Abstract
Background
Tramadol provokes oxidative stress that gives rise to apoptosis with alterations in the cellular structure and adversely influences male fertility. A specific inhibitor of nuclear factor kappa B (NF-κB), pyrrolidine dithiocarbamate (PDTC), has observable antioxidant and anti-inflammatory characteristics and enhances the improvement of organs damage caused by various agents. The impact of PDTC on testicular damage caused by tramadol has not been previously examined.
Objective
This study was designed to investigate the potential impact of pyrrolidine dithiocarbamate on testicular damage provoked by chronic tramadol usage.
Materials and methods
Forty healthy adult male albino rats were included in this study. Rats were randomly and equally divided into 4 groups: group (I), control group; group (II), pyrrolidine dithiocarbamate (PDTC) group; group (III), tramadol (Tr) group; and group (IV), Tr + PDTC group. This study measured serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), and free testosterone levels. Testicular malondialdehyde (MDA), interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) levels, and superoxide dismutase (SOD), glutathione peroxidase (GPX), and caspase-8 and caspase-3 activities were also detected. Immuno-histochemical evaluation of inducible NOS (iNOS) expression in testicular tissue along with histopathological examination of testicular tissue has been studied.
Results
Tramadol caused a significant reduction in serum FSH, LH and testosterone levels, epididymal sperm count, and motility, as well as testicular GPX and SOD activities. On the other hand, a significant elevation of testicular MDA, IL-1β, IL-6, and TNF-α levels and caspase-8 and caspase-3 activities were found. However, PDTC administration with tramadol showed significantly increased sperm production and motility and alleviated tramadol-induced disturbance in other measured parameters in the Tr + PDTC group compared to the Tr group. Moreover, co-administration of PDTC with tramadol significantly alleviated the histopathological structure of testicular tissue and the increased iNOS expressions noticed in the tramadol-treated group.
Conclusion
Considering the protective effects of PDTC against the reproductive toxicity induced by tramadol, this compound can be used as a possible protective and treating target for tramadol-induced reproductive toxicity.
Similar content being viewed by others
Background
One of the most widely used pain killers is tramadol hydrochloride that is considered centrally acting opioids with more potent pain killing effect. Regarding its pharmacokinetics, rapid absorption and rapid tissue distribution are its characteristics (Leppert and Łuczak 2005). Regarding its pharmacodynamics, as tramadol is considered a synthetic opioid, it gives action through selective agonistic effect on mu (μ) receptors with lower action through other opioid receptors: δ- and κ-. As a synthetic opioid, it produces similar side actions like other opioid drugs including hypotension, dizziness, nausea, dry mouth, constipation, delayed ejaculation, and respiratory depression (in rare cases) (Baghishani et al. 2018).
It was stated that chronic consumption of tramadol in males complaining of chronic pain resulted in decreased libido and erectile dysfunction (Ahmed and Kurkar 2014), and it was thought that reproductive toxicity associated with tramadol was associated with decreased sex hormones and sperm abnormalities in both morphology and count (Aloisi et al. 2011).
Oxidative stress and apoptosis in the spermatocytes are the recent theories explaining the mechanisms of testicular toxicity from chronic tramadol intake (Ibrahim and Salah-Eldin 2019a). On the other hand, it was reported that uncontrolled production of free radicals can act as an inflammatory effector by activating the transcription factor, nuclear factor-kappa B (NF-κB), which in turn results in the transcription of genes encoding cell adhesion molecules, nitric oxide synthase (NOS), proinflammatory cytokines (IL-1β, IL-12 and TNF), and cyclo-oxygenase-2 (Nafees et al. 2015).
Previous research found the possible protective action of antioxidants against tramadol reproductive adverse effects. For example, pre-treatment of rats with other antioxidants as selenium, garlic, curcumin (Sheweita et al. 2018), gallic acid (Abdel-Zaher et al. 2011), and/or nigella sativa oil before tramadol administration restored the inhibition in activities of antioxidant enzyme (Kopalli et al. 2022). Also, a promising role of melatonin was studied as it maintained mitochondrial functions and membrane integrity in testicular Sertoli cells (Koohsari et al. 2020).
On the other side, a specific inhibitor of NF-κB, pyrrolidine dithiocarbamate (PDTC), was a metal chelator thiol compound capable of producing intense anti-inflammatory and antioxidant actions (Mehdizadeh et al. 2017), and it was also studied that PDTC protects and mends histopathology in various organ damages caused by variable agents (Mehdizadeh et al. 2017 and Kabel 2018).
Up to our knowledge, no studies have examined the protective effect of PDTC on testicular damage caused by tramadol yet.
Therefore, this study is designed to investigate the histopathological and biochemical impacts of PDTC on testicular damage provoked by tramadol in rats. To elaborate the potential protective mechanisms, the study investigated its effect on the redox status of the testes by evaluating the levels of lipid peroxides and antioxidant enzyme activities in testes. Some inflammatory and apoptotic markers have also been assessed in testicular tissue.
Methods
Animals
This study involved 40 healthy adult male albino rats weighing 197–234 g (obtained from the animal house of Faculty of Veterinary Medicine, Zagazig University). Rats were kept in steel wired cages under hygienic conditions with water ad libitum at room temperature and on natural light/dark cycle (in the animal house in Faculty of Medicine, Zagazig University). The study protocol was approved by the ethics of the guiding standard for the use of research animals by ZU-IACUC committee, with number ZU-IACUC/3/F/119/2022.
Chemicals
Tramadol was obtained from Hikma Pharmaceutical Co. Giza, Egypt, in the form of tramadol hydrochloride tablets, while PDTC was obtained from Sigma Aldrich (St. Louis, MO, USA).
Experimental design
Rats were randomly divided into four equal groups, 10 rats in each group based on previous researches (El Bana et al. 2019 and Ramadan et al. 2020): group I, the control group, in which rats were intraperitoneally (IP) administered saline twice daily for 4 successive weeks; group II, the pyrrolidine dithiocarbamate (PDTC) group, in which rats received 100 mg/kg PDTC dissolved in saline (divided into two equal doses and administered intraperitoneally daily) for 4 successive weeks (Delen and Uz 2021); group III, the tramadol (Tr) group, in which rats received 40 mg/kg/day tramadol dissolved in distilled water orally by gastric gavage for 4 successive weeks; this dose is equivalent to human effective therapeutic dose (Ramadan et al. 2020); and group (IV), the Tr + PDTC group, in which rats had been concomitantly treated daily with the previous doses of PDTC and tramadol for 4 successive weeks (Delen and Uz 2021).
Sample collection
At the end of the experimental period, rats were anaesthetized with intraperitoneal thiopental, and then blood, epididymis, and testes samples were collected.
Biochemical testing
Hormonal parameters
-
Serum FSH level: Serum FSH level was measured using FSH ELISA kit (Catalog Number: BC-1029, BioCheck, Inc 323 Vintage Park Dr. Foster City, CA 94404) as described by Rebar et al. (1982)
-
Serum LH level: Serum LH level was measured using FSH ELISA kit (Catalog Number: BC-1031, BioCheck, Inc 323 Vintage Park Dr. Foster City, CA 94404) as described by Tietz (1995)
-
Serum-free testosterone level: Serum-free testosterone level was measured using testosterone ELISA kit (BioCheck, Dr. Foster City, CA 94404) as described by Zirkin and Chen (2000).
Epidydimal sSperm parameters analysis
The right cauda epididymis of each rat was dissected, cut, and minced in 2 ml of Hank’s buffer salt solution at 37 °C. After 5 min incubation at 37 °C, the epididymal sperm count was then determined using the standard hemocytometric method (Belsey et al., 1980). The percentage of sperm motility was calculated using the number of live sperm cells over the total number of sperm cells (Khaki et al., 2009).
Evaluation of testicular redox status
MDA level and SOD and GPX activities in testicular tissues were measured using specific ELISA kits (Biodiagnostic company, Egypt) according to Ohkawa et al. (1979), Kakkar et al. (1984), and Reddy et al. (1995) respectively.
Measurement of testicular inflammatory biomarkers
IL-1β, IL-6, and TNF-α concentrations in testicular tissues were quantified according to the manufacturer’s instructions and guidelines using their specific ELISA kit (Biodiagnostic company, Egypt).
Evaluation of apoptosis markers
Activities of caspases-8 and caspase-3 were measured in testicular homogenate using their specific colorimetric assays following their manufacturer’s recommendations (Sigma Aldrich Co., USA).
Histopathological examination
Right testes from all groups were fixed in Bouin’s solution and followed by dehydration in a descending series of ethyl alcohol and then cleared in xylene and embedded in paraffin. Paraffin sections of testes were cut at 5 μm on a rotary microtome, mounted on slides, and stained with hematoxylin and eosin (H&E) and examined under a light microscope by a blinded pathologist (Raghavendra et al. 2003).
Inducible nitric oxide synthase (iNOS) immunohistochemical study
Paraffin sections were deparaffinized in xylene and rehydrated using ascending grades of alcohol and used to evaluate the expression of iNOS using anti-inducible nitric oxide synthase antibody: mouse monoclonal reacts specifically with iNOS (Sigma Aldrich Co.-USA) as described by Kolasa et al. (2009).
Statistical analysis
Data were presented as mean ± SD. IBM SPSS statistics 20 (SPSS Inc., Chicago, IL, USA) was used for performing the statistical analysis. Analysis of variance (ANOVA) followed by LSD (least significant difference) post hoc test was performed to compare the means of the different groups. P values < 0.05 were considered statistically significant.
Results
Effect of PDTC on hormonal parameters in tramadol-treated rats
Regarding hormonal parameters, the current study estimated highly significant decrease (P < 0.001) in serum FSH, LH, and free testosterone levels in group III (tramadol group) in comparison with other groups, while these changes were reversed significantly (P < 0.001) with administration of PDTC in group IV (Tr + PDTC group) when compared with that in group III (tramadol group) (Table 1 and Fig. 1).
Serum sex hormones [follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (A)], free testosterone level (B), and epidydimal sperm parameters [count and motility (C)] of all studied groups. * Significant versus control, # significant versus pyrrolidine dithiocarbamate (PDTC) group, $ significant versus tramadol (Tr) group
Effect of PDTC on sperm parameters in tramadol-treated rats
Tramadol administration in group III (tramadol group) resulted in highly significant decrease (P < 0.001) in epidydimal sperm count and motility when compared with other groups, but this effect was improved significantly (P < 0.001) by PDTC in group IV (Tr + PDTC group) when compared with that in group III (tramadol group) (Table 1 and Fig. 1).
Effect of PDTC on testicular redox status in tramadol-treated rats
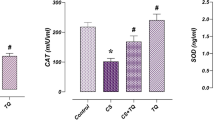
Statistical analysis of data regarding testicular oxidative stress/antioxidant parameters showed significant increase (P < 0.001) in MDA that was associated with high significant reduction (P < 0.001) of SOD and GPX activities in testicular tissues from rats of group III (tramadol group) compared with other groups. The specimens from rats of group IV (Tr + PDTC group) showed improvement in the form of highly significant reduction (P < 0.001) in MDA along with high significant elevation (P < 0.001) of testicular SOD and GPX activities when compared with that of group III (tramadol group) (Table 2 and Fig. 2).
Testicular oxidant/antioxidant [malondialdehyde (c) level and superoxide dismutase (SOD) and glutathione peroxidase (GPX) activities] parameters of all studied groups. * Significant versus control, # significant versus pyrrolidine dithiocarbamate (PDTC) group, $ significant versus tramadol (Tr) group
Effect of PDTC on testicular inflammatory biomarkers in tramadol-treated rats
Regarding testicular inflammatory biomarkers, IL-1β, IL-6, and TNF-α were highly significantly (P < 0.001) increased in group III (tramadol group) when compared to other groups. This elevation was highly significantly decreased (P < 0.001) in testicular tissues of rats in group IV (Tr + PDTC group) in comparison to in group III (tramadol group) (Table 3 and Fig. 3).
Testicular inflammatory [tumor necrosis factor alpha (TNFα), interleukin 6 (IL-6), and interleukin-1beta (IL-1β) (A)] and apoptotic [caspase-8 and caspase-3 (B)] parameters of all studied groups. * Significant versus control, # significant versus pyrrolidine dithiocarbamate (PDTC) group, $ significant versus tramadol (Tr) group
Effect of PDTC on apoptosis markers in tramadol-treated rats
Testicular activities of apoptotic factors and caspase 3 and 8 activities were increased significantly in group III (tramadol group) in comparison with other groups, while these effects were alleviated by PDTC administration in group IV (Tr + PDTC group) (Table 3 and Fig. 3).
Effect of PDTC on histopathological findings in tramadol-treated rats
Light microscopic examination (H&E) of specimens from rats receiving tramadol (group III) expressed histopathological changes in the form of distorted seminiferous tubules both in shape and structure with vacuolated germinal epithelium and wide interstitial spaces (Fig. 4a–c) comparable to that in both group I (control group) (Fig. 5) and group II (PDTC group) (Fig. 6) which had well-arranged normal shape and structure of seminiferous tubules, lined with stratified germinal epithelium consisted of germinal cells and supporting Sertoli cells. Narrow interstitial spaces presented in between the seminiferous tubules containing blood capillaries and clusters of interstitial Leydig cells.
a A photomicrograph of a rat testis section from the tramadol-treated group showing many distorted seminiferous tubules (T) with vacuolated germinal epithelium (arrow). The interstitial spaces are wide and contain few cells (I) (H&E × 100). b A photomicrograph of a rat testis section from the tramadol-treated group showing many distorted seminiferous tubules (T) with exfoliated germ cells (*). The interstitial spaces are wide (I) (H&E × 200). c A photomicrograph of a rat testis section from the tramadol-treated group showing distorted seminiferous tubules (T) with multiple vacuolations (arrow) (H&E × 400)
A photomicrograph of a rat testis section from control group showing the well-known histological picture of rat testis. The testis consists of multiple seminiferous tubules (T) with interstitial space between them (I). Sperms (S) are seen inside the tubules. Each tubule is bounded by a basal lamina (arrow) (H&E × 400)
A photomicrograph of a rat testis section from PDTC treated group showing the well-known histological picture of rat testis. The testis consists of multiple seminiferous tubules (T) with interstitial space between them (I). Sperms (S) are seen inside the tubules. Each tubule is bounded by a basal lamina (arrow) (H&E × 400)
On the other hand, histopathological changes in rats of group IV (Tr + PDTC group) showed a remarkable improvement in the histopathological alterations induced by tramadol; the testis regained near normal histological structure. The seminiferous tubules lumen was patent, and some showed aggregation of sperms. Narrow interstitial spaces were also noticed (Fig. 7).
A photomicrograph of rat testis section from the PDTC + tramadol-treated group showing near normal histological structure. The seminiferous tubules lumen was patent (T) and some showed aggregation of sperms. Few vacuolations in the germinal layer (arrow) were noticed. Profiles of seminiferous tubules are separated by narrow interstitial spaces (I) (H&E × 200)
Effect of PDTC on immunohistochemical findings in tramadol-treated rats
There was intense positive immunoreaction to iNOS in specimens taken from group III (tramadol group) (Fig. 8) opposite to that in group I (control group) (Fig. 9) and group II (DPTC group) which showed unnoticed reaction to iNOS (Fig. 10). However, PDTC administration decreased the immunoreaction in group IV (Tr + PDTC group) (Fig. 11).
Discussion
One of the most widely used pain killers is tramadol hydrochloride. It is considered centrally acting opioids with more potent pain killing effect. Regarding its pharmacokinetics, rapid absorption and rapid tissue distribution are its characteristics (Leppert and Łuczak 2005). Regarding its pharmacodynamics, as tramadol is considered a synthetic opioid, it gives action through selective agonistic effect on mu (μ) receptors with lower action through other opioid receptors: δ- and κ-. As a synthetic opioid, it produces similar side actions like other opioid drugs including hypotension, dizziness, nausea, dry mouth, constipation, delayed ejaculation, and respiratory depression (in rare cases) (Baghishani et al. 2018).
Reproductive toxicity associated with tramadol can be induced by decreased sex hormones and sperm abnormalities in both morphology and count (Aloisi et al. 2011).
Ahmed and Kurkar (2014) stated that chronic consumption of tramadol in males complaining of chronic pain resulted in decreased libido and erectile dysfunction.
Many researchers tried to rule out possible mechanisms involved in chronic tramadol testicular toxicity; from multiple mechanisms, oxidative stress and apoptosis of spermatocytes were accused (Ibrahim and Salah-Eldin 2019a).
Several research had studied the mechanism of reproductive toxicity of tramadol as an inducer of oxidative stress and the possible roles of antioxidants. From those, the natural product, resveratrol, is present in grapes. De la Lastra and Villegas (2007) stated that it produced antioxidant impacts by reducing lipid peroxidation and inducing release of antioxidant enzymes.
Nafees et al. (2015) reported that uncontrolled production of free radicals can act as an inflammatory effector by activating the transcription factor, nuclear factor-kappa B (NF-κB), which in turn results in the transcription of genes encoding cell adhesion molecules, nitric oxide synthase (NOS), proinflammatory cytokines (IL-1β, IL-12, and TNF), and cyclo-oxygenase-2.
Another recent study by Koohsari et al. (2020) proved that tramadol provoked mitochondrial-mediated apoptosis in rat testicular tissue, and its inflammatory responses were produced through NF-κB pathway.
A specific inhibitor of NF-κB, pyrrolidine dithiocarbamate (PDTC), is a metal chelator thiol compound capable of producing intense anti-inflammatory and antioxidant actions (Mehdizadeh et al. 2017).
Mehdizadeh et al. (2017) and Kabel (2018) researched that PDTC safeguards and mends histopathology in various organ injuries caused by variable agents.
Up to our knowledge, no research had studied the protective impact of PDTC on testicular injury caused by tramadol yet.
The current findings in the tramadol group revealed a significant reduction in the values of FSH, LH, and testosterone hormones. These findings are parallel to the findings of Adelakun et al. (2022) and Ramadan et al. (2022) who clarify this reduction by tramadol’s direct activity on the hypothalamic–pituitary axis inducing repression of both FSH and LH release. The noteworthy decrease of sexual hormones might be clarified through tramadol boost free radicals’ release and exerted harm on both cells of Leydig and Sertoli. Diminution of secretion of gonadotrophic hormones gives rise to atrophy of the testes, as well as a diminution in production of sperm cells (Tousson et al. 2012). Anywise, it was twigged that co-administration of pyrrolidine dithiocarbamate along with tramadol significantly elevated these hormonal parameters in the tramadol-treated group. Similar results have been declared by Delen et al. (2021).
Oxidative stress with overexpression of nitric oxide synthase (NOS) are declared as the most important possible pathophysiology for tramadol-induced suppressive impact on spermatogenesis added to spermiological parameters (El-Baky A. and Hafez M., 2017). Oxidative stress conditions have also been shown to initiate progressive aponecrosis, necrosis, and ferroptosis in male testicular tissue, resulting in male fertility loss (Alahmar 2019).
It is important to understand that oxidative stress-induced ROS can prompt lipid and protein peroxidation leading to mitochondrial-related and unrelated apoptosis, added to oxidative harm of DNA in the testicular cells: both somatic and germ cells (Chianese and Pierantoni 2021). In addition, oxidative stress-induced cell injury strikes up an enormous immune cell infiltration into the connective tissue, leading to inflammation in the testicles (Razi and Malekinejad 2015).
In our experimental model, MDA content was significantly rise, while there was a significant diminution in the antioxidant enzyme activities (SOD and GPX) in the tramadol group. This result can be declared by studies of Bisht et al. (2017) explaining that spermatozoa have a rich content of unsaturated fatty acids and a little content of antioxidants, and they are overmuch susceptible to the adverse impacts of reactive oxygen species (ROS). High amounts of ROS were reported to impair fertility by induction of lipid oxidation, DNA harm, and sperm apoptosis. Lipid peroxidation induced by tramadol can eventually result in dysfunction and structural damage of cells (Abd et al. 2020).
On the other hand, PDTC administration to tramadol-treated rats significantly reduced MDA level and significantly enhanced the antioxidant enzymes activities. Similarly, in other investigations, it was stated that PDTC prevented the lipid peroxidation and testicular damage induced by other oxidative stress conditions (Kemahli et al. 2016 and Ilbey et al. 2009). This antioxidant or free radicle scavenging properties is linked with its thiol group, which acts by neutralizing reactive oxygen species (Ding et al. 2014).
Moreover, our results demonstrated increased testicular TNF-α, IL-6, and IL-1β in the tramadol-treated group, whereas administration of PDTC with the tramadol ameliorated their levels. Similar results have been reported by Abd et al. (2020). The process of inflammation involves different signal transduction pathways, including NF-κB. NF-κB is a complex of transcriptional activator proteins, found in the cytoplasm of normal cells in an inactive state, dimers with the inhibitory kappa B (IKB) subunit proteins. Oxidative stress can activate NF-κB via phosphorylation of IKB by IKB-kinases (Mantawy et al. 2014). Free NF-κB binds to the corresponding DNA sequence of target genes, including TNF-α, IL-1β, and other genes, associated with increased ROS generation (Nafees et al. 2015). Pyrrolidine dithiocarbamate (PDTC) is recognized as the inhibitor of (NF-κB) in a variety of cells (Lu et al. 2017).
Moreover, the feedback of our experiment revealed that tramadol exposure increased levels of the initiator caspase-8 and the executioner caspase-3. Consequently, tramadol-induced ROS can also damage the integrity of mitochondrial membrane and opening of MPT pores and lead to apoptosis which agrees with Ibrahim and Salah-Eldin (2019) and Koohsari et al. (2020). Otherwise, PDTC administration significantly lessened both caspase-3 and caspase-8 activities in treated group. Caspase-3 is an executioner caspase and one of the key indicators of apoptosis because of its robust role in assortment the destruction of cellular structures such as DNA fragmentation or degradation of cytoskeletal proteins (McIlwain et al. 2015). PDTC protected against apoptosis induced by oxidative or inflammatory cytokines in other studied (song et al. 2013 and Zheng et al. 2016).
Previous histopathological studies of tramadol-treated rabbit testes showed disrupted immature spermatozoid with vacuolization which corresponds with the current results, while on administrations of antioxidants in the form of garlic and selenium, normal mitotic and meiotic divisions and healthy sperms were observed. Moreover, tramadol treatment decreased significantly the motility and number of sperms in testes of rabbits (Sheweita et al. 2022).
Added to the above, tramadol administration resulted in accretion of iNOS immunoreactivity in testicular cells. Similar results have been reported by Ramadan et al. (2022). Increment of expression of iNOS interposes the production of an excess amount of nitric oxide. Excess nitric oxide interacts with superoxide anion and composes a potent oxidant peroxynitrite accountable for cell damage by nitrating cellular macromolecules and elevating the vulnerability to oxidative stress by minimizing intracellular glutathione (Xu et al. 2014).
On the other hand, co-administration of PDTC along with tramadol ameliorated the iNOS immunoreactivity as compared to tramadol untreated group. Similarly, other investigations also recorded blocking of the NF-κB pathway and inhibition of iNOS activity by PDTC administration.
Consequently, according to previous literature search, PDTC treatment significantly improved serum FSH, LH, and testosterone hormone levels and had antioxidant and anti-inflammatory with a protective impact on testicular tissue by decreasing prokineticins and NFkB expressions and elevating expressions of Nrf2. So, it was thought that PDTC can participate in the management of methotrexate-induced testicular injury and infertility (Delen and Uz 2021).
PDTC prevented deoxynivalenol-induced mitochondrial dysfunction and apoptosis by blocking the translocation of NF-κB into the nucleus and inhibiting induced iNOS expression (Wan et al. 2018). Aras et al. (2010) also denoted diminished immunohistochemical iNOS expression by PDTC in renal tissue in streptozotocin-induced diabetic rats.
Conclusions
Conclusively, the present study demonstrated that tramadol therapy decreased epididymal sperm count and motility, induced testicular tissue damage, reduced FSH, LH, and testosterone levels, inhibited antioxidant enzyme activity, and increased inflammatory and apoptotic markers. Such changes were reversed and alleviated by pyrrolidine dithiocarbamate co-administration along with tramadol. However, there is need for further clinical trial studies to evaluate its possible clinical applicability in chronic tramadol usage.
Availability of data and materials
The authors declare that all necessary data supporting the findings of this study are available within the article.
Abbreviations
- NF-κB:
-
Nuclear factor kappa B
- PDTC:
-
Pyrrolidine dithiocarbamate
- Tr:
-
Tramadol
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- MDA:
-
Malondialdehyde
- IL-1B:
-
Interleukin-1B
- IL6:
-
Interleukin 6
- TNF:
-
Tumor necrosis factor
- SOD:
-
Superoxide dismutase
- GPx:
-
Glutathione peroxidase
- iNOS:
-
Inducible nitric oxide synthase
- ANOVA:
-
Analysis of variance
- LSD:
-
Least significant difference
References
Abd H, Ahmed H, Mutar T (2020) Moringa oleifera leaves extract modulates toxicity, sperms alterations, oxidative stress, and testicular damage induced by tramadol in male rats. Toxicol Res 9(2):101–106
Abdel-Zaher AO, Abdel-Rahman MS, Elwasei FM (2011) Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: Role of nitric oxide and oxidative stress. Neurotoxicology 32(6):725–733
Adelakun S, Akintunde O, Jeje S, Alao O (2022) Ameliorating and protective potential of 1-isothiocyanato-4-methyl sulfonyl butane on cisplatin induced oligozoospermia and testicular dysfunction via redox-inflammatory pathway: Histomorphometric and immunohistochemical evaluation using proliferating cell nuclear antigen. Phytomedicine Plus. 2(2):100268
Ahmed MA, Kurkar A (2014) Effects of opioid (tramadol) treatment on testicular functions in adult male rats: the role of nitric oxide and oxidative stress. Clin Exp Pharmacol Physiol 41:317–323
Alahmar A (2019) Role of oxidative stress in male infertility: an updated review. J Hum Reprod Sci 12(1):4–18
Aloisi AM, Ceccarelli I, Carlucci M, Suman A, Sindaco G, Mameli S (2011) Hormone replacement therapy in morphine-induced hypogonadic male chronic pain patients. Reprod Biol Endocrinol 1:9–26
Aras B, Tugcu V, Eren G, Mutlu B, Uhri M (2010) Protective effect of pyrrolidine dithiocarbamate on kidney tissue in streptozotocin-induced diabetic rats. Turk J Urol Istanbul 36(2):167–175
Baghishani F, Mohammadipour A, Hosseinzadeh H, Hosseini M, Ebrahimzadeh-Bideskan A (2018) The effects of tramadol administration on hippocampal cell apoptosis, learning and memory in adult rats and neuroprotective effects of crocin. Metab Brain Dis 33(3):907–916
Belsey MA, Moshissi KS, Eliasson R, Paulsen CA, Callegos AJ, Prasad MR (1980) Laboratory manual for the examination of human semen and semen cervical mucus interaction. Press concern. 1-43
Bisht S, Faiq M, Tolahunase M, Dada R (2017) Oxidative stress and male infertility. Nat Rev Urol 14(8):470–485
Cajú F, Queiroz G, Torres S (2011) Opioid system manipulation during testicular development: results on sperm production and sertoli cells population. Acta Sci Biol Sci 33:219–225
Ceccarelli I, De Padova AM, Fiorenzani P, Massafra C, Aloisi AM (2006) Single opioid administration modifies gonadal steroids in both the CNS and plasma of male rats. Neuroscience 140:929–937
Chianese R, Pierantoni R (2021) Mitochondrial reactive oxygen species (ROS) production alters sperm quality. Antioxidants. 10(1):92. https://doi.org/10.3390/antiox10010092
De la Lastra CA, Villegas I (2007) Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. BiochemSoc Trans 35:1156–1160
Delen O, Uz YH (2021) Protective effect of pyrrolidine dithiocarbamate against methotrexate-induced testicular damage. Hum Exp Toxicol 40(12):S164–S177
Ding H, Zhu T, Yin X, Liu J, Zhang L, Bernier L, Zhao R (2014) Pyrrolidine dithiocarbamate protects pancreatic β-cells from oxidative damage through regulation of FoxO1 activity in type 2 diabetes rats. Acta Biochim Biophys Sin (shanghai) 46:582. https://doi.org/10.1093/abbs/gmu034
El Bana E, Sarg N, Elwakeel E (2019) The histological and immunohistochemical study of tramadol induced testicular toxicity and protective effects of resveratrol in adult male albino rats. J Med Histology 3(2):207–215
El-Baky A., Hafez M. (2017) NOS expression in oxidative stress, neurodegeneration and male infertility induced by the abuse of tramadol. Biochem Pharmacol 6(1). https://doi.org/10.4172/2167-0501.1000223
Ibrahim M, Salah-Eldin A (2019a) Chronic addiction to tramadol and withdrawal effect on the spermatogenesis and testicular tissues in adult male albino rats. Pharmacology 103(3–4):202–211
Ilbey YO, Ozbek E, Simsek A (2009) Chemoprotective effect of a nuclear factor-kappaB inhibitor, pyrrolidine dithiocarbamate, against cisplatin induced testicular damage in rats. J Androl 30:505–514
Kabel AM (2018) Zinc/alogliptin combination attenuates testicular toxicity induced by doxorubicin in rats: Role of oxidative stress, apoptosis and TGF-beta1/NF-kappaB signaling. Biomed Pharmacother 97:439–449
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Kemahli E, Yildiz M, Firat T, Özyalvaçli ME, Üyetürk U, Yilmaz B, Gücük A (2016) An experimental study on effects of pyrrolidine dithiocarbamate on ischemia-reperfusion injury in testis. Can Urol Assoc J 10(3–4):E104–E109
Khaki A, Nouri M, Fathiazad F, Ahmadi-Ashtiani HR, Rastgar H, Rezazadeh, (2009) Protective effects of quercetin on spermatogenesis in streptozotocin induced diabetic rat. J Medicinal Plans. 8(5):57–64
Kolasa A, Marchlewicz M, Kurzawa R, Głabowski W, Trybek G, Wenda-Rózewicka L, Wiszniewska B (2009) The expression of inducible nitric oxide synthase (iNOS) in the testis and epididymis of rats with a dihydrotestosterone (DHT) deficiency. Cell Mol Biol Lett 14(3):511–527
Koohsari M, Ahangar N, Mohammadi E, Shaki F (2020a) Ameliorative effect of melatonin against reproductive toxicity of tramadol in rats via the regulation of oxidative stress, mitochondrial dysfunction, and apoptosis-related gene expression signaling pathway. Addic Health 12(2):118–129
Koohsari M, Ahangar N, Mohammadi E, Talebpour Amiri F, Shaki F (2020b) Effects of tramadol administration on male reproductive toxicity in Wistar rats. The role of oxidative stress, mitochondrial dysfunction, apoptosis-related gene expression, and nuclear factor kappa B signalling. Bratisl Lek Listy 121(6):400–410
Kopalli SR, Cha KM, Cho JY, Kim SK, Koppula S (2022) Cordycepin mitigates spermatogenic and redox related expression in H 2O2-exposed Leydig cells and regulates testicular oxidative apoptotic signalling in aged rats. Pharm Biol 60(1):404–416
Leppert W, Łuczak J (2005) The role of tramadol in cancer pain treatment-a review. Support Care Cancer 13(1):5–17
Lu CW, Lin Y, Lei YP, Wang L, He ZM, Xiong Y (17AD) Pyrrolidine dithiocarbamate ameliorates endothelial dysfunction in thoracic aorta of diabetic rats by preserving vascular DDAH activity. PLoS One 12(7):e0179908
Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E (2014) Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 728:107–118
McIlwain DR, Berger T, Mak TW (2015) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 7. https://doi.org/10.1101/cshperspect.a026716.
Mehdizadeh H, Pourahmad J, Taghizadeh G, Vousooghi N, Yoonessi A, Naserzadeh P (2017) Mitochondrial impairments contribute to spatial learning and memory dysfunction induced by chronic tramadol administration in rat: protective effect of physical exercise. Prog Neuropsychopharmacol Biol Psychiatry 79(Pt B):426–433
Nafees S, Rashid S, Ali N, Hasan SK, Sultana S (2015) Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFκB/MAPK pathway. Chem Biol Interact 231:98–107
Nna V, Osim E (2017) Testicular toxicity following separate and combined administration of PDE 5 inhibitors and opioid: assessment of recovery following their withdrawal. Andrologia 49(6):e12669
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animals and tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358
Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA (2003) Anti-hyperalgesic and morphine-sparing actions of propoentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain 104(3):655–664
Ramadan B, Schaalan M, Mahmoud E (2018) Protective effect of taurine on thiopurine-induced testicular atrophy in male albino rats. J Steroids Horm Sci 9(1):192
Ramadan O, Abd-Allah E, Amr I, Gomah T, Mahmoud M, Mohammad O, Abd El-Hay M, El Sayed S, Fayyad R, Darwish M, Damanhory A, Awad M, Elsify A (2020) Effect of tramadol hydrochloride on the testis of adult male albino rats and the possible ameliorating role of L-carnitine. Annals of R. S. C. B. 24(1):1449–1463
Ramadan R, Mohamed Abd El Rahman A, Ibrahim R, Yousef D (2022) ameliorative effect of nigella sativa on tramadol-induced testicular toxicity in adult rats. ESCTJ. 10(1):14–28. https://doi.org/10.21608/ESCTJ.2022.132234.1007
Razi M, Malekinejad H (2015) Varicocele-induced infertility in animal models. Int J Fertil Steril 9(2):141–149
Rebar RW, Morandini IC, Petze JE, Erickson GF (1982) Hormonal basis of reproductive defects in athymic mice: reduced gonadotropins and testosterone in males. Biol Repro 5:1267–1276
Reddy KP, Subhani SM, Khan PA, Kumar KB (1995) Effect of light and benzyl adenine on dark-treated growing rice leaves, II changes in peroxidase activity. Plant Cell Physiol 24:987–994
Sheweita SA, Almasmari AA, El-Banna SG (2018) Tramadol-induced hepato- and nephrotoxicity in rats: role of curcumin and gallic acid as antioxidants. PLoS ONE 13(8):e0202110
Sheweita SA, El-dafrawi YA, El-ghalid OA, Ghoneim AA, Wahid A (2022) Antioxidants (selenium and garlic) alleviated the adverse effects of tramadol on the reproductive system and oxidative stress markers in male rabbits. Sci Rep 12:13958. https://doi.org/10.1038/s41598-022-16862-4
Soliman T, Shaher H, Mohey A, El-Shaer W, Sebaey A (2021) Gonadotoxic effect of tramadol administration: a prospective controlled study. Arab J Urol 20(1):54–60
Song W, JiaY FY, Du M, Liu A (2013) PDTC antagonized polysaccharide-induced apoptosis in MCF-7 cells through a caspase-8 mediated Fas pathway. J Funct Foods 5:1270–1278
Tietz NW (1995) Clinical Guide to Laboratory Tests, 3rd edn. W.B. Saunders Company, Philadelphia, pp 509–580
Tousson E, El-Moghazy M, Massoud, (2012) Histopathological and immunohistochemical changes in the testes of rabbits after injection with the growth promoter boldenone. Reprod Sci 19:253–259
Udefa AL, Beshel FN, Nwangwa JN, Mkpe ID, Ofuru OS, Sam-Ekpe VG, Stephen GI (2020) Vitamin E administration does not ameliorate tramadolassociated impairment of testicular function in Wistar rats. Androl 52(1):e13454
Wan D, Wu O, Wei QuW, Liu G, Wang X (2018) Pyrrolidine dithiocarbamate (PDTC) inhibits DON-induced mitochondrial dysfunction and apoptosis via the NF-κB/iNOS pathway. Oxid Med Cell Longev. 1324173:8. https://doi.org/10.1155/2018/1324173
Xu M, Wei Q, Zheng K, Mao D, Zheng Y, Li Y, Shi F (2014) Protective effects of big-leaf mulberry and physiological roles of nitric oxide synthases in the testis of mice following water immersion and restraint stress. Acta Histo Chemica 116(8):1323–1330
Zheng QY, Cao ZH, Hu XB, Li GQ, Dong SF, Xu GL, Zhang KQ (2016) LIGHT/IFN-gamma triggers beta cells apoptosis via NF-kappa B/Bcl2-dependent mitochondrial pathway. J Cell Mol Med 20:1861–1871
Zirkin BR, Chen H (2000) Regulation of Leydig cell steroidogenic function during aging. Biol Reprod 63(4):977–981
Acknowledgements
Thanks to the team of histology department, Faculty of Medicine, Zagazig University, for helping in performing the histopathological and the immunohistochemical studies.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Abeer Ramzy Hussieny Mahmoud: designing the experiments, supervision, original draft preparation, investigation, data analysis, data acquisition, supervision, writing, reviewing and editing, formal analysis, revision of the manuscript. Suzan M. M. Moursi: designing the experiments, supervision, original draft preparation, reviewing, editing, formal analysis, visualization, and investigation, data analysis, data acquisition, visualization, and formal analysis. Safya E. Esmaeel: data analysis, data acquisition, visualization, and formal analysis. Nesma Ismail Sharawy Mohamed: data analysis, data acquisition, visualization, and formal analysis. Nagah El Sayed Mohamed Ali: data analysis, data acquisition, visualization, and formal analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics of the guiding standard for the use of research animals by ZU-IACUC committee, with number ZU-IACUC/3/F/119/2022. All the authors consent to participate in this research paper.
Consent for publication
All the authors consent to publish this research paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoud, A.R.H., Moursi, S.M.M., Esmaeel, S.E. et al. The possible protective effect of the nuclear factor kappa B inhibitor pyrrolidine dithiocarbamate on tramadol-induced testicular damage in rats. Egypt J Forensic Sci 13, 28 (2023). https://doi.org/10.1186/s41935-023-00351-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41935-023-00351-4