Abstract
Background
The use of plant-based formulations is an easy and economical method to control insect pests. The objectives of the present study were to evaluate ovicidal and larvicidal potential of ethanolic leaf extracts of four plants, Cyathocline purpurea, Blumea lacera, Neanotis lancifolia, and Neanotis montholonii, and their effects on gut histology of Aedes aegypti (Diptera: Culicidae) larvae. We identified the phytochemicals present in the ethanolic extracts of these plants by LC-MS analysis. The eggs and larvae of A. aegypti were exposed to four concentrations (0.05, 0.1, 0.2, and 0.3 mg/mL) of crude ethanol extracts for different time durations. We observed egg hatching for 48 h, mortality, and gut histology of the 3rd instar larvae after 24, 48, and 72 h of treatments.
Results
LC-MS analysis revealed the presence of various active compounds such as alkaloids, tannins, saponins, and flavonoids in the ethanol extracts of all these plants. The extracts of all plants showed ovicidal activity. The extracts of C. purpurea showed the highest larvicidal activity (LC50 at 72 h = 0.088 mg/mL) followed by B. lacera (LC50 at 72 h = 0.758 mg/mL) while N. lancifolia and N. montholonii extracts did not show significant larvicidal activity. All plant extracts affected gut morphology in concentration and treatment duration-dependent manner. The plant extracts used in the present study have ovicidal properties and affected the gut histology of A. aegypti larvae.
Conclusion
These results suggest that C. purpurea has the highest larvicidal activity against A. aegypti larvae and can be further evaluated for application purposes.
Similar content being viewed by others
Background
Mosquitoes are ecologically and economically important insects as they are the vectors of many life-threatening diseases. Aedes aegypti (Diptera: Culicidae) is a vector of many viral diseases such as dengue, chikungunya, Zika, and yellow fever which are the reason for millions of deaths worldwide and serious health problems (Christophers, 1960; Chapman, 2012; World Health Organization, 2017; Bonica et al., 2019; Kraemer et al., 2019). Despite their economic importance, there are no proper treatments or preventive measures available for these viral diseases. The vectors of these diseases have been major targets for disease eradicating programs (Kumar, Singh, Tomar, and Baijal, 2010). Due to their occurrence in limited space (small pools and puddles), embryonic and larval stages of mosquitoes have been a prime target for the researchers seeking drugs to control mosquito populations (Ignacimuthu and David, 2009; Becker et al., 2010; Day, 2016). Many synthetic pesticides including pyrethroids, carbamates, and organophosphates are available in the market for mosquito control. But, most of them are pollutants, detrimental to the ecosystem, and harmful to non-target animals (Ignacimuthu and David, 2009; Rhind, 2009; Benelli, 2015). Moreover, their repeated use has developed resistance in target animals (Després, David, and Gallet, 2007; Ignacimuthu and David, 2009). For example, larvae of Culex quinquefasciatus and A. aegypti developed resistance when exposed to widely used mosquito controlling chemicals permethrin and temephos, respectively (Muthusamy and Shivakumar, 2015; Ramkumar and Shivakumar, 2015). Therefore, there is a need of screening of novel drugs or combination of drugs to control mosquito populations.
Besides synthetic pesticides, there are several other biological controlling methods including the use of fungal pathogen, predators, traps, and plant-based drugs which are being practiced (Ignacimuthu and David, 2009; Benelli, 2015). Among biological methods used to control mosquitoes, plant-based pesticides are popular due to their low economic cost, easy availability, and ecofriendly nature. Plants of many species contain diverse phytochemicals which can be used to develop drugs against disease-causing insects (Pascual-Villalobos and Robledo, 1998; Benelli, 2015). In fact, chemicals identified in the plant extracts have been reported for their activity against various medically important insects (Rao, Chattopadhyay, and Reddy, 1990; Wachira et al., 2014). Phytochemicals are of diverse chemical nature, and therefore, their application can be the promising opportunity for controlling various insect-borne diseases (Traboulsi et al., 2005; Zhu and Tian, 2011; Wachira et al., 2014). Previously, solvent preparations of the plants or phytochemicals isolated have been reported to have ovicidal or larvicidal activities in several mosquito species (Gupta, Deshpande, Tare, and Sabharwal, 2011; Subramaniam, Kovendan, Kumar, Murugan, and Walton, 2012). In addition, many plant-based extracts/compounds induce changes in morphology, physiology, biochemical processes, and behavior of different life stages of mosquitoes suggesting their importance in controlling mosquito population (Al-Mehmadi and Al-Khalaf, 2010; Gupta et al., 2011; Lija-Escaline et al., 2015; Yu, Wong, Ahmad, and Jantan, 2015).
The aim of the present study is to screen the plants Cyathocline purpurea (Buch.-Ham. ex D.Don) Kuntze (family Compositae), Blumea lacera (Burm.f.) DC. (family Compositae), Neanotis montholonii (Hook.f.) W.H. Lewis (family Rubiaceae), and Neanotis lancifolia (Hook.f.) W.H. Lewis (family Rubiaceae) for their properties to control different stages of dengue vector A. aegypti (Fig. 1). All these plants are common weeds, widely distributed in South Asia, belonging to medicinally important families, and some of them are also known for medicinal properties. C. purpurea is a common weed having importance in traditional medicine. This plant is having various phytochemicals known for medicinal properties including antimicrobial, anticancer, and hypotensive properties (Zu-Qiang, Guo-Yi, Lei, and Xi-Tai, 2006; Ma, Chong, Li, Cheung, and Tattersall, 2009; Joshi, 2013). B. lacera is also a medicinally important plant and shows insecticidal activity (Roy et al., 2005; Hasan, Rahman, Guo, and Hirashima, 2015). The plants N. lancifolia and N. montholonii are native weeds belonging to the family reported to have plant species with medicinal properties, active metabolites, and unpleasant smell (Guerrero-Analco et al., 2007; Karou, Tchacondo, Ilboudo, and Simpore, 2011; Martins and Nunez, 2015). In addition, there are no reports on the anti-mosquito properties of these plants. The objective of the present study was to assess the activity of ethanolic leaf extract of these plants against different life stages of A. aegypti. In the present study, we prepared ethanol extracts of the leaves of these plants and identified phytochemicals present in it using LC-MS. We then studied ovicidal and larvicidal activities of these extracts on A. aegypti. We also studied the effect of these leaf extracts on the gut morphology of A. aegypti larvae.
Flowering stages of the four weeds used in the present study. A Cyathocline purpurea, voucher number - Bot/DNM/51/2020. B Blumea lacera , voucher number - Bot/DNM/52/2020. C Neanotis montholonii, voucher number - BSI/WRC/IDEN.CER./2016/800. D N.lancifolia, voucher number - BSI/WRC/IDEN.CER./2016/800
Methods
Plant material and extract preparation
Plants were collected from the northern Western Ghats during flowering stages (August–September 2018). Plants samples of C. purpurea, B. lacera, N. lancifolia, and N. montholonii were collected from Bhimashankar (19° 4′ 19.09″ N, 73° 32′ 8.5″ E), Savitribai Phule Pune University campus (18° 32′ 53.9″ N, 73° 49′ 28.9″ E), Bamnoli (17° 50′ 48.4″ N, 73° 52′ 49.7″ E) and Vani, Nashik (19° 59′ 50.8344″ N, 73° 47′ 23.2908″ E), respectively. The identity of the plants was confirmed from the expert authorities in Botanical Survey of India (BSI), Western Circle, Pune, Maharashtra, and Department of Botany, Savitribai Phule Pune University, Pune (sweetgum.nybg.org; Fig. 1). The plant materials were brought to the laboratory and cleaned with distilled water. Leaves were separated from the plants and spread on a filter paper for shade drying at room temperature 27 ± 2 °C and humidity 75–80%. Dried leaves were grounded into fine powder using Wiley mill. The powder obtained was passed through 2 mm sieve. We mixed 100 g sieved powder in 1000 mL ethanol and kept in a shaker overnight at 25 °C and 60 rpm. The sample was then filtered using Whatman filter paper no. 1. The solvent from the filtrate was removed under reduced pressure on a rotary evaporator at 35–40 °C. Then, the extract was dissolved in distilled water to make 1 g per 100 mL stock solution. The required amount of stock solution was dissolved further in tap water to make desired concentrations.
LC-MS analysis and identification of compounds
For preliminary screening of phytochemicals, ethanolic leaf extracts of all four plants were subjected to liquid chromatography coupled with mass spectrometry (LC-MS). LC-MS analysis was done by injecting 10 μL filtered samples on a Dionex system composed of P680 pump and ASI-100 autosampler and UVD170U UV detector. This was coupled to a Thermo Finnigan Surveyor MSQ mass spectral detector. Separation was performed on a Doinex Acclaim 120 C18 columns (5 mm, 4.6 × 150 mm) using gradient elution. Compounds were eluted at a 0.7 mL/min flow rate for three minutes at 10% B, a linear gradient to 90% B over 40 min, and held at 90% B for 8 min. UV detection was recorded at 254, 280, and 310 nm. Ionization for MS analysis was performed in both positive and negative ion mode using electro-spray ionization with a nitrogen flow at 80 psi, a cone voltage of 70 V, needle voltage of 3 Kv, and cone temperature of 600 °C. Mass data were collected over the range of the gradient program at a rate of one scan per second.
Culture of Aedes aegypti
A mother culture of A. aegypti was obtained from Ross Life Sciences Pvt. Ltd., Pune, India, and maintained in a laboratory following established guidelines (Das, Garver, and Dimopoulos, 2007; World Health Organization, 2005). The larvae were maintained in steel trays (35 × 26 × 6 cm) with 600 mL de-chlorinated tap water from a public water supply at 27 ± 2 °C, relative humidity 75–85% under 12:12 h light and dark condition. A pinch of finely powdered fish food was provided daily as food for larvae. Adult mosquito colonies were maintained in rearing cages (60 cm × 60 cm × 60 cm) with 50 μm mesh size. Adult mosquitoes were maintained on 10% sugar solution, and female mosquitoes were fed on chicken for blood meal once in a week at least for 6 h. After 72 h of blood feeding, female mosquitoes were allowed to lay eggs in a container (height 6 cm, base diameter 9.5 cm). Fresh dried egg stripes were used for ovicidal assays. Third instar larvae were used for larvicidal assays and histopathological study. Ovicidal, larvicidal assays and histopathological study were carried out in the Department of Zoology, SPPU.
Ovicidal activity
Ovicidal activity of leaf extracts was studied following the modified method described previously (Su and Mulla, 1998). The whole egg strips were kept in the water containing desired concentrations (0.05, 0.1, 0.2, and 0.3 mg/mL) of each plant extracts. The assay was carried out in four replicates for each concentration. Ovicidal activity was carried out in plastic containers (height 6 cm, base diameter 9.5 cm with water level 2.5 cm). Hatching success in each replicate of each concentration was monitored up to 48 h. After 48 h, hatched and unhatched eggs were counted under Leica stereozoom microscope (Leica-MZ75). We combined the hatching data of all four replicates to calculate the total number of hatched and unhatched eggs from each concentration. The ovicidal activity of each concentration was expressed in percent mortality (number of unhatched eggs).
Larvicidal activity
Larval susceptibility tests were carried out by following the protocol recommended by WHO (World Health Organization, 2005). Based on preliminary tests, we treated third instar larvae with 0.05, 0.1, 0.2, and 0.3 mg/mL concentrations in triplicate (25 larvae in each replicate). Untreated larvae were considered as a control. The larvae were reared in 500 mL beakers with 250 mL water containing desired concentrations of extracts. We recorded mortality of larvae treated with all concentrations at 24, 48, and 72 h. Larvae were considered dead when they stopped activity for long period. We confirmed the death of the larvae using a spatula as a probe. If the larva does not move even after gently probing, it was considered as dead. We combined the data from all the replicate of each concentration and calculated LC50 and LC90. LC50 and LC90 values were calculated for each concentration at 24, 48, and 72 h using Probit analysis in SPSS 20.
Histopathological study
To study the effects of leaf extracts on gut morphology of A. aegypti larvae, third instar larvae were treated with 0.1 and 0.3 mg/mL (N. montholonii N. lancifolia, and B. lacera) or 0.05 and 0.2 mg/mL (C. purpurea) concentrations using the same protocol described above. Six live larvae from each treatment were sampled at 24 h, 48 h, and 72 h and fixed in Bouin’s fluid for 24 h. Then, they were passed through the series of ascending grades of ethanol (30, 50, 70, 90, and 100%) for dehydration, passed through xylene, and embedded in paraffin wax at 58–60 °C (MERCK). Tissues (anterior part of the mid-gut of larvae) were sectioned at 7 μm using Leica RM 223 rotary microtome and stained with hematoxylin and eosin. Stained sections were observed and photographed under a microscope (Leica-DF450) to analyze the changes in the gut morphology.
Results
LC-MS analysis and identification of compounds
Preliminary analysis revealed the presence of phytochemicals of diverse chemical nature in the leaf extracts of all plants (Supplementary data). The major constituents in the extracts of C. purpurea, B. lacera, N. lancifolia, and N. montholonii were alkaloids, flavonoids, phenols, saponins, tannins, etc. Some of the major phytochemicals identified in these plant extracts are mytiloxanthin (6.023%), 1,2-dipentadecanoyl-sn-glycero-3-phospho-(1′-sn-glycerol) (6.457%), picrotoxinin (6.457%), 3-n-decyl acrylic acid (11.066%), C16 sphinganine (9.74%), cosmosiin (7.18%), 1,4,5,8-tetrahydroxy-2,6-dimethylanthroquinone (7.010%), phytosphingosine (11.14%), (6RS)-6,19-epidioxy-24,24 difluoro-25-hydroxy-6,19-dihydrovitamin D3 (12%), 1-dodecanoyl-2-octadecanoyl-glycero-3-phospho-(1′-sn-glycerol) (11.87%), Fipexide (7.18%), and 8-hydroxymianserin (13.60%) (Supplementary data).
Ovicidal activity
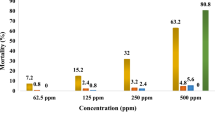
Results showed that the leaf extracts of all plants have ovicidal activity. Extract of all the plants caused 70–90% mortality at higher concentrations (Fig. 2).
Larvicidal activity
Among four leaf extracts used in the present study, C. purpurea had the highest larvicidal activity. LC50 values for C. purpurea extract at 24, 48, and 72 h were 0.72, 0.18, and 0.088 mg/mL while LC50 values for B. lacera leaf extract were 7.349, 2.457, and 0.758 mg/mL, respectively (Table 1). The leaf extracts of N. montholonii and N. lancifolia did not show significant larvicidal activities (Table 1). The larvae from the control group and the groups treated with different concentrations of N. montholonii and N. lancifolia started pupation generally after 48 h.
Histopathological study
All plant extract treatments showed a similar pattern of larval gut damage. Therefore, we categorized the deterioration of midgut epithelium/cells integrity into four stages/groups based on the damage severity (Fig. 3 and Table 2). In the first stage (control), gut epithelial cells (cuboidal/flattened cells) with round nucleus and clear cytoplasm were observed (Fig. 3A). In the midgut of stage 2, swollen cells or vesicles of different sizes were visualized (Fig. 3B). The third stage consists of a midgut with lost cell integrity, damaged nucleus and cell membrane, cells detached from basal lamina, and loss of nuclear and cytoplasmic materials (Fig. 3C). In the fourth stage, complete damage of mid-gut epithelium/cells could be seen. It included the cells detached from basal lamina as well as loss of cell-cell contact and cellular integrity (Fig. 3D).
Cross-sections of A. aegypti larvae midgut depicting different stages of deterioration after weed extract treatment. A Normal midgut or control (stage 1 = −) with intact flattened cells, their dark nucleus, and clear cytoplasm. B Swelling of cells cytoplasm or vesicle formation (stage 2 = +). Arrow indicates swelling of cells. C Loss of cell-cell contact, damage of cell membrane, and distortion of cell and nucleus integrity (stage 3 = ++). Arrow indicates cell membrane/contact damage. D Complete damage of epithelium, disorganized cells, loss of cell-cell contact, and detachment of cells and basal lamina (stage 4 = +++). Note the cell and basil lamina detachment. Arrow indicates loss of cell integrity (L = intestinal lumen, IE = intestinal epithelium, BL = basal lamina; N = nucleus; DN = cell with damages nucleus)
All the four leaf extracts used in the study affected gut morphology in treatment duration and concentration-dependent manner. Among all the plant extracts used, C. purpurea leaf extract induced severe gut damage followed by N. montholonii, N. lancifolia, and B. lacera (Table 2; Fig. 3).
Discussion
The discovery of pharmaceutical drugs is the first step towards the control of parasitic vector insects. Plant-based formulations provide an easy and economical method to screen novel paste controlling drugs. Moreover, mosquito eggs and larvae are the ideal systems for screening novel insecticides present in the plant-based formulations (Rawani, Haldar, Ghosh, and Chandra, 2009; Subramaniam et al., 2012; Benelli et al., 2017). In the present study, we used ethanolic leaf extracts of four common weeds and showed their stage and concentration-dependent effects on A. aegypti.
The results revealed that the plant extracts used in the present study contain diverse phytochemicals. Many of the compounds having similar chemical nature to these phytochemicals have been reported to be present in other plant extracts and found to have activity against various disease-causing insects (Lee, 2000; Senthilkumar, Kannathasan, and Venkatesalu, 2008; Rawani et al., 2009; Adhikari, Singha, and Chandra, 2012). The results also showed that the leaf extracts of C. purpurea and B. lacera have the highest ovicidal and larvicidal activities among the extracts used in the present study whereas all plant extracts affected gut morphology at different concentrations. C. purpurea and B. lacera are known to have medicinal properties such as anti-cancer, anti-inflammatory, antibacterial, hypoglycemic, analgesic, anti-arthritic, cytotoxic, and antiviral (Chiang, Cheng, Chen, and Lin, 2004; Ma et al., 2009; Joshi, 2013; Bihani, Rojatkar, and Bodhankar, 2014; Hasan et al., 2015; Tambewagh et al., 2017). Ethanolic leaf extracts of these plants possess various types of phytochemicals including essential oils, terpenoid, alkaloids, phenols, flavonoids, anthraquinones, cardiac glycosides, cyanogenetic compound, steroids, and terpenes. Similar to these phytochemicals, compounds present in the other plant extracts have been reported for their larvicidal activities in insects (Liu, Liu, Du, and Deng, 2012; Gautam, Kumar, and Poonia, 2013; Govindarajan, Sivakumar, Rajeswary, and Veerakumar, 2013; Benelli et al., 2017). Previous studies reported the presence of various phytochemicals in the essential oils obtained from hydrodistillation of C. purpurea (Joshi, 2013). In addition, we first time identified the phytochemicals present in B. lacera, N. montholoni, and N. lancifolia. Further studies need to direct the focus on the isolation, characterization, and insecticidal properties of the phytochemicals present in these plant extracts and evaluate their insecticidal activities and effects on non-target animals as well.
In the present study, we observed considerable differences in the ovicidal and larvicidal activities of the leaf extracts of these plants. For instance, C. purpurea and B. lacera show high larvicidal activity while N. montholonii and N. lancifolia did not show significant larvicidal activity. Among these plants, B. lacera extract reported to have insect repellent and toxic properties. Petroleum ether extract of B. lacera is a strong repellent and toxicant for Lesser Grain Borer and Rice Weevil (Roy et al., 2005). The plants C. purpurea, N. montholonii, and N. lancifolia have never been explored for insecticidal properties although they have medicinal properties or belong to the families known for the plants with medicinal properties or unpleasant smell. Surprisingly, plant extracts of N. montholonii and N. lancifolia did not show significant larvicidal activity but affected hatching and gut morphology of A. aegypti larvae. Similarly, previous studies reported that the plant extracts or compounds isolated from the plants have stage-dependent effects in mosquitoes (Autran et al., 2009; Gupta et al., 2011). For example, a-amylase inhibitor isolated from Macrotyloma uniflorum possesses larvicidal activity but not ovicidal activity (Gupta et al., 2011). The differences in the activity patterns of the plant extracts in mosquitoes can be partially attributed to the chemical composition of the extracts and the chemical nature of the compounds (AhbiRami, Zuharah, Thiagaletchumi, Subramaniam, and Sundarasekar, 2014; Porto et al., 2017). Plant extracts (combination of chemicals) may have a variety of effects and show solvent-dependent larvicidal or ovicidal activities (Traboulsi et al., 2005; Talontsi, Matasyoh, Ngoumfo, and Chepkorir, 2011; Zhu and Tian, 2011; Chapman, 2012; Lija-Escaline et al., 2015). For instance, acetone extract of Ipomoea carica showed high mortality as compared to those of the methanol extract (AhbiRami et al., 2014). Phytochemicals are also known to affect different systems insects including physiology, behavior, and morphology (Broadway and Duffey, 1988; Lija-Escaline et al., 2015; Procópio et al., 2015; Yu et al., 2015). Gut histology of mosquito larvae has been one of the most promising methods to evaluate insecticidal effects of plant extracts (Al-Mehmadi and Al-Khalaf, 2010; Lija-Escaline et al., 2015; Procópio et al., 2015). In the present study, all plant extracts affected gut morphology of A. aegypti larvae. Higher concentrations of N. lancifolia and N. montholonii affected gut morphology while low concentrations of B. lacera and C. purpurea were sufficient to induce gut damage. The susceptibility of A. aegypti larvae gut to different plant extracts has been reported in some previous studies. The extracts of some plants affect gut morphology at high concentrations while some plant extracts show their effects at very low concentrations (Lija-Escaline et al., 2015; Procópio et al., 2015; Yu et al., 2015). The concentrations of green seaweed extracts used in the previous study (Yu et al., 2015) were within the range of the concentration used in the present study.
Aqueous preparations of the pesticides can be made available easily and applied for the target animals in the water bodies. Since mosquitoes breed and their embryonic and larval stages occur in pools and puddles, the application of phytochemicals for their control is manageable (Becker et al., 2010; World Health Organization, 2017). Many studies have reported the larvicidal activities of plant extracts, and their direct applications seem to be promising (Ghosh, Chowdhury, and Chandra, 2012; Vivekanandhan, Senthil-Nathan, and Shivakumar, 2018a; Vivekanandhan, Usha-Raja-Nanthini, Valli, and Subramaniam Shivakumar, 2018b). The present study reports the effects of some weed extracts on different life aspects of A. aegypti and demands further application-oriented studies considering less harm to non-target species. Additionally, the carryover effects of sub-lethal concentrations of plant extracts (N. montholoni and N. lancifolia) on pupae and adult mosquitos need to be further evaluated. The plants used in the present study can be a valuable source of various medicinally important phytochemicals as these plants are widely distributed as common weeds (Almeida, 1996; Singh, Lakshminarasimhan, Karthieyan, and Prasanna, 2001).
Conclusions
The leaf extracts of all the studied plants have ovicidal activity against A. aegypti. The leaf extracts of the weeds C. purpurea and B. lacera have lervicidal properties and induce gut damage. The leaf extracts of N. montholonii and N. lancifolia affected gut morphology although they do not have larvicidal activities. All four extracts affected gut morphology of A. aegypti larvae in a concentration-dependent manner and their severity increased with treatment duration. The weeds C. purpurea and B. lacera can be further evaluated for immediate application purposes.
Availability of data and materials
The data supporting the conclusions are included in the article. Additional supporting data is with the corresponding author and will be made available on request.
References
Adhikari, U., Singha, S., & Chandra, G. (2012). In vitro repellent and larvicidal efficacy of Swietenia mahagoni against the larval forms of Culex quinquefasciatus Say. Asian Pacific Journal of Tropical Biomedicine, 2(1), S260–S264. https://doi.org/10.1016/S2221-1691(12)60171-3.
AhbiRami, R., Zuharah, W. F., Thiagaletchumi, M., Subramaniam, S., & Sundarasekar, J. (2014). Larvicidal efficacy of different plant parts of railway creeper, Ipomoea cairica extract against dengue vector mosquitoes, Aedes albopictus (Diptera: Culicidae) and Aedes aegypti (Diptera: Culicidae). Journal of Insect Science, 14(1), 180–186.
Al-Mehmadi, R. M., & Al-Khalaf, A. A. (2010). Larvicidal and histological effects of Melia azedarach extract on Culex quinquefasciatus Say larvae (Diptera: Culicidae). Journal of King Saud University - Sci, 22(2), 77–85. https://doi.org/10.1016/j.jksus.2010.02.004.
Almeida, M. (1996). Flora of Maharashtra. Mumbai: Blatter Herbarium, St. Xavier’s College.
Autran, E. S., Neves, I. A., da Silva, C. S. B., Santos, G. K. N., da Câmara, C. A. G., & Navarro, D. M. A. F. (2009). Chemical composition, oviposition deterrent and larvicidal activities against Aedes aegypti of essential oils from Piper marginatum Jacq. (Piperaceae). Bioresource Technology, 100(7), 2284–2288. https://doi.org/10.1016/j.biortech.2008.10.055.
Becker, N., Petric, D., Zgomba, M., Boase, C., Dahl, C., Madon, M., & Kaiser, A. (2010). Mosquitoes and their control. Heidelberg, Dordrecht, London, New York: Springer. https://doi.org/10.1007/978-3-540-92874-4.
Benelli, G. (2015). Research in mosquito control: current challenges for a brighter future. Parasitology Research, 114(8), 2801–2805. https://doi.org/10.1007/s00436-015-4586-9.
Benelli, G., Govindarajan, M., Rajeswary, M., Senthilmurugan, S., Vijayan, P., Alharbi, N. S., … Khaled, J. M. (2017). Larvicidal activity of Blumea eriantha essential oil and its components against six mosquito species, including Zika virus vectors: the promising potential of (4E,6Z)-allo-ocimene, carvotanacetone and dodecyl acetate. Parasitology Research, 116(4), 1175–1188. https://doi.org/10.1007/s00436-017-5395-0.
Bihani, G. V., Rojatkar, S. R., & Bodhankar, S. L. (2014). Anti-arthritic activity of methanol extract of Cyathocline purpurea (whole plant) in Freund’s complete adjuvant-induced arthritis in rats. Biomedicine and Aging Pathology, 4(3), 197–206. https://doi.org/10.1016/j.biomag.2014.04.007.
Bonica, M. B., Goenaga, S., Martin, M. L., Feroci, M., Luppo, V., Muttis, E., … Levis, S. (2019). Vector competence of Aedes aegypti for different strains of Zika virus in Argentina. PLoS Neglected Tropical Diseases, 13(6), e0007433. https://doi.org/10.1371/journal.pntd.0007433.
Broadway, R. M., & Duffey, S. S. (1988). The effect of plant protein quality on insect digestive physiology and the toxicity of plant proteinase inhibitors. Journal of Insect Physiology, 34(12), 1111–1117. https://doi.org/10.1016/0022-1910(88)90213-2.
Chapman, R. F. (2012). The insects structure and function, (5th ed., ). Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9781139035460.
Chiang, L.-C., Cheng, H.-Y., Chen, C.-C., & Lin, C.-C. (2004). In vitro anti-leukemic and antiviral activities of traditionally used medicinal plants in Taiwan. The American Journal of Chinese Medicine, 32(5), 695–704. https://doi.org/10.1142/S0192415X04002284.
Christophers, S. R. (1960). Aedes aegypti (L.) The yellow fever mosquito: its life history, bionomics bionomics and structure. New York: Cambridge University Press.
Das, S., Garver, L., & Dimopoulos, G. (2007). Protocol for mosquito rearing (A. gambiae). Journal of Visualized Experiments, 5(5), 221. https://doi.org/10.3791/221.
Day, J. (2016). Mosquito oviposition behavior and vector control. Insects, 7(4), 65. https://doi.org/10.3390/insects7040065.
Després, L., David, J. P., & Gallet, C. (2007). The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecology & Evolution, 22(6), 298–307. https://doi.org/10.1016/j.tree.2007.02.010.
Gautam, K., Kumar, P., & Poonia, S. (2013). Larvicidal activity and GC-MS analysis of flavonoids of Vitex negundo and Andrographis paniculata against two vector mosquitoes Anopheles stephensi and Aedes aegypti. Journal of Vector Borne Diseases, 50(3), 171–178.
Ghosh, A., Chowdhury, N., & Chandra, G. (2012). Plant extracts as potential mosquito larvicides. Indian Journal of Medical Research, 135(5), 581–598.
Govindarajan, M., Sivakumar, R., Rajeswary, M., & Veerakumar, K. (2013). Mosquito larvicidal activity of thymol from essential oil of Coleus aromaticus Benth. against Culex tritaeniorhynchus, Aedes albopictus, and Anopheles subpictus (Diptera: Culicidae). Parasitology Research, 112(11), 3713–3721. https://doi.org/10.1007/s00436-013-3557-2.
Guerrero-Analco, J., Medina-Campos, O., Brindis, F., Bye, R., Pedraza-Chaverri, J., Navarrete, A., & Mata, R. (2007). Antidiabetic properties of selected Mexican copalchis of the Rubiaceae family. Phytochemistry, 68(15), 2087–2095. https://doi.org/10.1016/j.phytochem.2007.05.006.
Gupta, L., Deshpande, S., Tare, V., & Sabharwal, S. (2011). Larvicidal activity of the α-amylase inhibitor from the seeds of Macrotyloma uniflorum (Leguminosae) against Aedes aegypti (Diptera: Culicidae). International Journal of Tropical Insect Science, 31(1-2), 69–74. https://doi.org/10.1017/S1742758411000087.
Hasan, M. N., Rahman, M. H., Guo, R., & Hirashima, A. (2015). Hypoglycemic activity of methanolic leaf extract of Blumea lacera in Swiss-albino mice. Asian Pacific Journal of Tropical Disease, 5(3), 195–198. https://doi.org/10.1016/S2222-1808(14)60652-6.
Ignacimuthu, S., & David, B. (2009). Ecofriendly insect pest management. Delhi: Elite Publishing House.
Joshi, R. K. (2013). Chemical constituents and antibacterial property of the essential oil of the roots of Cyathocline purpurea. Journal of Ethnopharmacology, 145(2), 621–625. https://doi.org/10.1016/j.jep.2012.11.045.
Karou, S. D., Tchacondo, T., Ilboudo, D. P., & Simpore, J. (2011). Sub-Saharan Rubiaceae: a review of their traditional uses, phytochemistry and biological activities. Pakistan Journal of Biological Sciences, 14(3), 149–169. https://doi.org/10.3923/pjbs.2011.149.169.
Kraemer, M. U., Reiner, R. C., Brady, O. J., Messina, J. P., Gilbert, M., Pigott, D. M., … Marczak, L. B. (2019). Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nature Microbiology, 4(5), 854–863. https://doi.org/10.1038/s41564-019-0376-y.
Kumar, K., Singh, P. K., Tomar, J., & Baijal, S. (2010). Dengue: epidemiology, prevention and pressing need for vaccine development. Asian Pacific Journal of Tropical Medicine, 3(12), 997–1000. https://doi.org/10.1016/S1995-7645(11)60017-5.
Lee, S. E. (2000). Mosquito larvicidal activity of pipernonaline, a piperidine alkaloid derived from long pepper, Piper longum. Journal of the American Mosquito Control Association, 16(3), 245–247.
Lija-Escaline, J., Senthil-Nathan, S., Thanigaivel, A., Pradeepa, V., Vasantha-Srinivasan, P., Ponsankar, A., … Abdel-Megeed, A. (2015). Physiological and biochemical effects of botanical extract from Piper nigrum Linn (Piperaceae) against the dengue vector Aedes aegypti Liston (Diptera: Culicidae). Parasitology Research, 114(11), 4239–4249. https://doi.org/10.1007/s00436-015-4662-1.
Liu, Z. L., Liu, Q. Z., Du, S. S., & Deng, Z. W. (2012). Mosquito larvicidal activity of alkaloids and limonoids derived from Evodia rutaecarpa unripe fruits against Aedes albopictus (Diptera: Culicidae). Parasitology Research, 111(3), 991–996. https://doi.org/10.1007/s00436-012-2923-9.
Ma, G., Chong, L., Li, Z., Cheung, A. H., & Tattersall, M. H. (2009). Anticancer activities of sesquiterpene lactones from Cyathocline purpurea in vitro. Cancer Chemotherapy and Pharmacology, 64(1), 143–152. https://doi.org/10.1007/s00280-008-0863-y.
Martins, D., & Nunez, C. V. (2015). Secondary metabolites from Rubiaceae species. Molecules, 20(7), 13422–13495. https://doi.org/10.3390/molecules200713422.
Muthusamy, R., & Shivakumar, M. S. (2015). Susceptibility status of Aedes aegypti (L.) (Diptera: Culicidae) to temephos from three districts of Tamil Nadu, India. Journal of Vector Borne Diseases, 52(2), 159–165.
Pascual-Villalobos, M. J., & Robledo, A. (1998). Screening for anti-insect activity in Mediterranean plants. Industrial Crops and Products, 8(3), 183–194. https://doi.org/10.1016/S0926-6690(98)00002-8.
Porto, K. R. D. A., Motti, P. R., Yano, M., Roel, A. R., Cardoso, C. A. L., & Matias, R. (2017). Screening of plant extracts and fractions on Aedes aegypti larvae found in the state of Mato Grosso do Sul (linnaeus, 1762) (culicidae). Annals of the Brazilian Academy of Sciences, 89(2), 895–906. https://doi.org/10.1590/0001-3765201720150017.
Procópio, T., Fernandes, K., Pontual, E., Matos Ximenes, R., Rafaella Cardoso de Oliveira, A., de Santana Souza, C., … Henrique Napoleão, T. (2015). Schinus terebinthifolius leaf extract causes midgut damage, interfering with survival and development of Aedes aegypti Larvae. PLoS One, 10(5), e0126612. https://doi.org/10.1371/journal.pone.0126612.
Ramkumar, G., & Shivakumar, M. S. (2015). Laboratory development of permethrin resistance and cross-resistance pattern of Culex quinquefasciatus to other insecticides. Parasitology Research, 114(7), 2553–2560. https://doi.org/10.1007/s00436-015-4459-2.
Rao, K. V., Chattopadhyay, S. K., & Reddy, G. C. (1990). Flavonoids with mosquito larval toxicity. Journal of Agricultural and Food Chemistry, 38(6), 1427–1430. https://doi.org/10.1021/jf00096a028.
Rawani, A., Haldar, K. M., Ghosh, A., & Chandra, G. (2009). Larvicidal activities of three plants against filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). Parasitology Research, 105(5), 1411–1417. https://doi.org/10.1007/s00436-009-1573-z.
Rhind, S. M. (2009). Anthropogenic pollutants: a threat to ecosystem sustainability? Philosophical Transactions of the Royal Society, B: Biological Sciences, 364(1534), 3391–3401. https://doi.org/10.1098/rstb.2009.0122.
Roy, B., Amin, R., Uddin, M., Islam, A. T. M. S., Islam, M. J., & Halder, B. (2005). Leaf extracts of shiyalmutra (blumea lacera DC.) as botanical insecticides against lesser grain borer and rice weevil. Journal of Biological Sciences, 5(2), 201–204.
Senthilkumar, A., Kannathasan, K., & Venkatesalu, V. (2008). Chemical constituents and larvicidal property of the essential oil of Blumea mollis (D. Don) Merr. against Culex quinquefasciatus. Parasitology Research, 103(4), 959–962. https://doi.org/10.1007/s00436-008-1085-2.
Singh, P., Lakshminarasimhan, P., Karthieyan, S., & Prasanna, P. (2001). Flora of Maharashtra. Calcutta: The Director, Botanical survey of India.
Su, T., & Mulla, M. S. (1998). Ovicidal activity of neem products (azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). Journal of the American Mosquito Control Association, 14(2), 204–209.
Subramaniam, J., Kovendan, K., Kumar, P. M., Murugan, K., & Walton, W. (2012). Mosquito larvicidal activity of Aloe vera (Family: Liliaceae) leaf extract and Bacillus sphaericus, against Chikungunya vector, Aedes aegypti. Saudi Journal of Biological Sciences, 19(4), 503–509. https://doi.org/10.1016/j.sjbs.2012.07.003.
Talontsi, F. M., Matasyoh, J. C., Ngoumfo, R. M., & Chepkorir, R. (2011). Mosquito larvicidal activity of alkaloids from Zanthoxylum lemairei against the malaria vector Anopheles gambiae. Pesticide Biochemistry and Physiology, 99(1), 82–85. https://doi.org/10.1016/j.pestbp.2010.11.003.
Tambewagh, U. U., Kandhare, A. D., Honmore, V. S., Kadam, P. P., Khedkar, V. M., Bodhankar, S. L., & Rojatkar, S. R. (2017). Anti-inflammatory and antioxidant potential of Guaianolide isolated from Cyathocline purpurea: Role of COX-2 inhibition. International Immunopharmacology, 52, 110–118. https://doi.org/10.1016/j.intimp.2017.09.001.
Traboulsi, A. F., El-Haj, S., Tueni, M., Taoubi, K., Nader, N. A., & Mrad, A. (2005). Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Management Science, 61(6), 597–604. https://doi.org/10.1002/ps.1017.
Vivekanandhan, P., Senthil-Nathan, S., & Shivakumar, M. S. (2018a). Larvicidal, pupicidal and adult smoke toxic effects of Acanthospermum hispidum (DC) leaf crude extracts against mosquito vectors. Physiological and Molecular Plant Pathology, 101, 156–162. https://doi.org/10.1016/j.pmpp.2017.05.005.
Vivekanandhan, P., Usha-Raja-Nanthini, A., Valli, G., & Subramanian Shivakumar, M. (2018b). Comparative efficacy of Eucalyptus globulus (Labill) hydrodistilled essential oil and temephos as mosquito larvicide. Natural Product Research, 34(8), 1–4.
Wachira, S. W., Omar, S., Jacob, J. W., Wahome, M., Alborn, H. T., Spring, D. R., … Torto, B. (2014). Toxicity of six plant extracts and two pyridone alkaloids from Ricinus communis against the malaria vector Anopheles gambiae. Parasites & Vectors, 7(1), 312. https://doi.org/10.1186/1756-3305-7-312.
World Health Organization (2005). Guidelines for laboratory and field testing of mosquito larvicides.
World Health Organization (2017). Dengue vaccine: WHO position paper, July 2016 – recommendations. Vaccine, 35(9), 1200–1201. https://doi.org/10.1016/j.vaccine.2016.10.070.
Yu, K. X., Wong, C. L., Ahmad, R., & Jantan, I. (2015). Larvicidal activity, inhibition effect on development, histopathological alteration and morphological aberration induced by seaweed extracts in Aedes aegypti (Diptera: Culicidae). Asian Pacific Journal of Tropical Medicine, 8(12), 1006–1012. https://doi.org/10.1016/j.apjtm.2015.11.011.
Zhu, L., & Tian, Y. (2011). Chemical composition and larvicidal effects of essential oil of Blumea martiniana against Anopheles anthropophagus. Asian Pacific Journal of Tropical Medicine, 4(5), 371–374. https://doi.org/10.1016/S1995-7645(11)60106-5.
Zu-Qiang, L., Guo-Yi, M., Lei, L., & Xi-Tai, Z. (2006). Sesquiterpene lactones from Cyathocline purpurea. Chemical Journal of Chinese Universities, 27(5), 859–862.
Acknowledgements
The authors are thankful to Sophisticated Analytical Instrument Facility (SAIF), Indian Institute of Technology (IIT), Bombay for LC-MS analysis and Ross Life Sciences Pvt. Ltd., Pune for providing mosquito culture. ST and RA received research fellowship from Savitribai Phule Pune University (SPPU).
Funding
The study was partially funded by the Departmental Research Development Project (DRDP) grant awarded to DM and RP and Centre for Advanced Studies (CAS) awarded to RP.
Author information
Authors and Affiliations
Contributions
Study design—ST, DM, RA, RP, and SP; experimentation—ST, RA, and SP; MS writing—SP and ST. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The experiments were carried out following the guidelines established for animal ethics by Savitribai Phule Pune University.
Consent for publication
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. LC-MS profile of the ethanol extract of C. purpurea. Table S1 Chemical compounds identified in the ethanol extracts of C. purpurea using LC-MS (*Concentration of the constituents was derived from the calibration curve of the authentic compound)). Figure S2. LC-MS profile of the ethanol extract of B. lacera. Table S2 Chemical compounds identified in the ethanol extracts of B. lacera using LC-MS (*Concentration of the constituents was derived from the calibration curve of the authentic compound). Figure S3. LC-MS profile of the ethanol extract of N. montholonii. Table S3 Chemical compounds identified in the ethanol extracts of N. montholonii using LC-MS (*Concentration of the constituents was derived from the calibration curve of the authentic compound). Figure S4. LC-MS profile of the ethanol extract of N. lancifolia. Table S4 Chemical compounds identified in the ethanol extracts of N. lancifolia using LC-MS (*Concentration of the constituents was derived from the calibration curve of the authentic compound).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torawane, S., Andhale, R., Pandit, R. et al. Screening of some weed extracts for ovicidal and larvicidal activities against dengue vector Aedes aegypti. JoBAZ 82, 36 (2021). https://doi.org/10.1186/s41936-021-00233-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-021-00233-y