. Herbal supplements have been reported to stimulate digestion of feeds (Adeniyi et al., 2018a, 2021, 2022; Bhosale et al., 2010; El-Dakar et al., 2015; Jiang et al., 2016) in cultured fishes. Thus, the growth-promoting effects of PBP in C. gariepinus in the present study could be ascribed to the activities of its phytochemicals which might have enhanced activities of digestive enzymes, gut health, and nutrient absorption with consequent higher nutrient utilization and the growth performance.
The present study has shown that dietary PBP significantly affected the whole-body proximate composition of C. gariepinus. The results of the fish whole-body proximate composition coincided with the earlier researchers who reported higher crude protein (Dada, 2015; Dong-Hoon et al., 2014; Maniat et al., 2014; Wafaa et al., 2014), lipids (Abdel-Tawwab et al., 2010; Ahmad and Abdel-Tawwab 2011); and ash (Wafaa et al., 2014). On the contrary, lower crude protein (Ahmad & Abdel-Tawwab, 2011; Fawole et al., 2020) and ash (Ahmad and Abdel-Tawwab (2011) were obtained in fish fed diets supplemented with phytoadditives. The higher whole-body protein composition obtained in the current study might be related to the role of PBP in enhancing higher utilization of dietary protein in C. gariepinus (Halver & Hardy, 2002) resulting to higher growth and protein retention. The higher body protein and lipids could also be associated with higher serum protein and cholesterol obtained in the present study.
Haematological and serum biochemical parameters are indispensable tools that have been widely used to evaluate the physiological status of fish in response to herbal-supplemented diets in several previous studies (Adeshina et al., 2021; Amirkhani & Firouzbakhsh, 2013; Bahrami et al., 2015; Farsani et al., 2019; Maldonado-Garcia et al., 2019; Mohammadi et al., 2020; Sudagar & Hajibeglou, 2010). Red blood cells and haemoglobin are responsible for transportation of oxygen in body tissue of fish, while hematocrit is the percentage of red blood cells in circulation. Although there were no significant differences in the red blood cell counts of PBP-fed fishes in the present study, the levels of haemoglobin and haematocrit seemed to be higher at 1.0–1.5 PBP supplementation; this could have contributed to tissue oxygenation and higher survival of fish recorded at these inclusion levels of PBP in the current study. The insignificant difference in the red blood cell counts in the present study is similar to the observations in recent studies (Farsani et al., 2019; Rajabiesterabadi et al., 2020; Zemheri-Navruz et al., 2019). On the other hand, Sudagar and Hajibeglou (2010), Mohammadi et al. (2020) and Adeshina et al. (2021) reported higher RBC counts in fishes fed herbal-supplemented diets. The enhanced haematrocrit and haemoglobin at 1.0–1.5% PBP coincided with observations of Sudagar and Hajibeglou (2010), Maldonado-Garcia et al. (2019), Mohammadi et al. (2020) and Adeshina et al. (2021).
White blood cells are the cells mediating the innate and adaptive responses in animals (Mak & Saunders, 2006); providing protection against both chemical and microbial-diseases (Pakravan et al., 2012). The higher lymphocytes in the PBP-fed fishes could indicate the immune-stimulatory role of PBP and this might have been responsible for the higher survival and productivity of the fish fed PBP-supplemented diets in the present study. The lower neutrophil–lymphocyte ratio in the present study could also be associated with higher neutrophil and lower lymphocytes obtained in fish fed the control diet. Several previous studies (Adeshina et al., 2021; Amirkhani & Firouzbakhsh, 2013; Fawole et al., 2020; Maldonado-Garcia et al., 2019; Mohammadi et al., 2020; Saleh et al., 2014; Sudagar & Hajibeglou, 2010; Tiamiyu et al., 2019) also reported higher lymphocytes in fishes fed dietary herbal supplements.
Albumin and globulin are components of blood proteins, playing significant roles in osmoregulation, transportation of substances as well as defense against pathogens (Peyghan et al., 2014). Dietary 1.0–2.0% PBP meal stimulated synthesis of total serum protein and albumin in the present study. Stimulation of the synthesis of these proteins, usually done by the liver, would have enhanced the immune system of the fish (Ahmadi et al., 2012) and contributed to the higher productivity and wellbeing of the fish. Higher values of proteins was similarly observed in previous studies (Amirkhani & Firouzbakhsh, 2013; Bahrami et al., 2015; Sudagar & Hajibeglou, 2010) in carp fed herbal-supplemented diets. On the other hand, the study of Farsani et al (2019) showed insignificant differences in the values of serum protein in rainbow trout fed coriander seed extract, compared to the control group. Albumin-globulin ratio provides a means of assessing the relative contribution of albumin and globulin to the total protein. Higher albumin-globulin ratios were obtained from PBP-fed fishes; however, the values fall within recommended ranges of 30–50% for fishes (Eckersall, 2008).
The activities of liver enzymes (aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase) were measured to detect the hepatic injury (Tennant and Center, 2008), which might consequently disrupt hepatocellular membranes; making these enzymes to leak out of the hepatocytes (Reda et al., 2013). Supplementing the diets of C. gariepinus with PBP significantly reduced the levels of these enzymes in the blood, except the alkaline phosphatase that was higher at 2.0% PBP level. The reduction in the concentrations of these enzymes could be ascribed to the hepatoprotective role of PBP in the present study; the higher level of alkaline phosphatase at 2.0% PBP could be an indication of negative effects of higher dose of phytochemical at this level, even though the highest growth performance was observed at this inclusion level. However, high alkaline phosphatase might not be an indication of damaged liver cells, but due to reduction in biliary excretion (Tennant and Center, 2008). The blood urea nitrogen, creatinine and bilirubin are metabolic nitrogenous waste products from the kidney; elevated levels of these metabolic wastes are indicators of impaired glomerular filtration or severe tubular dysfunction (Brien & Walterson, 2009) in animals. The significant reduction of blood urea nitrogen in PBP-fed fishes and insignificant differences in the values of creatinine and total bilirubin in the present study could be associated with the contribution of PBP to healthy functioning of kidney in the experimental fish. Cholesterol is an essential part of cell membrane, playing significant role in the maintenance of cellular fluidity and structural integrity of animals. The increased level of serum total cholesterol in the present study is similar to the observation of El-Dakar et al. (2015) and has been associated with good quality diet and healthy fish.
Conclusions
The results of the present study showed that dietary Parkia biglobosa pulp significantly enhanced nutrient utilization, growth performance, whole-body proximate composition, lymphocytes production, and reduced levels of urea nitrogen and liver enzymes (aspartate aminotransferase and alanine aminotransferase) in Clarias gariepinus at 1.0–2.0% inclusion levels. The best growth performance was at 2.0% and the reduced concentration of liver enzymes at this level indicated the healthy condition of the fish. Therefore, supplementation of Parkia biglobosa pulp meal as feed additive in the basal diet of Clarias gariepinus at 2.0% is recommended. Further studies to ascertain the optimum inclusion level and the utilization of Parkia biglobosa pulp as therapeutic agents in fish culture against common fish pathogenic organisms are also recommended.
Availability of data and materials
All the data generated and analyzed are available and have been included in this article.
Abbreviations
- AOAC:
-
Association of Official Analytical Chemistry
- DO:
-
Dissolved oxygen
- CLSI:
-
Clinical and Laboratory Standards Institute
- NRC:
-
National Research Council
- FAO:
-
Food and Agriculture Organization
- PBP:
-
Parkia biglobosa pulp
References
Abdel-Tawwab, M., Adesina, I., Jenyo-Oni, A., Ajani, E. K., & Emikpe, B. O. (2018). Growth, antioxidant and immune response of African catfish, Clarias gariepinus (B), to dietary clove basil, Ocimum gratissimum, leaf extract and its susceptibility to Listeria monocytogenes infection. Fish and Shellfish Immunology, 78, 346–354. https://doi.org/10.1016/j.fsi.2018.04.057
Abdel-Tawwab, M., Ahmad, M. H., Medhat, E. A., & Saleh, F. M. (2010). Use of green tea, Camellia sinensis L. in practical diet for growth and protection of Nile tilapia, Oreochromis niloticus (L) against Aeromonas hydrophila infection. Journal of the World Aquaculture Society, 41, 203–213. https://doi.org/10.1111/j.1749-7345.2010.00360.x
Adeniyi, O. V. (2020). Growth performance of Clarias gariepinus (Burchell 1822) fed diets fortified with lemongrass (Cymbopogon citratus). Acta Veterinaria Eurasia, 46, 21–29. https://doi.org/10.5152/actavet.2020.19023
Adeniyi, O. V., & Lawal, A. O. (2017). Growth performance of African catfish fed diet supplemented with Gossypium herbacium powder as dietary feed additive. Journal of Agriculture and Ecological Research International, 11(2), 1–7. https://doi.org/10.9734/JAERI/2017/31639
Adeniyi, O. V., Olaifa, F. E., & Emikpe, B. O. (2017b). Effect of Tamarindus indica (Linn, 1753) pulp and leaf-fortified diets on experimental Aeromonas hydrophila infection in Clarias gariepinus (Burchell, 1822). Bulletin of Animal Health and Production in Africa, 65(4), 623–634.
Adeniyi, O. V., Olaifa, F. E., & Emikpe, B. O. (2018a). Growth performance and nutrient digestibility of Clarias gariepinus (Burchell 1822) fed diets fortified with Tamarindus indica pulp and leaf meal. Asian Fisheries Science, 31, 17–31. https://doi.org/10.33997/j.afs.2018.31.1.002
Adeniyi, O. V., Olaifa, F. E., & Emikpe, B. O. (2022). Effects of dietary tamarind pulp extract on growth performance, nutrient digestibility, intestinal morphology, and resistance to Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus L.). Journal of Applied Aquaculture, 34(1), 43–63. https://doi.org/10.1080/10454438.2020.1785984
Adeniyi, O. V., Olaifa, F. E., Emikpe, B. O., & Ogunbanwo, S. T. (2017a). Phytochemical components and antibacterial activity of Tamarindus indica Linn. extracts against some pathogens. Biotechnology Journal International, 17(2), 1–9. https://doi.org/10.9734/BJI/2017/30618
Adeniyi, O. V., Olaifa, F. E., Emikpe, B. O., & Ogunbanwo, S. T. (2021). Effects of dietary tamarind (Tamarindus indica L.) leaves extract on growth performance, nutrient utilization, gut physiology, and susceptibility to Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus L.). International Aquatic Research, 13, 37–51. https://doi.org/10.22034/IAR.2021.1916077.1115
Adeniyi, O. V., Olaifa, F. E., Emikpe, B. O., & Oyagbemi, A. A. (2018b). Experimental evaluation of the wound-healing and antioxidant activities of tamarind. Tamarindus indica pulp and leaf meal in the African catfish (Clarias gariepinus). Acta Veterinaria Eurasia, 44, 63–72. https://doi.org/10.26650/actavet.2018.011
Adeshina, I., Abdel-Tawwab, M., Tijjani, Z. A., Tiamiyu, L. O., & Jahanbakhshi, A. (2021). Dietary Tridax procumbens leaves extract stimulated growth, antioxidants, immunity, and resistance of Nile tilapia, Oreochromis niloticus, to monogenean parasitic infection. Aquaculture, 532, 736047. https://doi.org/10.1016/j.aquaculture.2020.736047
Adeshina, I., Adewale, Y. A., & Tiamiyu, L. O. (2017). Growth performance and innate immune response of Clarias gariepinus infected with Aeromonas hydrophila fed diets fortified with Curcuma longa leaf. West African Journal of Applied Ecology, 25(2), 79–90.
Ahmad, M. H., & Abdel-Tawwab, M. (2011). The use of caraway seed meal as a feed additive in fish diets: Growth performance, feed utilization and whole body composition of Nile tilapia, Oreochromis niloticus (L) fingerlings. Aquaculture, 314, 110–114. https://doi.org/10.1016/j.aquaculture.2011.01.030
Ahmadi, K., Banaee, M., Vosoghei, A. R., Mirvaghefei, A. R., & Ataeimehr, B. (2012). Evaluation of the immunomodulatory effects of silymarin extract (Silybum marianum) on some immune parameters of rainbow trout (Oncorhynchus mykiss). Acta Ichthyology Piscatoria, 42, 113–120. https://doi.org/10.3750/AIP2011.42.2.04
Ajaiyeoba, E. O. (2002). Phytochemical and antibacterial properties of Parkia biglobosa and Parkia bicolor leaf extracts. African Journal of Biomedical Research, 5, 125–129. https://doi.org/10.4314/ajbr.v5i3.54000
Al-Dohail, M. A., Hashim, R., & Paiko, M. A. (2009). Effects of the probiotic, Lactobacillus acidophilus, on the growth performance, haematology parameters and immunoglobulin concentration in African catfish (Clarias gariepinus, Burchell 1822) fingerling. Aquaculture Research, 40, 1642–1652. https://doi.org/10.1111/j.1365-2109.2009.02265.x
Amirkhani, N., & Firouzbakhsh, F. (2013). Protective effects of basil (Ocimum bacilicum) ethanolic extract supplementation diets against experimental Aeromonas hydrophila injection in common carp (Cyprinus carpio). Aquaculture Research, 46(3), 716–724. https://doi.org/10.1111/are.12217
AOAC (Association of Official Analytical Chemistry) International. (2005). Official methods of analysis (18th ed.). AOAC International.
Audu, B. S., Adamu, K. M., & Bing, S. A. (2008). Effects of substituting fishmeal diets with varying quantities of ensiled parboiled beniseed (Sesanum indicum) and raw African locust bean (Parkia biglobosa) on the growth response and feed utilization of Nile tilapia, Oreochromis niloticus. International Journal of Zoological Research, 4(1), 42–47.
Babalola, O. A., Odu-Onikosi, S. G., Adam, O. B., & Ogunyomi, O. R. (2019). Effects of replacing soya bean meal with fermented African locust bean (Parkia biglobosa) meal on the growth performance and condition factor of Tilapia zilli fingerlings. Global Journal of Fisheries, 1(1), 9–14. https://doi.org/10.31248/GJFS2019.005
Bahrami, B. S., Paykan, H. F., Dorafshan, S., Mahboobi, S. N., & Vahabi, M. R. (2015). Effect of dietary wood betony, Stachys lanvandulifolia extract on growth performance, haematological and biochemical parameters of common carp, Cyprinus carpio. Iranian Journal of Fisheries Sciences, 14(4), 805–817.
Bhosale, S. V., Bhilave, M. P., & Nadaf, S. B. (2010). Formulation of fish feed using ingredients from plant sources. Research Journal of Agricultural Sciences, 1(3), 284–287.
Biobaku, K. T., Thomas, F. C., Aremu, A., Asogwa, N. T., Ameen, S. A., Akorede, G. J., & Basiru, A. (2017). Nutriceutical effects of fermented Parkia biglobosa seeds on recovery of malnourished rats. Ceylon Journal of Science, 46(3), 47–53. https://doi.org/10.4038/cjs.v46i3.7442
Brien, P. J. O., & Walterson, C. L. (2009). Assessment of hepatoxicity. In G. O. Evans (Ed.), Animal clinical chemistry, a practical guide for toxicologists and biomedical researchers (2nd ed., pp. 37–57). Boca Raton: CRC Press.
Campbell-Platt, G. (1980). African locust beans (Parkia spp) and its West African fermented food product, ‘dawadawa.’ Ecology of Food & Nutrition, 9, 123–132. https://doi.org/10.1080/03670244.1980.9990590
CLSI (Clinical and Laboratory Standards Institute). (2012). Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute’s Twenty-Second Information Supplement, 32, 44–160.
Dacie, J. V., & Lewis, S. M. (1991). Practical haematology (6th ed.). Churchill.
Dada, A. A. (2015). Use of fluted pumpkin (Telfairia occidentalis) leaf powder as feed additive in African catfish (Clarias gariepinus) fingerlings. International Journal of Biological and Chemical Sciences, 9(1), 301–307. https://doi.org/10.4314/ijbcs.v9i1.27
Dong-Hoon, L., Seong-Ryul, L., Jung-Jo, H., Sang-Woo, L., Chang-six, R., & Jeong-Dae, K. (2014). Effect of dietary garlic powder on growth, feed utilization and whole-body composition changes in fingerling sterlet sturgeon. Asian-Australasian Journal of Animal Sciences, 27(9), 1303–1310. https://doi.org/10.5713/ajas.2014.14087
Eckersall, P. D. (2008). Proteins, proteomics and the dysproteinemias. In J. J. Kaneko, J. W. Harvey, & M. L. Bruss (Eds.), Clinical biochemistry of domestic animals (6th ed., pp. 117–155). Amsterdam: Elsevier.
El-Dakar, A. Y., Sakr, E. M., & Toutou, M. M. (2015). Possibility of using basil (Ocimum basilicum) supplementation in gilthead sea bream (Sparus aurata) diet. Egyptian Journal of Aquatic Research, 41, 203–210. https://doi.org/10.21608/maj.2008.2662
Espin, J. C., Gonzalez-Sarrias, A., & Tomas-Barberan, F. A. (2017). The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochemistry and Pharmacology, 139, 82–93. https://doi.org/10.1016/j.bcp.2017.04.033
FAO (Food and Agriculture Organization). (2018). The state of the world fisheries and aquaculture: Meeting the sustainable development goal, 227. FAO of the United Nations.
FAO. (2020). The state of the world fisheries and aquaculture: Sustainability in action, 224. FAO of the United Nations.
Farsani, M. N., Hoseinifar, S. H., Rashidian, G., Farsani, H. G., Ashouri, G., & Doan, H. V. (2019). Dietary effects of Coriandrum sativum extract on growth performance, physiological and innate immune responses and resistance of rainbow trout (Oncorhynchus mykiss) against Yersinia ruckeri. Fish and Shellfish Immunology, 91, 233–240. https://doi.org/10.1016/j.fsi.2019.05.031
Fawole, F. J., Adeoye, A. A., Tiamiyu, L. O., Samuel, F. C., Omosuyi, O. M., & Amusa, M. T. (2020). Dietary combination of pawpaw seed and onion peel powder: Impact on growth, haematology, biochemical and antioxidant status of Clarias gariepinus. Aquaculture Research. https://doi.org/10.1111/are.14629
Gernmah, D. I., Atolagbe, M. O., & Echegwo, C. C. (2007). Nutritional composition of the African locust bean (Parkia biglobosa) fruit pulp. Nigerian Food Journal, 25(1), 190–196. https://doi.org/10.4314/nifoj.v25i1.33669
Halver, J. E., & Hardy, I. W. (2002). Nutrient flow and retention. In R. W. Hardy (Ed.), Fish nutrition (3rd ed., pp. 755–770). Elsevier.
Harikrishnan, C., Balasundaram, C., & Heo, M. (2010). Herbal supplementation diets on hematology and innate immunity in goldfish against Aeromonas hydrophila. Fish and Shellfish Immunology, 28(2), 354–361. https://doi.org/10.1016/j.fsi.2009.11.013
Ibraheem, S. A., Audu, E. A., Jaafar, M., Adudu, J. A., Barmina, J. T., Ochigbo, V., Igunnu, A., & Malomo, S. O. (2019). Novel pectin from Parkia biglobosa pulp mediated green route synthesis of hydroxyapatite nanoparticles. Surfaces and Interfaces. https://doi.org/10.1016/j.surfin.2019.100360
Jiang, J., Wu, X., Zhou, X., Feng, L., Liu, Y., Jiang, W., Wu, P., & Zhao, Y. (2016). Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquaculture, 463, 174–180. https://doi.org/10.1016/j.aquaculture.2016.05.040
Karl, J. P., Hatch, A. M., Arcidiacono, S. M., Pearce, S. C., Pantoja-Feliciano, I. G., Doherty, L. A., & Soares, J. W. (2018). Effects of psychological, environmental and physical stressors on the gut microbiota. Frontiers in Microbiology, 9, 2013. https://doi.org/10.3389/fmicb.2018.02013
Mak, T. W., & Saunders, M. E. (2006). The cells and tissues of the immune response. In T. W. Mak & M. E. Saunders (Eds.), The immune response: Basic and clinical principles (pp. 35–67). Elsevier.
Maldonado-Garcia, M., Angulo, C., Vazquez-Martinez, J., Sanchez, V., Lopez, M. G., & Reyes- Becerril, M. (2019). Antioxidant and immunostimulant potentials of Chenopodium ambrosioides L. in Pacific red snapper (Lutjanus peru). Aquaculture, 513, 734414. https://doi.org/10.1016/j.aquaculture.2019.734414
Maniat, M., Ghotbeddin, N., & Ghatrami, E. R. (2014). Effect of garlic on growth performance and body composition of benni fish (Mesopotamichthys sharpeyi). International Journal of Biosciences, 5(4), 269–277. https://doi.org/10.12692/ijb/5.4.269-277
Mohammadi, G., Rashidian, G., Hoseinifar, S. H., Naserabad, S. S., & Doand, H. V. (2020). Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio). Fish & Shellfish Immunology, 99, 267–273. https://doi.org/10.1016/j.fsi.2020.01.032
Naiel, M. A. E., Ismael, N. E. M., Negm, S. S., Mohamed, S., Ayyat, M. S., & Al-Sagheer, A. A. (2020). Rosemary leaf powder-supplemented diet enhances performance, antioxidant properties, immune status, and resistance against bacterial diseases in Nile tilapia (Oreochromis niloticus). Aquaculture. https://doi.org/10.1016/j.aquaculture.2020.735370
NRC (National Research Council). (1993). Nutrient requirement of fish. Committee on Animal Nutrition, Board on Agriculture (p. 123). National Academy Press.
Odebunmi, E. O., Oluwaniyi, O. O., & Bashiru, M. O. (2010). Comparative proximate analysis of some food condiments. Journal of Applied Sciences Research, 6(3), 272–274.
Odunfa, S. A. (1986). Dawadawa. In N. R. Reddy, M. D. Pierson, & D. K. Salunkhe (Eds.), Legume based fermented of foods. CRC Press.
Oladunmoye, M. K. (2007). Effects of fermentation on nutrient enrichment of locust beans (Parkia biglobosa, Robert bam). Research Journal of Microbiology, 2(2), 185–189. https://doi.org/10.3923/jm.2007.185.189
Oso, J. A., Idowu, E. O., & Agoi, O. F. (2011). Growth response of Clarias gariepinus fingerlings fed Parkia biglobosa diet as protein source. Indian Journal of Science & Technology, 4(2), 82–84. https://doi.org/10.17485/IJST%2F2011%2FV4I2%2F29938
Pakravan, S., Hajimoradloo, A., & Ghorbani, R. (2012). Effect of dietary willow herb, Epilobium hirsutum, extract on growth performance, body composition, haematological parameters and Aeromonas hydrophila challenge on common carp, Cyprinus Carpio. Aquaculture Research, 43(6), 861–869. https://doi.org/10.1111/j.1365-2109.2011.02901.x
Peyghan, R., Khadjeh, G. H., & Enayati, A. (2014). Effect of water salinity on total protein and electrophoretic pattern of serum proteins of grass carp, Ctenopharyngodon idella. Veterinary Research Forum, 3, 225–229.
Rajabiesterabadi, H., Yousefi, M., & Hoseini, S. M. (2020). Enhanced haematological and immune responses in common carp Cyprinus carpio fed with olive leaf extract-supplemented diets and subjected to ambient ammonia. Aquaculture Nutrition, 26, 763–771. https://doi.org/10.1111/anu.13035
Reda, R. M., Ibrahim, R. E., Ahmed, E. G., & El-Bouhy, Z. M. (2013). Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egyptian Journal of Aquatic Research, 9, 241–248. https://doi.org/10.1016/j.ejar.2013.12.001
Reverter, M., Bontemps, N., Lecchini, D., Banaigs, B., & Sasal, P. (2014). Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture, 433, 50–61. https://doi.org/10.1016/j.aquaculture.2014.05.048
Reverter, M., Tapissier-Bontemps, N., Sasal, P., & Saulnier, D. (2017). Use of medicinal plants in aquaculture. 9. In B. Austin & A. Newaj-Fyzul (Eds.), Diagnosis and control of diseases of fish and shellfish (pp. 223–261). Wiley.
Saleh, M. S. M., Jalil, J., Zainalabidin, S., Asmadi, A. Y., Mustafa, N. H., & Kamisah, Y. (2021). Genus Parkia: Phytochemical, medicinal uses, and pharmacological properties. International Journal of Molecular Sciences, 22, 618. https://doi.org/10.3390/ijms22020618
Saleh, N. E., Micheal, F. R., & Toutou, M. M. (2014). Evaluation of garlic and onion powder as phytoadditives in the diets of sea bass (Dicentrarcus labrax). Egyptian Journal of Aquatic Research, 41, 211–217. https://doi.org/10.1016/j.ejar.2015.03.008
Santos, L., & Ramus, F. (2018). Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. International Journal of Antimicrobial Agents, 52(2), 135–143. https://doi.org/10.1016/j.ijantimicag.2018.03.010
Scalbert, A., & Williamson, G. (2000). Dietary intake and bioavailability of polyphenols. Journal of Nutrition, 130(8S Suppl), 2073S-2085S. https://doi.org/10.1093/jn/130.8.2073S
Setufe, S. B., Ajani, E. K., Emikpe, B. O., & Ogunbanwo, S. T. (2018). Growth performance of Clarias gariepinus on diets fortified with Lactobacillus plantarum and Psidium guajava leaf. Acta Veterinaria Eurasia, 44, 105–111. https://doi.org/10.26650/actavet.2019.412319
Sofowora, A. (1993). Medicinal plants and traditional medicine in Africa (p. 150). Spectrum Books Limited.
Steinkraus, K. H. (1996). Handbook of indigenous fermented foods (2nd ed., pp. 103–109). Marcel Dekker.
Strauch, S. M., Wenzel, L. C., Bischoff, A., Dellwig, O., Klein, J., Schüch, A., Wasenitz, B., & Palm, H. W. (2018). Commercial African catfish (Clarias gariepinus) recirculating aquaculture systems: Assessment of element and energy pathways with special focus on the phosphorus cycle. Sustainability. https://doi.org/10.3390/su10061805
Sudagar, M., & Hajibeglou, A. (2010). Effect of plant extracts supplemented diets on immunity and resistance to Aeromonas hydrophila in common carp (Cyprinus carpio). Research Journal of Animal Sciences, 4(1), 26–34. https://doi.org/10.3923/rjnasci.2010.26.34
Tennant, B. C., & Center, S. A. (2008). Hepatic function. In J. J. Kaneko, J. W. Harvey, & M. L. Bruss (Eds.), Clinical biochemistry of domestic animals (Vol. 13, pp. 378–412). Amsterdam: Elsevier.
Tiamiyu, A., Olatoye, I., & Adedeji, O. (2019). Blood indices of African catfish (Clarias gariepinus) following dietary administration of Talinum triangulare. International Journal of Research, 7(4), 185–198. https://doi.org/10.29121/granthaalayah.v7.i4.2019.888
Trease, G. E., & Evans, W. C. (1989). Pharmacognosy (11th ed., pp. 176–180). Macmillian Publishers.
Wafaa, E., Doaa, I., El-murr, A., & Mahmoud, R. (2014). Effects of dietary inclusion of black cumin seeds, green tea and propolis extraction on growth parameters, body composition and economic efficiency of Nile tilapia, Oreochromis niloticus. World Journal of Fisheries & Marine Science, 6(5), 447–452.
Wang, J., Meng, X., Lu, R., Wu, C., Luo, Y., Yan, X., Li, X., Kong, X., & Nie, G. (2014). Effects of Rehmannia glutinosaon growth performance, immunological parameters and disease resistance to Aeromonas hydrophila in common carp (Cyprinus carpio L.). Aquaculture, 435, 293–300. https://doi.org/10.1016/j.aquaculture.2014.10.004
Zemheri-Navruz, F., Acar, U., & Yılmaz, S. (2019). Dietary supplementation of olive leaf extract increases haematological, serum biochemical parameters and immune related genes expression level in common carp (Cyprinus carpio) juveniles. Fish and Shellfish Immunology, 89, 672–676. https://doi.org/10.1016/j.fsi.2019.04.037
Acknowledgements
The authors thank Dr Theophilus Jarike and Mrs. Josephine Ademakinwa for their supports during blood sampling and analysis.
Funding
No financial support was received for the study.
Author information
Authors and Affiliations
Contributions
OVA designed the study, participated in data collection, statistical analysis, general supervision, and drafted the manuscript. NS and OS participated in the experimentation and data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare that all applicable international, national, and institutional guidelines for the welfare and use of fish were followed. The ethical approval for the current study was given by the Research Ethics Committee of Kwara State University, Malete, Nigeria (KWASU/CRIT/REA/2016/0004).
Consent for publication
Not applicable.
Competing interests
The authors declared that they do not have any competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adeniyi, O.V., Norman, A.S. & Onojobi, S. Effects of dietary supplementation of Parkia biglobosa pulp on growth performance and physiological status of Clarias gariepinus fingerlings. JoBAZ 84, 19 (2023). https://doi.org/10.1186/s41936-023-00340-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-023-00340-y