Abstract
Introduction
Although short-term clinical trials have demonstrated that switching from infliximab (INF) bio-originator to its biosimilar is safe with no significant loss of efficacy, there are limited real-world data comparing their patterns of use and adherence.
Methods
Using 2015–2018 IBM Marketscan data, we established 4 cohorts of patients with at least one administration or pharmacy claim for INF bio-originator or biosimilar in 2017, including INF naïve biosimilar users, INF prevalent biosimilar users, INF naïve bio-originator users, and INF prevalent bio-originator users, defined according to their prior use of INF from 2015 to their first INF administration in 2017. The proportion of days covered (PDC) was calculated for patients with at least 6, 12, or 18 months of follow-up time. Factors associated with optimal adherence (PDC > 80%) were evaluated using log-binomial models.
Results
We identified 96 INF naïve biosimilar users, 223 INF prevalent biosimilar users, 2,149 INF naïve bio-originator users, and 10,970 INF prevalent bio-originator users. At the end of 18 months of follow-up, 64% of INF prevalent bio-originators, 48% of INF naïve biosimilars, 41% of INF naïve bio-originators, and 36% of INF prevalent biosimilars had optimal adherence. Depression, previous hospitalization, and greater use of prior biologics were negatively associated with adherence, whereas IBD diagnoses (referent to RA) and age 55–64 (referent to < 35) were positively associated with high adherence.
Conclusion
INF prevalent users had higher adherence in our analyses than INF naïve users. However, further studies with larger sample size are needed to evaluate INF biosimilar users’ adherence.
Similar content being viewed by others
Introduction
Infliximab (INF) is one of the five tumor necrosis factor-α (TNF) inhibitors that is routinely used for indications of chronic inflammatory diseases such as rheumatoid arthritis (RA), inflammatory bowel disease (IBD), ankylosing spondylitis (AS), and psoriatic arthritis (PsA). However, INF treatment can be expensive [1,2,3,4,5,6], with an estimated annual cost of $21,000 for new initiates, and those continuing therapy paying close to $26,000 per year [2] largely due to dose escalation [1, 3, 4, 6]. Hence, biosimilars were introduced to encourage treatment options and reduce treatment costs through competition [7].
During 2016–2017, the Food and Drug Administration (FDA) approved several INF biosimilars for similar indications as the INF bio-originator, based on shared similarities in the mechanisms of action, routes of administration, dosage form, and strength [8]. These included inflectra (infliximab-dyyb; Celltrion, Inc.) approved in April 2016 [9]; renflexis (infliximab-abda; Samsung Bioepis Co., Ltd.) approved in April 2017 [10]; and ixifi (infliximab-qbtx; Pfizer Inc.) approved in December 2017 [11]. However, optimal adherence, paramount for preventing the associated morbidity and mortality of chronic inflammatory diseases [12,13,14,15,16,17,18,19,20,21,22], is seldom factored in the approval of these drugs.
Sub-optimal adherence (< 80% adherence rate) is common among patients with chronic inflammatory diseases, especially for patients taking biologics. In a Danish study of RA patients switching to biologics after failing disease‐modifying antirheumatic drug (DMARD), adherence was 56% for etanercept, 52% for adalimumab, and 41% for INF. Treatment discontinuation was greater among INF takers regardless of reasons for withdrawal [23]. Similarly, an overall non‐adherence rate of 54% was noticed in a French study for IBD patients continuing INF therapy [24]. Clinical trials have demonstrated that switching from INF to its biosimilar (i.e., inflectra) is safe with no significant loss of efficacy [25]. However, there are limited real-world data that compares their utilization and adherence patterns. Therefore, we compared medication adherence between INF biosimilar and INF bio-originator users and evaluated factors that affected medication adherence using national administrative data.
Methods
Data source and study design
We performed a retrospective cohort study using 2015–2018 IBM Marketscan commercial and Medicare claims data, which included de-identified person-level information for over 200 million individuals, encompassing employees, their spouses, and dependents who were covered by employer-sponsored private health insurance or Medicare insurance in the US. These datasets covered enrollment and healthcare utilization across different settings, including demographics, outpatient prescriptions, and diagnostic claims codes for physician office visits, hospital stays, and procedures [26].
Study cohort
We identified patients with at least one administration or pharmacy claim for INF bio-originator or biosimilar in 2017. We used data from 2015–2017 to classify patients into four groups based on their use of INF before 2017 (index date). These included INF-naïve biosimilar users, prevalent INF biosimilar users, INF-naïve bio-originator users, and prevalent INF bio-originator users. We defined naïve users of INF bio-originator or biosimilar as patients without prior use of INF bio-originator or INF biosimilar before the index date using all available data. Prevalent users of INF bio-originator or biosimilar were defined based on prior exposure to INF bio-originator and no previous use of INF biosimilar. We used 2017 as the index year because we only identified 10 users of INF biosimilar in 2016, of which in 2017, 6 switched to INF bio-originator and the remaining 4 were not defined as naïve biosimilar users. We used the National Drug Code (NDC) and the Healthcare Common Procedure Coding System (HCPCS) to identify claims for INF bio-originator (HCPCS: J1745; NDC: 57894003001) or INF biosimilar (HCPCS; Q5103 and Q5102. NDC; 00069080901 and 32228000101).
To be eligible, patients were also required to be ≥ 18 years of age at the index date and continuously enrolled with full medical and pharmacy health insurance coverage for 2 years preceding their index date (baseline) and through follow up. Follow-up started on the index date and ended on the earliest date of insurance disenrollment, switching from INF bio-originator to INF biosimilar or vice versa or 12/31/2018.
Assessment of medication adherence
We used the proportion of days covered (PDC) to measure medication adherence rate, which was our primary outcome of interest. The standard dosage schedule for INF bio-originator or biosimilar at time of initiation includes the first administration, and then subsequent treatment at 2 weeks, 6 weeks, and then every 8 weeks subsequently. However, to avoid underestimating adherence, we assigned 8 weeks of medication supply (56 days) per administration [27, 28]. We evaluated the PDC at 6 months, 12 months, and 18 months of follow up. PDC was calculated by dividing each patient’s total days of medication supplied for an interval by the total days of coverage in the interval (183 days, 365 days, and 548 days, respectively).
Covariates of interest
We included sociodemographic, concurrent medication, and comorbid diseases as potential factors associated with medication adherence. The baseline sociodemographic variables were sex, region (Midwest, Northeast, South, West, and Unknown), and age, which was categorized (< 35, 35–44, 45–54, 55–64, ≥ 65 years) to compare adherence across age groups. We captured past use of biologics (prior to 2017) as at least one NDC or HCPCS code for abatacept, adalimumab, certolizumab, etanercept, anakinra, belimumab, canakinumab, golimumab, ixekizumab, rituximab, sarilumab, secukinumab, tocilizumab, and ustekinumab. To compare adherence between rheumatoid arthritis and other autoimmune diseases, the autoimmune diseases that may have been the underlying indication for INF were hierarchically defined using ICD9/10 codes during baseline. According to order of highest hierarchy, we included rheumatoid arthritis (RA), psoriasis/psoriatic arthritis (PsO/PsA), inflammatory bowel disease (IBD), and others.
Baseline comorbidities were dichotomous and included cancer of any form, chronic kidney disease, chronic obstructive pulmonary disease (COPD), chronic heart disease (CHD), and depression. We identified these baseline autoimmune diseases and comorbidities using physician diagnosed ICD-9 and ICD-10 codes. We included all-cause hospitalization, which was dichotomously defined as any inpatient visit at baseline. Similarly, using a series of ICD9 and ICD 10 diagnoses codes, we identified patients with baseline infections and dichotomously defined them as any inpatient or outpatient infection vs neither. The medications used at baseline that we assessed included steroids, antibiotics, beta-blockers, hormone therapy, opioids, Non-steroidal anti-inflammatory drugs (NSAIDs), and statin. We identified these medications with NDC codes from pharmacy claims.
Statistical analysis
All variables were categorical and summarized with frequencies and percentages. The chi-square test was used to examine differences in the nominal variables between treatment groups and non-parametric one-way ANOVA (Analysis of variance) was used for ordinal variables. We calculated the PDC of patients with at least 6, 12, or 18 months of follow-up time and categorized their PDC into three groups at these intervals (< 50%, 50–80%, > 80%), choosing conventions commonly used in the literature [29,30,31,32]. We defined high adherence as a PDC greater than or equal to 80%, moderate adherence as a PDC of 50–80%, and low adherence as a PDC less than 50% [31].
Log-binomial regression models were used to analyze the baseline characteristics associated with high adherence (i.e., adherence > 80%) adjusting for treatment groups, sociodemographic, number of other biologics at baseline, type of autoimmune disease, comorbidities, baseline medications, and baseline all-cause hospitalization. Both the crude and adjusted relative risks with their 95% confidence intervals were reported. Subgroup analyses were conducted based on patients with prior use of infliximab bio-originator and duration of prior use, and due to sample size, we adjusted for age, number of other biologics at baseline, type of autoimmune disease, and all-cause hospitalization. We reported the relative risks with their 95% confidence intervals. All analyses were conducted with SAS version 9.4 (SAS Institute Inc. Cary, NC).
Results
We identified 527 INF biosimilar users and 25,875 INF bio-originator users. After applying the inclusion and exclusion criteria, our final cohort consisted of 13,438 patients, of which 319 were INF biosimilar users and 13,119 were INF bio-originator users (Fig. 1). Among INF biosimilar users, we identified 96 naïve users and 223 prevalent users. Whereas among INF bio-originators, 2,149 were naïve users and 10,970 were prevalent users (Fig. 1).
Flow chart for the selection of four INF (infliximab) groups. 96 INF-naïve biosimilar users and 2,149 INF-naïve bio-originator users were ≥ 18 years at index date, had consistent baseline medical and pharmacy coverage and without baseline use of INF bio-originator or biosimilar. 223 prevalent INF biosimilar users and 10,970 prevalent INF bio-originator users were ≥ 18 years at index date, had consistent baseline medical and pharmacy coverage, and had baseline use of INF bio-originator and no baseline use of INF biosimilar
The baseline characteristics are presented in Table 1. As shown, there were more females than males. Among cohorts, prevalent biosimilar users were older, while naïve bio-originator users were the youngest. Overall, a higher proportion of the INF biosimilar groups were in the west of the United States compared to the bio-originator groups, who were more from the south and the mid-west (Table 1). Regarding autoimmune diseases, the INF biosimilar groups had more RA patients (51%) than the bio-originators (39%), whereas there were more IBD patients in the bio-originator groups (46%) than the biosimilars (36%). In terms of the all-cause hospitalization during baseline, both naïve INF biosimilar (28.1%) and bio-originator groups (28.6%) have higher proportion of patients with baseline hospitalization compared to their corresponding prevalent users. Among these hospitalizations, 7.6% (prevalent biosimilar group) to 23.1% (naïve bio-originator group) of them were due to severe infection, with top four types of infections including abdominal infection, sepsis/bacteremia septicemia, skin and soft issue infection, and pneumonia (data not shown). The proportion of patients with baseline infection was also higher in both naïve INF biosimilar (60.4%) and bio-originator groups (63.9%) than that of prevalent INF biosimilar (54.3%) and bio-originator groups (57.3%). Overall, a larger percentage of the INF-naïve groups had used other biologics at baseline (41%) compared to the prevalent groups (11%).
The distribution of baseline biologics (excluding infliximab) is shown in Fig. 2. As shown, adalimumab was more frequently used across groups (45–52%). For naïve biosimilars, the proportion of patients who previously used abatacept (14%) and etanercept (15%) were comparable, with 7% using certolizumab. However, prevalent biosimilar users were equally inclined to use certolizumab (15%) and etanercept (15%) compared to abatacept (5%). Among the INF bio-originator groups, the proportions of patients who used other baseline biologics were similar, with adalimumab being the most frequent, followed by etanercept, certolizumab, and abatacept (Fig. 2).
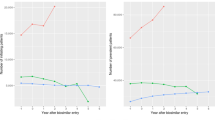
As the primary outcome, Table 2 shows the adherence rates at three intervals of follow-up (6 months, 12 months, and 18 months), unadjusted for differences in age, sex, or clinical factors. Overall, the proportions of patients with PDC of > 80% decreased in all groups as follow-up progressed. At the 12-month follow-up, prevalent bio-originators had the highest adherence (73% with PDC > 80%), followed by naïve bio-originators (52% with PDC > 80%), prevalent biosimilars (46%), and naïve biosimilar users (43%). At the end of 18 months, prevalent bio-originators still had the highest adherence (64%), whereas the prevalent biosimilars became the lowest (Table 2). However, the adherence for naïve biosimilar users was numerically higher than those of the naïve bio-originator group (48% vs. 41%).
Regarding their switching patterns, those who switched to another biologic, or from INF bio-originator to INF biosimilar, or vice versa, were 39% of prevalent biosimilars, 23% of naïve biosimilars, 16% of naïve bio-originators, and13% of prevalent bio-originators. Among these switchers, a large percentage of the INF bio-originator groups and naïve biosimilars switched to another biologic, whereas most of the prevalent biosimilars returned to INF bio-originator (data not shown).
We examined the baseline factors associated with high adherence (Table 3). Among the groups, prevalent bio-originators had higher adherence compared to naïve bio-originators (reference) at all intervals of follow-up (Table 3, 6-months not shown). Prevalent biosimilars were less likely to have high adherence than naïve bio-originators at 12-months (adjusted RR: 0.82 [95% CI 0.68–0.98]). However, there was no significant difference in adherence between the naïve groups in all intervals of follow-up (Table 3). Across age groups, those of ages 55–64 years maintained high adherence as follow-up progressed compared to those younger than 35 years (Table 3). Likewise, IBD patients had better adherence than RA patients throughout follow-up (Table 3). Having depression, any hospitalization, and using other biologics at baseline were negative associates of high adherence.
In a subgroup analysis, we examined high adherence between the prevalent groups by the length of previous use of INF bio-originator, adjusting for age, type of autoimmune disease, baseline all-cause hospitalization, and number of other biologics at baseline (Table 4). Among the prevalent bio-originator group, those who previously used INF bio-originator for less than 12 months were less likely to have high adherence compared to those whose previous use were ≥ 12 months (Table 4). However, in the prevalent biosimilar group, there was no significant difference in adherence by the length of previous use of INF bio-originator (Table 4). Similarly, there was no significant difference in adherence between the prevalent groups who previously used INF bio-originator for less than 12 months (Table 4). However, in those who previously used INF bio-originator for ≥ 12 months, the biosimilar group were less likely to have high adherence compared to the bio-originator group (Table 4).
Discussion
Multiple factors that affect medication adherence have been reported, including limiting access to health care, using restricted formulary, switching to a different formulary, high cost for drugs, and high copayment [33]. However, the effects of switching from biosimilar to its bio-originators were not well studied in the real-world. Therefore, our study evaluated the adherence patterns among infliximab biosimilar naïve and prevalent users and compared them with its bio-originators.
We performed a retrospective analysis that compared the adherence rate between INF bio-originator and INF biosimilar users and examined the baseline factors potentially associated with high adherence. In all four groups, we found that INF prevalent bio-originators had the highest adherence, and INF prevalent biosimilars had the lowest. Among the baseline factors that might be potentially associated with adherence, we found that patients with depression, previous hospitalization, and using other biologics were less likely to reach optimal adherence, whereas patients who had IBD (reference to RA) and of age group 55–64 (reference to < 35 years old) were positively associated with high adherence.
Our results were consistent with similar studies on adherence to the INF bio-originator used in our work. Kane S.V. et al. reported a 34% non-adherence rate among patients with Crohn’s disease in the first year of infusion-based infliximab therapy [34]. Likewise, Martelli L. et al. found an overall non-adherence of 54% for infliximab among patients with IBD [24]. Also, the adherence rate for infliximab users has been reported as 43% among patients with RA [35]. These studies are consistent with the overall decrease in adherence that we observed.
In our work, we found that IBD patients were positively associated with high adherence than RA patients. This could be due to differences in the presentation of IBD compared to RA. For example, ulcerative colitis (UC), which is a form of IBD and an inflammation of the colon’s mucosa, presents with abdominal pain, hematochezia, and diarrhea [36]. Also, about 33% of UC sufferers experience extraintestinal pain, with arthritis being the most common [36]. However, RA, which is an inflammation of the joints, presents with multiple joint pain and stiffness [36]. A high severity and malaise of IBD over RA could explain the difference in adherence. Another explanation could be the large proportion of IBD patients that we recorded among INF bio-originators compared to INF biosimilars (46% vs. 36%), of which INF bio-originators reported better adherence than INF biosimilars.
Current study also found that all-cause hospitalization was negatively associated with adherence after adjustment. Given that patients with multiple comorbidities and medications were less likely to be adherent and patients with baseline hospitalizations were more likely to have acute conditions and comorbidities that need more medications after discharge, we considered this negative association as consistent with our expectation. We did not find the significant association between baseline infection and adherence, which might have been because we included all inpatient or outpatient infections, and therefore, the infections could simply be due to multiple factors rather than specifically from the use of infliximab.
INF prevalent bio-originators had the highest adherence across cohorts, which was consistent with our expectation. Since these patients used INF bio-originator at baseline and the index date, they were likely to continue due to strong familiarities with their current treatment. Several studies have reported an early higher infection risk to accompany the initiation of biologics [27, 37,38,39]. Because INF prevalent bio-originators have passed through this early treatment phase where discontinuations due to side effects, tolerability, and lack of efficacy are more common, they were more likely to retain treatment. In fact, INF prevalent bio-originators recorded the least percentage of switchers (13%) compared to other cohorts.
On the other hand, we found that INF prevalent biosimilars users had the largest proportion of switchers (39%), from which most of them returned to the INF bio-originator. A transitory use of INF biosimilar as a substitute for the bio-originator could explain why these users had the least adherence. Among INF prevalent biosimilar users, a strong preference for INF bio-originator could perpetually return some users to the bio-originator. Also, within these users, similar therapeutic effects between INF bio-originator and biosimilar could cause some to alternate between these treatments. Since we censored patients if there was a switch between the medications of interest, we did not capture the degree of alternation or switching from INF bio-originator to biosimilar, and vice versa. However, this is the focus of an ongoing analysis.
In our work, patients from the west reported lower adherence compared to those from the south, although the confidence interval included the null. In contrast, optimal adherence was virtually the same for patients in the mid-west and the south. Geographical differences in physician practice and marketing strategies may be reasons for this phenomenon. Due to the small sample size of individuals who used more than one biologic at baseline, we compared adherence between those who used other biologics at baseline versus those who did not. We found that using another biologic at baseline was negatively associated with optimal adherence, which could be due to a habit of switching among these users. Since these patients were already switchers at baseline, they had an increased tendency of switching during follow-up.
Since several studies have found depression associated with non-adherence [40,41,42,43,44], we expected to see it negatively impact medication adherence. Non-adherence has also been associated with higher odds of previous hospitalizations [35], which we also found in our study. Adherence has been shown to increase with age [45, 46], with those younger than 50 years being more likely to report poor adherence [47]. Advanced age also has a negative effect on adherence due to the accompanied age-related comorbidities like cognitive impairment and physical difficulties [45, 48, 49]. Like these studies [48, 50, 51], we found individuals of ages 55–64 years were more likely to have higher adherence.
Strengths and limitations
PDC has been used to study adherence to a class of treatment [48, 52,53,54] and has been shown to provide a conservative estimate of adherence than the medication possession ratio (MPR), especially when patients are likely to switch medications within a class or simultaneously use multiple drugs in a class [52]. Our use of national administrative data ensured geographical representation, with large sample size. And we were also among the first handful of studies to compare medication adherence between an INF bio-originator and its biosimilar. However, our study has several limitations. We assigned 8 weeks of medication exposure (56 days) per administration, which might have over-estimated adherence for the first several months of follow-up. However, the impact of the potential misclassification of exposure for follow-up of 12 and 18 months on adherence was insignificant. Second, we were not able to evaluate dose escalation due to the lack of body weight, absence of drug dose units that was used to count for the strength and unavailable valid algorithms to identify dose escalation based on dose frequency changes, so the residual confounding could exist. Third, the reasons for low adherence were not available, even though we made efforts to adjust for numerous confounders, it does not compensate for imbalance in factors that were not measured and therefore could not be controlled for, such as the prior duration of therapy among the prevalent users and total drug cost. However, in the stratified analysis of adherence between the prevalent groups by the length of previous use of INF bio-originator, the length of previous use of INF bio-originator did not modify adherence between the prevalent groups. In addition, the proportion of patients with hospitalized infections during follow up was less than 5% in all four groups, the potential impact of therapy interruptions should be minimal. Lastly, the small sample size among INF biosimilars (2%) is a limitation that was caused by the recency of its approval and limited uptake in the US.
Conclusion
In summary, optimal adherence was more common among INF bio-originators, with INF prevalent bio-originators reporting better adherence among cohorts. Among naïve users, naïve bio-originators showed greater adherence, especially in the first 12 months. Further studies with large sample sizes are needed to evaluate the adherence of INF biosimilar users. However, we found that non-adherence was still common in patients with autoimmune diseases, which is a hindrance to preventing the complications accompanying the long-term management of chronic inflammatory diseases. Our future work is examining the real-life health outcomes between INF bio-originators and biosimilars.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the data use agreement but are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- AS:
-
Ankylosing spondylitis
- CHD:
-
Chronic heart disease
- COPD:
-
Chronic obstructive pulmonary disease
- DMARD:
-
Disease modifying antirheumatic drug
- FDA:
-
Food and drug administration
- HCPCS:
-
Healthcare common procedure coding system
- IBD:
-
Inflammatory bowel disease
- IBM:
-
International business machines corporation
- ICD:
-
International classification of disease
- INF:
-
Infliximab
- MPR:
-
Medication possession ratio
- NDC:
-
National drug code
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PDC:
-
Proportion of days covered
- PsA:
-
Psoriatic arthritis
- PsO:
-
Psoriasis
- RA:
-
Rheumatoid arthritis
- TNF:
-
Tumor necrosis factor
- UC:
-
Ulcerative colitis
References
Baumgartner SW, Chiou C-F. Claims data analysis of dosing and cost of TNF antagonists. Am J Pharmacy. 2010;2(6):351–9.
Bonafede MMK, Gandra SR, Watson C, Princic N, Fox KM. Cost per treated patient for etanercept, adalimumab, and infliximab across adult indications: a claims analysis. Adv Ther. 2012;29(3):234–48.
Harrison DJ, Huang X, Globe D. Dosing patterns and costs of tumor necrosis factor inhibitor use for rheumatoid arthritis. Am J Health Syst Pharm. 2010;67(15):1281–7.
Ollendorf DA, Klingman D, Hazard E, Ray S. Differences in annual medication costs and rates of dosage increase between tumor necrosis factor-antagonist therapies for rheumatoid arthritis in a managed care population. Clin Ther. 2009;31(4):825–35.
Schmier J, Ogden K, Nickman N, Halpern MT, Cifaldi M, Ganguli A, Bao Y, Garg V. Costs of providing infusion therapy for rheumatoid arthritis in a hospital-based infusion center setting. Clin Ther. 2017;39(8):1600–17.
Weycker D, Yu EB, Woolley JM, Oster G. Retrospective study of the costs of care during the first year of therapy with etanercept or infliximab among patients aged > or =65 years with rheumatoid arthritis. Clin Ther. 2005;27(5):646–56.
Biosimilars. https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars
FDA approves Inflectra, a biosimilar to Remicade https://www.fda.gov/news-events/press-announcements/fda-approves-inflectra-biosimilar-remicade]
FDA Approves Inflectra https://www.biosimilarsresourcecenter.org/fda-approves-inflectra/]
FDA Approves Renflexis, Second Biosimilar Infliximab https://www.biosimilarsresourcecenter.org/fda-approves-renflexis-second-biosimilar-infliximab/]
FDA Approves Ixifi, Third Biosimilar Infliximab https://www.biosimilarsresourcecenter.org/fda-approves-ixifi/
Bykerk V, Emery P. Delay in receiving rheumatology care leads to long-term harm. Arthritis Rheum. 2010;62(12):3519–21.
Costedoat-Chalumeau N, Pouchot J, Guettrot-Imbert G, Le Guern V, Leroux G, Marra D, Morel N, Piette J-C. Adherence to treatment in systemic lupus erythematosus patients. Best Pract Res Clin Rheumatol. 2013;27(3):329–40.
Goekoop-Ruiterman YPM, De Vries-Bouwstra JK, Allaart CF, Van Zeben D, Kerstens PJSM, Hazes JMW, Zwinderman AH, Ronday HK, Han KH, Westedt ML, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis Rheum. 2005;52(11):3381–90.
Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. The Lancet. 2009;373(9664):659–72.
Lard LR, Visser H, Speyer I, vander Horst-Bruinsma IE, Zwinderman AH, Breedveld FC, Hazes JMW,. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111(6):446–51.
Li-Yu J, Clayburne G, Sieck M, Beutler A, Rull M, Eisner E, Schumacher HR Jr. Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout? The Journal of rheumatology. 2001;28(3):577–80.
Möttönen T, Hannonen P, Korpela M, Nissilä M, Kautiainen H, Ilonen J, Laasonen L, Kaipiainen-Seppänen O, Franzen P, Helve T, et al. Delay to institution of therapy and induction of remission using single-drug or combination–disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 2002;46(4):894–8.
Neogi T, Hunter DJ, Chaisson CE, Allensworth-Davies D, Zhang Y. Frequency and predictors of inappropriate management of recurrent gout attacks in a longitudinal study. J Rheumatol. 2006;33(1):104.
Pelajo CF, Sgarlat CM, Lopez-Benitez JM, Oliveira SKF, Rodrigues MCF, Sztajnbok FR, Diniz CC, Miller LC. Adherence to methotrexate in juvenile idiopathic arthritis. Rheumatol Int. 2012;32(2):497–500.
Quinn MA, Conaghan PG, O’Connor PJ, Karim Z, Greenstein A, Brown A, Brown C, Fraser A, Jarret S, Emery P. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52(1):27–35.
Wu EQ, Patel PA, Mody RR, Yu AP, Cahill KE, Tang J, Krishnan E. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36(5):1032.
Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, Kollerup G, Linde L, Lindegaard HM, Poulsen UE, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: Results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62(1):22–32.
Martelli L, Lopez A, Strobel S, Danese S, Roblin X, Baumann C, Peyrin-Biroulet L. Adherence to infliximab therapy in inflammatory bowel disease patients in a real-life setting. J Dig Dis. 2017;18(10):566–73.
Blair HA, Deeks ED. Infliximab biosimilar (CT-P13; infliximab-dyyb): a review in autoimmune inflammatory diseases. BioDrugs. 2016;30(5):469–80.
IBM MarketScan Research Databases https://www.ibm.com/products/marketscan-research-databases/resources
Yun H, Xie F, Delzell E, Chen L, Levitan EB, Lewis JD, Saag KG, Beukelman T, Winthrop K, Baddley JW, et al. Risk of hospitalised infection in rheumatoid arthritis patients receiving biologics following a previous infection while on treatment with anti-TNF therapy. Ann Rheum Dis. 2015;74(6):1065–71.
Yun H, Xie F, Delzell E, Levitan EB, Chen L, Lewis JD, Saag KG, Beukelman T, Winthrop KL, Baddley JW, et al. Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in medicare. Arthritis Rheumatol. 2016;68(1):56–66.
Doshi JA, Takeshita J, Pinto L, Li P, Yu X, Rao P, Viswanathan HN, Gelfand JM. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol. 2016;74(6):1057-1065.e1054.
Stolshek BS, Wade S, Mutebi A, De AP, Wade RL, Yeaw J: Two-year adherence and costs for biologic therapy for rheumatoid arthritis. The American journal of managed care 2018, 24(8 Spec No.):Sp315-sp321.
Curtis JR, Yun H, Lange JL, Matthews R, Sharma P, Saag KG, Delzell E. Does medication adherence itself confer fracture protection? An investigation of the healthy adherer effect in observational data. Arthritis Care Res. 2012;64(12):1855–63.
Siris ES, Selby PL, Saag KG, Borgström F, Herings RM, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122(2 Suppl):S3-13.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97.
Kane SV, Chao J, Mulani PM. Adherence to infliximab maintenance therapy and health care utilization and costs by Crohn’s disease patients. Adv Ther. 2009;26(10):936–46.
Li P, Blum MA, Von Feldt J, Hennessy S, Doshi JA. Adherence, discontinuation, and switching of biologic therapies in medicaid enrollees with rheumatoid arthritis. Value Health. 2010;13(6):805–12.
Attalla MG, Singh SB, Khalid R, Umair M, Epenge E. Relationship between ulcerative colitis and rheumatoid arthritis: a review. Cureus. 2019;11(9):e5695–e5695.
Curtis JR, Xi J, Patkar N, Xie A, Saag KG, Martin C. Drug-specific and time-dependent risks of bacterial infection among patients with rheumatoid arthritis who were exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56(12):4226–7.
Dixon W, Symmons D, Lunt M, Watson K, Hyrich K, Silman A. Serious infection following anti–tumor necrosis factor α therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheumatism. 2007;56(9):2896–904.
Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, Griffin MR, Herrinton LJ, Liu L, Ouellet-Hellstrom R, et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306(21):2331–9.
Gascón JJ, Sánchez-Ortuño M, Llor B, Skidmore D, Saturno PJ. Group ftTCiHS: Why hypertensive patients do not comply with the treatment: Results from a qualitative study. Fam Pract. 2004;21(2):125–30.
Kilbourne AM, Reynolds CF, Good CB, Sereika SM, Justice AC, Fine MJ. How does depression influence diabetes medication adherence in older patients? Am J Geriatr Psychiatry. 2005;13(3):202–10.
Lopez A, Billioud V, Peyrin-Biroulet C, Peyrin-Biroulet L. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis. 2013;19(7):1528–33.
Stilley CS, Sereika S, Muldoon MF, Ryan CM, Dunbar-Jacob J. Psychological and cognitive function: predictors of adherence with cholesterol lowering treatment. Ann Behav Med. 2004;27(2):117–24.
Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the california medicaid program. Value in Health. 2005;8(4):495–505.
Jin J, Sklar GE, Min Sen OhV, Chuen Li S. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269–86.
Lacasse Y, Archibald H, Ernst P, Boulet L-P. Patterns and determinants of compliance with inhaled steroids in adults with asthma. Can Respir J. 2005;12: 375454.
Caspard H, Chan AK, Walker AM. Compliance with a statin treatment in a usual-care setting: retrospective database analysis over 3 years after treatment initiation in health maintenance organization enrollees with dyslipidemia. Clin Ther. 2005;27(10):1639–46.
Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–61.
Cooper C, Carpenter I, Katona C, Schroll M, Wagner C, Fialova D, Livingston G. The AdHOC study of older adults’ adherence to medication in 11 countries. Am J Geriatr Psychiatry. 2005;13(12):1067–76.
Leggat JE Jr, Orzol SM, Hulbert-Shearon TE, Golper TA, Jones CA, Held PJ, Port FK. Noncompliance in hemodialysis: predictors and survival analysis. Am J Kidney Dis. 1998;32(1):139–45.
Loong TW. Primary non-compliance in a Singapore polyclinic. Singapore Med J. 1999;40(11):691–3.
Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann Pharmacother. 2009;43(1):36–44.
Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value in Health. 2007;10(1):3–12.
Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–40.
Acknowledgements
Not applicable.
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
CJA and HY had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: CJA, HY, SS, CYL, SB, JRC. Acquisition of data: HY and JRC. Analysis and Interpretation of Data CJA, HY, SS, CYL, SB, JRC. All authors were involved drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and later amendments. IBM Marketscan data are completely de-identified administrative claims data, and it does not contain any human participants or animals, therefore, the ethical approval is not required by the institutional review board at the university of Alabama at Birmingham. As the data is fully anonymized, the University of Alabama at Birmingham institutional review board exempted the informed consent, and the formal informed consent was not needed.
Consent for publication
Not applicable.
Competing interests
Financial competing interests: No for all authors. Non-financial competing interests: Huifeng Yun: While working on this project at UAB, Dr.Yun received research support from Pfizer. Inc for unrelated work. She recently joined GSK, but the entire work was conducted at UAB. None for other coauthors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alanaeme, C.J., Sarvesh, S., Li, C.Y. et al. Adherence patterns in naïve and prevalent use of infliximab and its biosimilar. BMC Rheumatol 6, 65 (2022). https://doi.org/10.1186/s41927-022-00295-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41927-022-00295-7