Abstract
Background
In the lab type-1 Polysomnography (PSG) is the gold standard for the diagnosis of Obstructive sleep apnea (OSA). But in view, the increasing prevalence, highly expensive, and the presence of a pandemic state make it sometimes impractical to perform PSG in all patients suspected of OSA. The objective of our study was to compare the correlation between the gold standard Autoscored Apnea Hypopnea Index (AHI) and Oxygen desaturation index (ODI) based classification of the severity of OSA.
Methodology
It was a single-centered hospital-based cross-sectional study in which a total of 176 Subjects suspected of OSA were recruited and underwent overnight 7 channels of in-lab polysomnography at AIIMS Patna. Demographic details, comorbidities, and lab data were collected for all enrolled patients. BMI, STOP-BANG score, and Epworth sleepiness score (ESS) were also calculated for all subjects. They were categorized into mild, moderate, and severe OSA based on AHI and ODI values of 5–14.9, 15–29.9, and ≥ 30 events per hour, respectively.
Recordings of polysomnography (PSG) were presented with descriptive statistics. Data is presented as the “mean ± standard deviations” for continuous variables and percentage frequencies for categorical variables. The Chi-square test was used to compare the discrete variables. Statistical significance was set at an alpha level of 0.05 with P < 0.05, with a two-tailed probability. Spearman's rank correlation analysis was used to test the strength and direction of the association between OSA severity (using AHI classification) and the Oxygen Desaturation Index.
Results
The Mean (SD) age of patients enrolled in our study was 47.42(12.60) years with 57.95% males. Among comorbidities, 31.8% (n = 56) were known cases or incidentally diagnosed with Diabetes Mellitus Type 2, 43.18% (n = 76) were hypertensive and 31.8% (n = 56) had hypothyroidism. The mean HbA1c level among Diabetic patients was 6.05 (1.27) and the mean TSH level among Hypothyroidism patients was 12.24 (27.34). There was a positive correlation between AHI and ODI in our study (Pearson’s correlation coefficient = 0.897).
Conclusion
Good concordance between AHI and ODI makes nocturnal oximetry a less expensive tool to confidently screen patients with severe OSA. This may be applicable in smaller centers where facilities and expertise for a full night PSG may not be easily available.
Similar content being viewed by others
Introduction
In the landscape of obstructive sleep apnea (OSA), recent population-based research underscores its pervasive prevalence, ranging from 5 to 23% in women and 14% to 50% in men (Heinzer et al. 2015). Notably, among Indians, the data indicates a particularly elevated prevalence, around 32% (Goyal et al. 2023). As sedentary lifestyles become more prevalent, OSA is emerging as a significant global health concern, with its true extent believed to be underestimated due to underdiagnosis and undertreatment (Wali et al. 2017). The standard parameter for the diagnosis of the disease as per existing guidelines is in-lab polysomnography (PSG), which uses auxiliary signals to monitor sleep position and leg movements in addition to cardio-respiratory signals to monitor changes in breathing and cardiac output (such as oximetry, pulse, airflow, snoring, and respiratory effort). There are electrophysiological channels that are required to stage sleep and include electroencephalograms (EEGs), electrooculograms (EOGs), and electromyograms (EMGs). The apnea–hypopnea index (AHI) recorded during PSG helps in defining and categorizing OSA into mild, moderate, and severe categories. Despite its high prevalence, the best part of the disease is that it can be treated. However, there is still controversy as to which parameter best defines the severity of OSA. Many promising metrics have been proposed, such as the desaturation severity parameter, hypoxic burden, time spent with oxygen saturation < 90% (T90), lowest oxygen saturation (LSpO2), saturation impairment time (SIT), approximate entropy (ApEn) of oxygen saturation, Cardiopulmonary coupling (CPC), heart rate variability (HRV), odds ratio product (ORP), Flow drive ratio, Expiratory time constant, sleep breathing impairment index, and cardiac risk index, even though the problem of accurately diagnosing and assessing the severity of OSA is still unresolved (Cao et al. 2020). The average length of apnea–hypopnea episodes, for example, which is influenced by variables including gender, BMI, and sleeping posture, offers details on ventilatory control and airway collapsibility that are not included in the AHI. Some researchers believe that oxygen saturation parameters may play a major role in classifying the severity of OSA, and this conclusion might be derived by the fact that, compared to AHI, measurements such as the oxygen desaturation index (ODI) better reflect the degree of hypoxia during sleep (Tkacova et al. 2014). A major problem associated with AHI is that the morphology consisting of the duration and depth of abnormal respiratory events is not considered. As a result, AHI continues to be a quantitative indicator, and the physiological stress levels of various patients can differ significantly even when their AHI values are comparable (Kulkas et al. 2013). Two patients with the same AHI may experience different degrees of oxygen desaturation, arousal frequency, and hemodynamic changes, leading to varied levels of physiological stress. The traditional practice of treating OSA entails performing a sleep study with emphasis on a single metric, the AHI, followed by CPAP treatment for most patients. However, it is increasingly evident that OSA is a heterogeneous condition. Wide variances exist among OSA patients with similar AHIs in terms of their prognosis, cardiovascular risk, and adherence to and reaction to CPAP and other treatments. Hence, the AHI score alone is not sufficient to rank OSA severity; indicating the need to include hypoxic events while diagnosing and classifying OSA. Researchers have gained interest in the past few years regarding the ODI as a better predictor of hypoxemia and in the diagnosis of OSA. However, as we conducted an extensive literature review, a notable gap emerged – there is a scarcity of recent Indian data comparing AHI with ODI in the diagnosis of OSA. Diversity in population characteristics, prevalence patterns of OSA, healthcare resource optimization, are few reasons making the dearth of Indian-specific research is particularly crucial. Addressing this gap through our study is essential for advancing our understanding of OSA within the unique Indian context, ultimately contributing to improved diagnostic precision and personalized healthcare strategies for individuals in this population.
Methodology
This was a single-center, hospital-based cross-sectional study conducted after ethical approval from the Institute Ethics Committee (AIIMS/Pat/IEC/PGTh/July 20/28), and patients were recruited after informed written consent was obtained from March 2021 to May 2022. The study included the conductance of overnight in-lab nocturnal polysomnography in all patients suspected of obstructive sleep apnea attending Pulmonary Medicine OPD at All India Institute of Medical Sciences, Patna. We included all the patients aged > 18 years, who were attending the Pulmonary Medicine OPD at AIIMS Patna, were suspected of OSA on the basis of clinical history, STOP-BANG score (≥ 3 considered as high risk), and Epworth sleepiness score (≥ 9 considered as positive) and were willing to give consent to participate in this study.
Patients who denied voluntary consent, were hemodynamically unstable, had a normal AHI on polysomnography (AHI < 5) or only snore were excluded from the study. Patients with congestive heart failure (CHF), long term home oxygen therapy (LTOT)/ home noninvasive ventilation (NIV), COVID-19 active infection, known cases of neuromuscular disease or other sleep disorders (Parasomnias, Insomnias, Narcolepsy) were also excluded.
Data were collected using a predesigned, structured proforma (Supplementary material) covering information related to demography, anthropometric data, lab parameters, and polysomnographic data. Sleep studies were performed using either of the two machines, Embletta MPR PSG Sleep Study System and Alice 6 LDxN base station.
For sample size calculation, we used data from a Tasmanian longitudinal health study published in the Journal of Sleep Research (Senaratna et al. 2019), a sixth-decade follow‐up that used a random subsample of 296 from a population-based cohort to determine the concordance between flow-based AHI and ODI, which was found to be fair (k = 0.32). The overall prevalence of OSA was found to be 30%. Keeping the margin of error as 20%, the sample size was 234. However, in view of the COVID-19 pandemic, we were able to enroll 200 patients only, out of whom 24 patients were excluded in view of AHI < 5. Hence, a total of 176 patients were studied.
The SPSS (Statistical Package for the Social Science)—22 version statistical program for Microsoft Windows was used. Quantitative data are expressed as the mean ± standard deviation or median (interquartile range), according to data distribution. The distribution of the variables was analysed with the Shapiro‒Wilk test. Qualitative data were expressed as counts and percentages. The significant differences between the two groups were tested with independent samples t tests or Mann‒Whitney U tests to determine whether the data were normally distributed. The chi-square test was used to compare discrete variables. Statistical significance was set at an alpha level of 0.05 with P (probability value) < 0.05, with a two-tailed probability. Spearman’s rank correlation analysis was used to test the strength and direction of the association between OSA severity (using AHI classification) and the Oxygen Desaturation Index.
Definitions (American Academy of Sleep Medicine 2014)
APNOEA—defined as a 90% reduction in peak signal deviation from the baseline before the incident, using an alternate apnea sensor, PAP device flow, or oronasal heat sensor (diagnostic study), and the duration of the 90% decline in the sensor signal is less than 10 s.
HYPOPNEA is defined as a ≥ 30% reduction in peak signal deviation from the baseline before the incident, using an oronasal heat sensor (diagnostic study), PAP device flow (titration study, or alternative apnea sensor (diagnostic study) for a duration of ≥ 10 s and a ≥ 3% oxygen desaturation from the baseline before the event or an arousal connected to the event.
APNOEA HYPOPNEA INDEX—(No. of apnoeas + No. of hypopnoeas) × 60) /Total sleep time (min) [TST].
OXYGEN DESATURATION INDEX—(No. of oxygen desaturations ≥ 3% × 60) /TST in min.
The AHI and ODI were used to categorize OSA severity. According to the 2012 American Academy of Sleep Medicine guidelines, patients were categorized as mild (AHI = 5–14 events/hour), moderate (AHI = 15–29 events/hour), or severe (AHI > 30 events/hour) (American Academy of Sleep Medicine 2014). Patients were categorized into three categories based on their ODI scores: mild (ODI = 5–14 incidents/hour), moderate (ODI = 15–29 incidents/hour), and severe (ODI > 30 incidents/hour) (Mediano et al. 2007; Spuy et al. 2017; Torre-Bouscoulet et al. 2007; Temirbekov et al. 2018).
Results
A total of 200 subjects underwent an in-lab nocturnal polysomnography study after meeting the inclusion criteria and ruling out exclusion criteria. Approximately 24 subjects had an AHI < 5 on polysomnography and hence were excluded from the study. Finally, 176 patents were studied and evaluated for primary and secondary outcomes. Patient demographic data have been described in two groups: mild to moderate OSA and severe OSA based on AHI (Table 1). Therefore, based on the AHI, OSA was classified as mild to moderate in 51 (28.98%) patients and severe in 125 (71.02%) patients. A total of 106 (60.2%) subjects were male, and 70 (39.8%) patients were female, giving a male/female ratio of OSA of 1.5: 1 in our study. The mean age of the study participants was 47.42 ± 12.60 years (mean + standard deviation), with a mean BMI of 33.89 ± 5.37 kg/m2. Male gender (28% vs 73.68%; p < 0.05). Nineteen patients (10.8%) were diagnosed with mild OSA, 32 patients (18.18%) with moderate OSA, and 125 patients (71.02%) with severe OSA, according to the AHI results of the recruited patients. Each parameter's range of OSA grades (AHI, ESS, STOP BANG, and ODI) has been shown in Table 2.
None of the parameters, including AHI (Spearman’s rho = 0.040, p value = 0.597), ODI (Spearman’s rho = -0.004, p value = 0.960), STOP BANG (Spearman’s rho = 0.087, p value = 0.249), and ESS (Spearman’s rho = 0.072, P value = 0.345), showed a significant correlation with age. Similarly, none of the parameters, including AHI (Spearman’s rho = -0.079, p value = 0.297), ODI (Spearman’s rho = 0.054, p value = 0.475), STOP BANG (Spearman’s rho = 0.138, p value = 0.067), and ESS (Spearman’s rho = -0.049, P value = 0.519), showed a significant correlation with neck circumference. However, there were positive correlations seen in AHI (Spearman’s rho = 0.175, p—value = 0.02) and ODI (Spearman’s rho = 0.151, p value = 0.045) with body mass index (BMI), but both questionnaires showed no significant correlation with BMI (p > 0.05).
AHI AND ODI
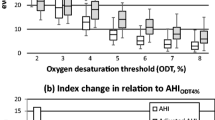
Among 176 patients, the values of AHI and ODI were distributed normally as tested by visual inspection of the Q‒Q scatter plot and box plot with few outliers (< 3). On applying Pearson’s correlation test, a statistically significant positive correlation could be traced between AHI and ODI values in overall patients of OSA enrolled in the study and among the patients with AHI > 15 events/hour, i.e., moderate and severe OSA (p0.05) in patients with mild OSA. (Table 3).
Between AHI and ODI, Cohen's kappa study revealed a significant concordance of 75.5% (k = 0.7), which implies substantial agreement between AHI and ODI in classifying the severity of OSA as mild, moderate, and severe (Table 4). Despite good correlation and cohesion between AHI and ODI, multiple interpretations can be withdrawn among the same patients; only 11 (57.8%) of 19 patients classified as having mild OSA based on AHI met the criteria for mild OSA based on ODI, 12 (37.5%) met criteria for moderate OSA, and 110 (88%) met criteria for severe OSA according to the ODI.
AHI and ODI Correlation in Obese Individuals
Taking the cut off for obesity [43] as 30 kg/m2, 70.4% (n = 124) were obese, and 29.6% (n = 52) had a BMI < 30 kg/m2. Both groups showed a statistically significant positive correlation between AHI and ODI. (p -value < 0.05).
STOP BANG and OSA
On the basis of the STOP BANG score, 11 patients were categorized as low risk for OSA, with 26.3%, 3%, and 4% belonging to the mild, moderate, and severe categories of OSA on the basis of AHI, respectively, and 165 patients were classified as high risk for OSA, of which 73.7%, 97%, and 96% were classified as mild, moderate, and severe OSA, respectively. These data were statistically significant (p < 0.05). Similarly, comparing the mean values of STOP BANG scores in mild to moderate OSA with severe OSA, showed significantly higher values in severe OSA (p value = 0.028) (Table 1). This implies that the patients screened as high risk for OSA on the basis of the STOP BANG Score (cut-off > 3) will have higher chances of increased AHI on polysomnography, especially moderate to severe OSA.
On further evaluating the STOP BANG score in predicting a high risk of OSA, it was found to have high sensitivity (96.18%) and specificity (26.31%), positive predictive value (91.5%), and negative predictive value (45.4%).
STOP-BANG also looked forward if it had any correlation with increasing severity of OSA on the basis of AHI and ODI, but no correlation was found between STOP BANG when compared with either AHI or ODI -based OSA severity, calculated via Spearman’s test. (p value > 0.05) (Table 5).
ESS and OSA
The mean value of ESS score overall in all study participants irrespective of severity was (10.26 + 3.617), and it did not differ significantly in the two groups of OSA, with 10.03 (3.84) in mild to moderate OSA and 10.23 (3.5) in severe OSA (p value > 0.05). Taking a cut off as 14 for this parameter, only 11 (8%) patients out of 125 severe OSA patients, scored ESS > 14. The overall sensitivity and specificity of ESS in predicting the severity of OSA (based on AHI) were less than those of the STOP BANG score (Table 6).
When ESS correlation was examined with AHI and ODI-based OSA grading, no association was detected (p > 0.05) (Table 5).
Discussion
OSA is one of the most common presentations from the spectra of SRBD. The gravity of OSA has come to the forefront with instances like the unfortunate passing of the legendary Indian singer, Bappi Lahiri, attributed to this sleep disorder. While such cases bring public attention to the serious implications of OSA, they also highlight the need for increased awareness and effective management of this widespread health issue. These real-world examples serve as poignant reminders that OSA is not confined to statistical estimates but has tangible and impactful consequences on individuals. By understanding the broader implications, we can appreciate the urgency of addressing OSA comprehensively. In our study, the ratio of males to females was 1.5:1, which is in accordance with previous studies (Young 1993), explaining the reason for the structure and physiological behavior of the upper airway, craniofacial morphology, sex hormones, and the pattern of fat deposition (Pillar et al. 2000). Compared to severe OSA, mild and moderate degrees were slightly more prevalent in females in our study, which is contrary to the rest. Exact reasons are not fully understood, but discrepancy can be attributed to clinical significance, and the potential of morbidity for having a high AHI in the general population is lower in females along with sociocultural factors or differences in clinical expression of this disorder and hence underdiagnosis of OSA in females (Quintana-Gallego et al. 2004). Sociocultural dynamics are multifaceted, encompassing societal norms, gender roles (Stigma and Gender Stereotypes), healthcare Accessibility / awareness and healthcare-seeking behavior (cultural expectations and prioritization of health). Our study showed that hypertension was a significant risk factor associated with OSA (p value 30 kg/m2), and a statistically significant correlation between BMI and AHI and ODI was observed. A similar correlation could be traced between AHI and ODI in patients with BMI < 30 kg/m2. This is in agreement with past studies (Sharma et al. 2006) and reviews (Romero-Corral et al. 2010), but few studies have shown no correlation between OSA and BMI (Ciavarella et al. 2018). A study conducted by Sharma et al. (Sharma et al. 2006) showed obesity defined by high BMI as a significant risk factor for OSA. It hereby explains the rationale of weight loss in OSA patients. The apnea–hypopnea index (AHI) is currently considered the gold standard of choice in polysomnographic studies for the estimation of the severity of OSA. Cohen’s weighted Kappa analysis in our study showed a good level of concordance (k = 0.7) and proportional agreement (75.5%) between AHI and ODI (p < 0.001) in predicting the severity of OSA.
This is more emphasized by the R2 value of 0.93 in the linear regression plot. An R2 value of 0.93 indicates the proportion of the variance in the dependent variable (ODI) that can be explained by the independent variable (AHI) in the linear regression model. It implies that approximately 93% of the variation in ODI can be accounted for or predicted by changes in AHI. These results are in agreement with those of the studies by (Temirbekov et al. 2018) and (Varghese et al. 2022), where a concordance of 72.3% and 87.32% between AHI and ODI was observed, respectively. In view of the above findings, we postulate that ODI could be used as a useful alternative parameter for determining the severity of OSA. However, since ODI cannot explain the origin of apnea/hypopnea, whether central or obstructive, taking ODI alone will not serve the purpose. It is essential to underscore the continued importance of polysomnography (PSG) in the diagnostic process. ODI can serve as a valuable screening tool due to its relative simplicity and cost-effectiveness. ODI-based assessments provide a convenient initial step, offering a glimpse into potential respiratory disturbances during sleep. Hence, it is recommended that patients who are categorized as moderate to severe OSA based on ODI should be subjected to PSG. PSG has financial implications, and limited availability, and is expertise dependent, which restricts its frequent use. In India, there is a dire need or a convenient, inexpensive, and accurate tool that can identify at risk patients for SDB at the earliest. The results from our study suggest that nocturnal ODI calculation via pulse oximetry during sleep can be a very useful tool in assisting the same, which is in agreement with past studies (Temirbekov et al. 2018; Varghese et al. 2022; Levendowski et al. 2019; Duarte et al. 2019a; Ernst et al. 2016). The present study demonstrates that the STOP-Bang questionnaire is useful in identifying patients at high risk for OSA, but the same cannot be seen with ESS. We found that both the STOP-Bang questionnaire and ESS had higher sensitivity (especially the STOP Bang score) and a low specificity, which is in accordance with other studies (Duarte et al. 2019a, 2019b; Marti-Soler et al. 2016; Hong et al. 2018). Excessive daytime sleepiness (EDS) is a well-known consequence of obstructive sleep apnea. ESS is one of the questionnaires indicating the severity of EDS. ESS in our study showed a poor correlation to the severity of OSA and had a lower sensitivity and specificity compared to the STOP BANG score, which had a low sensitivity (Veugen et al. 2021). The predictive ability of the STOP-BANG questionnaire to differentiate the various grades of OSA was similar in relation to both the AHI and ODI. The STOP-BANG questionnaire, however, may have a high rate of false positives and increase the number of pointless overnight recordings due to its limited specificity and strong positive predictive value. ESS relies on self-reported subjective measures of sleepiness based on a respondent's perception of their likelihood to doze off in various situations which can likely have various potential limitations including subjectivity and recall bias, adaptation to chronic sleepiness, limited assessment of respiratory disturbances. On the other hand, the simplicity and accessibility of the STOP-Bang questionnaire, coupled with its notable predictive power, make it a valuable screening tool in various healthcare settings. Its ability to capture multiple risk factors, including snoring, tiredness, observed apneas, high blood pressure, body mass index (BMI), age, neck circumference, and gender, contributes to its effectiveness in identifying individuals who may benefit from further sleep evaluation.
There are some limitations to our study as well, which includes a single-center study with a small sample size that also had a very small sample size in mild OSA and excluded patients with AHI < 5, so before projecting this result on a large population, more studies with large sample sizes are needed in this direction. Taking ODI alone as a diagnostic marker of OSA will be wrong to consider, as it lacks the ability to predict the origin of sleep apnea, so all the patients who are diagnosed as moderate to high risk on the basis of ODI should always be subjected to in-lab polysomnography. The influence of a few other factors, such as low hemoglobin, hypotension, and increased frequency of movements during sleep, can act as a confounding factor and can be the cause of misleading results from pulse oximetry. Further researches with inclusion of comprehensive health assessments, multivariate analysis, matching / stratification, and standardized measurement protocols are needed to escape the effect of these confounding factors. Additionally, the subjects of this study included all patients who were referred to the Pulmonary Medicine OPD with a suspected diagnosis of OSA, therefore, the non-OSA group was not studied here; causing a selection bias.
Conclusion
We recommend that in addition to AHI, ODI can be used as an important parameter for the diagnosis and classification of OSA and should not be neglected in PSG assessment of OSA patients. In fact, in the era of the pandemic, in a developing country where OSA is increasing in prevalence and there is a lack of proper and sufficient laboratory sleep study availability, screening OSA patients via ODI can be beneficial.
Availability of data and materials
The dataset(s) supporting the conclusions of this article is(are) included within the article. The datasets generated and/or analysed during the current study are not publicly available to maintain the confidentiality but are available from the corresponding author on reasonable request.
Abbreviations
- TPS:
-
Test of Pragmatic Skills
- AHI:
-
Apnea Hypopnea Index
- ODI:
-
Oxygen Desaturation Index
- OSA:
-
Obstructive Sleep apnea
- PSG:
-
Polysomnography
- EEG:
-
Electroencephalogram
- EOG:
-
Electrooculogram
- EMG:
-
Electromyogram
- SRBD:
-
Sleep related breathing disorders
- CPAP:
-
Continuous positive airway pressure
- UARS:
-
Upper airway resistance syndrome
- EDS:
-
Excessive daytime sleepiness
- p – Value:
-
Probability value
- OPD:
-
Out-patient Department
- PAP:
-
Positive airway pressure
- TST:
-
Total sleep time
- HSAT:
-
Home Sleep Apnea Testing
- BMI:
-
Body Mass Index
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- OHS:
-
Obesity Hypoventilation syndrome
- ESS:
-
Epworth sleepiness score
- S.D.:
-
Standard deviation
- LTOT:
-
Long-term oxygen therapy
- CHF:
-
Congestive heart failure
- NIV:
-
Non-invasive Ventilation
- SPSS:
-
Statistical Package for the Social Science
References
American Academy of Sleep Medicine. International Classification of Sleep Disorders. Darien, IL: American Academy of Sleep Medicine; 2014, 2018.
Cao W, Luo J, Xiao Yi. A Review of Current Tools Used for Evaluating the Severity of Obstructive Sleep Apnea. Nature and Science of Sleep. 2020;12:1023–31. https://doi.org/10.2147/NSS.S275252.
Ciavarella D, Tepedino M, Chimenti C, Troiano G, Mazzotta M, Foschino Barbaro MP, et al. Correlation between body mass index and obstructive sleep apnea severity indexes - A retrospective study. Am J Otolaryngol. 2018;39(4):388–91. Epub 2018 Mar 27
Duarte RLM, Mello FCQ, Magalhães-da-Silveira FJ, Oliveira-e-Sá TS, Rabahi MF, Gozal D. Comparative performance of screening instruments for obstructive sleep apnea in morbidly obese patients referred to a sleep laboratory: a prospective cross-sectional study. Sleep Breath. 2019a;23:1123–32. https://doi.org/10.1007/s11325-019-01791-w.
Duarte RLM, Magalhães-da-Silveira FJ, Oliveira-e-Sá TS, Rabahi MF, Mello FCQ, Gozal D. Predicting obstructive sleep apnea in patients with insomnia: a comparative study with four screening instruments. Lung. 2019b;197:451–8. https://doi.org/10.1007/s00408-019-00232-5.
Ernst G, Bosio M, Salvado A, Dibur E, Nigro C, Borsini E. Difference between apnea-hypopnea index (AHI) and oxygen desaturation index (ODI): proportional increase associated with degree of obesity. Sleep Breath. 2016;20(4):1175–83. https://doi.org/10.1007/s11325-016-1330-3. (Epub 2016 Mar 30 PMID: 27026417).
Goyal A, Pakhare A, Joshi A. Prevalence of OSA in Indian population and diagnostic accuracy of different tools for OSA. ERJ Open Research. 2023;9(suppl 11):58. https://doi.org/10.1183/23120541.sleepandbreathing-2023.58.
Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–8.
Hong C, Chen R, Qing S, Kuang A, Yang HJ, Su X, et al. Validation of the NoSAS score for the screening of sleep-disordered breathing: a hospital-based retrospective study in China. J Clin Sleep Med. 2018;14:191–7. https://doi.org/10.5664/jcsm.6930.
Kulkas A, Tiihonen P, Julkunen P, Mervaala E, Töyräs J. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med Biol Eng Comput. 2013;51(6):697–708.
Levendowski DJ, Hamilton GS, St Louis EK, Penzel T, Dawson D, Westbrook PR. A comparison between auto-scored apnea-hypopnea index and oxygen desaturation index in the characterization of positional obstructive sleep apnea. Nat Sci Sleep. 2019;11:69–78. Published 2019 Jul 12. https://doi.org/10.2147/NSS.S204830.
Marti-Soler H, Hirotsu C, Marques-Vidal P, Vollenweider P, Waeber G, Preisig M, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4:742–8. https://doi.org/10.1016/S2213-2600(16)30075-3.
Mediano O, Barceló A, de la Pe-a M, Gozal D, Agustí A, Barbé F. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J. 2007;30:110–3. https://doi.org/10.1183/09031936.00009506.
Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Respir Crit Care Med. 2000;162:1627–32.
Quintana-Gallego E, Carmona-Bernal C, Capote F. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Resp Med. 2004. https://doi.org/10.1016/j.rmed.2004.03.002.
Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–9. https://doi.org/10.1378/chest.09-0360.PMID:20202954;PMCID:PMC3021364.
Senaratna CV, Lowe A, Perret JL, Lodge C, Bowatte G, Abramson MJ, et al. Comparison of apnoea-hypopnoea index and oxygen desaturation index when identifying obstructive sleep apnoea using type-4 sleep studies. J Sleep Res. 2019;28(5). https://doi.org/10.1111/jsr.12804. Epub 2018 Dec 18. PMID: 30565351
Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi. India Chest. 2006;130(1):149–56.
Temirbekov D, Güneş S, Yazıcı ZM, Sayın İ. The Ignored Parameter in the Diagnosis of Obstructive Sleep Apnea Syndrome: The Oxygen Desaturation Index. Turk Arch Otorhinolaryngol. 2018;56(1):1–6. https://doi.org/10.5152/tao.2018.3025. Epub 2018 Mar 1. PMID: 29988275; PMCID: PMC6017211.
Tkacova R, McNicholas WT, Javorsky M, Fietze I, Sliwinski P, Parati G, et al. Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. European Sleep Apnoea Database study collaborators. Eur Respir J. 2014;44(4):931–41.
Torre-Bouscoulet L, Castorena-Maldonado A, Ba-os-Flores R, Vázquez-García JC, Meza-Vargas MS, Pérez-Padilla R. Agreement between oxygen desaturation index and apnea-hypopnea index in adults with suspected obstructive sleep apnea at an altitude of 2240 m. Arch Bronconeumol. 2007;43:649–54.
Van der Spuy I, Karunanayake CP, Dosman JA, McMullin K, Zhao G, Abonyi S, et al. Determinants of excessive daytime sleepiness in two First Nation communities. BMC Pulm Med. 2017;17:192. https://doi.org/10.1186/s12890-017-0536-x.
Varghese L, Rebekah G, N P, Oliver A, Kurien R. Oxygen desaturation index as alternative parameter in screening patients with severe obstructive sleep apnea. Sleep Sci. 2022;15(Spec 1):224–8. https://doi.org/10.5935/1984-0063.20200119. PMID: 35273770; PMCID: PMC8889990
Veugen, C.C.A.F.M., Teunissen, E.M., den Otter, L.A.S, et al. Prediction of obstructive sleep apnea: comparative performance of three screening instruments on the apnea-hypopnea index and the oxygen desaturation index. Sleep Breath. 2021;25(3):1267–75.
Wali SO, Abalkhail B, Krayem A. Prevalence and risk factors of obstructive sleep apnea syndrome in a Saudi Arabian population. Ann Thorac Med. 2017;12(2):88–94.
Young T. Analytic epidemiology studies of sleep-disordered breathing: what explains the gender difference in sleep disordered breathing? Sleep. 1993;16:S1–2.
Acknowledgements
No other acknowledgement to be declared.
Funding
No funding is available to declare while designing of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All the authors have equally contributed in collecting data, analysing and interpreting data as well as writing this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval from institute Ethical committee has been taken and written informed consent was taken from all participants included in study. Name and number of the ethics committee has been mentioned in methodology as AIIMS/Pat/IEC/PGTh/July 20/28.
Consent for publication
Consent for publication has also been taken in the informed consent by all the participants enrolled. No images and videos of patients has been taken.
Competing interests
We declare no financial and non-financial competing interests with this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharma, P., Thakur, S., Rai, D.K. et al. Connecting the dots: analysing the relationship between AHI and ODI in obstructive sleep apnea. Sleep Science Practice 8, 9 (2024). https://doi.org/10.1186/s41606-024-00102-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41606-024-00102-x