Abstract
More than a decade ago, PIWI-interacting RNA (piRNA) was discovered almost simultaneously by four different research groups. The length of this type of single stranded noncoding RNA is 24~31 nucleotides (nt), with most of the piRNAs fall into the range of 29~30 nt. PiRNAs form specific RNA-induced silencing complex with PIWI subfamily proteins, which is how piRNA got its name. PiRNAs are initially famous for the crucial roles they played in germline cells. Binding with PIWI family proteins, piRNAs is able to affect methylation of genomic DNA in germline cells and, therefore, maintaining genomic stability and suppressing transposons. Since mammalian PIWI subfamily proteins are mainly germline specific, it was once thought that piRNAs might only function in the gonadal cells. However, evidence from the later research suggest that piRNAs are broadly expressed in many kinds of somatic cells and are involved in numerous pathological condition far beyond those have been reported in the germline. For example, piRNAs were discovered to be abnormally expressed in several kinds of cancers. PiRNAs are also shown to be promising prognostic markers for various types of cancers. Interestingly, a recent research indicated that piRNAs are also regulators for pancreatic beta cell function. PiRNAs are promising regulators for the development of type 2 diabetes. From a disease-oriented point of view, this review will focus on both confirmed and proposed biological functions of piRNAs mostly in those fields beyond germline cells. Meanwhile, some of the underlying molecular mechanisms will also be mentioned.
Similar content being viewed by others
Background
Mammalian Argonaute proteins, which are segregated into two subfamilies named AGO and PIWI, are indispensable components for the execution of the small non-coding RNA (snoRNA) function [1]. AGO clade of Argonaute proteins are famous for the critical role they played in the micoRNA (miRNA) and short interference RNA (siRNA) pathways [2]. Complexed with AGO protein, siRNA and miRNA could bind perfectly with the target RNAs and trigger the cleavage and degradation of target mRNA. The AGO-miRNA complex could also bind imperfectly to the 3’UTR region of the target mRNAs, which results in their de-adenylation, translational suppression, and subsequent degradation [3, 4]. It was initially reported that PIWI clade of the Argonaute proteins are important players in the formation, development and meiosis of germline stem cells. The studies on miRNAs and siRNAs gave the scientists great motivation to find out the potential RNA guides for PIWI proteins. Excitingly, they found out a novel class of RNA sized 24~31 nt, which is clearly longer than the well-characterized 21~23 nt of miRNAs or siRNAs [5,6,7,8]. Different from miRNAs, piRNAs are 2′-O-methylated at the 3′-termini [9]. They coined the name PIWI-interacting RNA (piRNA) for this particular class of small noncoding RNAs.
Since many of the members of PIWI family proteins are mainly germline restricted, early research on piRNA after its discovery were mainly focused on germline cells. Tremendous amount of germline-related research of piRNA exhibited that piRNAs are functional related to germline development, maintaining germline genome integrity, silencing of selfish DNA element, etc. [10,11,12]. One icebreaking research that investigated the function of piRNA beyond germline cells reported that PIWI-piRNA complex could bind to specific genomic sites in somatic cells and regulate target DNA locus epigenetically. It was revealed that PIWI also binds to the Heterochromatin Protein 1A (HP1A) and is responsible for the methylation of H3K9 at numerous genomic sites in somatic cells, which suggests that piRNA could contribute to the epigenetic modification of the genome in somatic cells [13]. Other studies, for example, identified that piRNAs exist in the mouse hippocampus [14]. The mouse hippocampal piRNAs are localized in both neurons and dendrites. Suppression of one of the piRNAs, DQ541777, could cause defects in the morphogenesis of spine [14]. Until now, the biological functions of piRNAs have been widely expanded into the fields such as metabolism, cardiology, and especially oncology. The disease-related biological function of piRNAs will be the main focus in this review article. Experimental results, prospective insights as well as some of the underlying molecular mechanisms will be listed and discussed. Through this review, we hope to broaden our view on the function of piRNA in various types of diseases, and more importantly, to provide some new insights for the future piRNA research field.
Biogenesis and functions of piRNAs
To better understand the clinical roles of piRNAs, the biogenic pathways as well as the general functioning mechanism of piRNAs in mouse germline tissue are briefly discussed firstly. The detailed review of this area could be found in several other review articles [15,16,17].
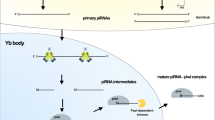
The piRNA production process is mainly composed of two pathways: the primary processing pathway and the secondary ping-pong cycle. The precursor piRNAs are firstly transcribed as long unidirectional single-stranded transcripts mostly from piRNA clusters which have a number of sequences complementary to transposons (transposon-derived piRNA). Researchers later found that besides those transposon-derived piRNA, some piRNAs have different origin. Bioinformatics study revealed that there are three different source of piRNA: transposon-derived, protein coding region-derived and intergenic region-derived piRNA. The ratio of these three different originated piRNAs varies during different phases of spermatogenesis [18]. The long single-stranded transcripts are subsequently cut into piRNA pieces containing various lengths, the underlying mechanism of which still remains elusive. Owing to the loop construct within the MID domain of PIWI proteins, PIWI protein has strong binding preference with small RNA pieces that have uridine residue located in the 5′ ends [19, 20]. The 3′ end of the small RNA pieces are then trimmed by exonuclease after binding to PIWI protein [21]. The size of different PIWI proteins determines the length of the mature piRNAs. The piRNA precursor is characterized by the tendency of having uridine residue at 5′ ends, and different sized piRNAs bind to specific PIWI protein species. Finally, the process of 2′-O-methylation at the 3′ end of piRNA by Hen1 methyltransferase marks the maturation of PIWI-piRNA complexes [17]. The production process of secondary piRNAs is well-known as ping-pong cycle. The primary piRNA recognizes mRNAs that are reverse transcribed from transposon through base-paring. Mili protein cut the complimentary mRNA at 5′ ends of primary piRNA between 10 and 11 nt position and results in the RNA product that displays a strong bias for adenine at the 10th positon. The RNA piece then associates with miwi2 and goes through the same 3′ end trimming and modification processes as described in primary piRNA production, which gives rise to mature secondary piRNA. The piRNA, which associated with miwi2, undergoes similar procedures in target mRNA recognition, 5′ end trimming, binding with mili protein, 3′ end trimming and modification. Large amount of piRNAs are obtained as ping-pong cycle repeats (Fig. 1) [17, 22].
Biogenic pathways of piRNA in mice. PiRNA precursor is transcribed from piRNA cluster, protein coding region or intergenic region, and it is further cut into small pieces that varies in length. After RNA pieces associate specifically with PIWI, they undergo 3′ end trimming process. The 3′ end is further modified by the RNA methyltransferase Hen1, which marks the maturation of primary piRNA. The secondary biogenesis process of piRNA is known as ping-pong cycle: mili firstly binds to primary piRNA, which is followed by the association between primary piRNA and mRNA through base-paring. Mili cut the paired mRNA between 10 and 11 nt position at 5′ end. The resultant RNA piece binds to miwi2 and goes through the same 3′ end trimming and modification procedure as described above. The miwi2-associated piRNA recognize target RNA in a similar manner, which is again followed by 5′ end trimming, mili association, 3′ end trimming and modification. The cycle then repeats
There are two major functions of piRNA. At transcriptional level, the miwi2/piRNA complex could go into the nuclear, recognize the chromosomal loci that are undergoing transcription, recruit modification enzymes and alter the methylation level on the corresponding region, which results in the silencing of either transposon genes or protein coding genes on the transcriptional level (Fig. 2). At post-transcriptional level, a great deal of mRNAs transcribed by transposons in the genome is cut during the process of piRNA generation, which leads to the stabilization of the genome. PiRNA may as well recognizes mRNAs beyond those derived from transposon through imperfect base pairing, a process that is similar to microRNA (miRNA) regulate its target mRNA, which leads to the subsequent mRNA deadenylation and degradation. It is worth to mention that piRNA may target multiple genes simultaneously (Fig. 2) [23,24,25]. From the way of piRNA functioning mentioned above, we could deduce the general role of piRNA played in various types of disease. In different diseases, the altered expression of piRNAs could affect the expression of many genes through either transcriptional inhibition or epigenetic modification. Protein encodes by those genes could be involved in many disease-related signaling cascades that regulated numerous cellular events such as proliferation, apoptosis, migration, invasion etc.
The functional pathways of piRNA. (1) The PIWI-antisense piRNA complex cleaves sense transposon, piRNA precursor and protein-coding transcripts, the process of which leads to the suppression of transposon and gene expression. (2) PIWI-piRNA complex could suppress translation of target mRNA through imperfect binding to the 3’UTR region, a process similar to how miRNA functions. (3) PIWI-piRNA complex could enter the nucleus and influence DNA methylation level in the promoter region of target genes. “Me”, methylation
PiRNAs in cancer
The most intensively studied disease in which piRNAs take parts is cancer. Large amount of pioneer researches which paved the way for finding the link between piRNA and cancer deal with the function of PIWI family proteins in different types of cancer. One study reported that Hiwi, a member of the PIWI family protein, is found to be expressed in human gastric cancer cells. Hiwi is able to induce gastric cancer cell growth, while inhibition of Hiwi could promote cell cycle arrest in G2/M phase. It was thus suggested that Hiwi might be a promising drug target for gastric cancer therapy [26]. Similar results were obtained in the lung cancer as well. The protein and mRNA levels of Hiwi were significantly upregulated in the intra-tumor non-small cell lung cancer (NSCLC) samples. The expression of Hiwi had close relationship with cancer cell proliferation [27]. In pancreatic cancer, on the other hand, altered mRNA expression of hiwi had poorer clinical prognosis [28]. Other researches showed that mouse and human PIWI proteins were widely expressed in numerous types of cancer including lung, gastrointestinal, liver, breast, colorectal, and ovarian cancer [29,30,31,32,33,34]. Further immunohistochemistry (IHC) investigations with different stages of human cancer sample suggested that PIWI is a potential biomarker for cervical cancer and breast cancer [35,36,37]. Mechanistically, the human PIWI family protein Piwil2 is able to influence two signaling cascades, Stat3/Bcl-X and Stat3/cyclinD1 signaling, and could act as an oncogene in tumorigenesis. Inhibition of Piwil2 expression suppresses tumor cells growth both in vitro and in vivo [38]. It is worth to mention that Tudor domain containing protein 9 (TDRD9), a helicase that is involved in the biosynthesis of piRNA plays a crucial role in lung adenocarcinoma. The expression of TDRD9 is linked with poor prognosis in lung adenocarcinoma. On the contrary, knock-down of TDRD9 could lead to a decrease in apoptosis, cell cycle arrest and proliferation [39]. Although all the research above are not directly on piRNAs, they are closely related to piRNA study and provided invaluable evidences and ideas for the piRNA research field.
The study that really linked piRNAs with cancer was performed in Hela cells, a well-known human cervical cancer cell line. After examine the whole small RNA library in Hela cells, Lu and colleagues (2010) discovered the existence of piRNAs within the library. In situ hybridization results showed that piR-49322 localizes in both nucleolus and cytoplasm in Hela cells, especially gathers around the periphery of nuclear membrane [40].
In the year of 2015, a bioinformatics study systematically explored piRNA transcriptomes of 6260 human tissues samples from both normal and cancerous tissues of many organs. The piRNA expression pattern in twelve different tumor types was analyzed. Overall, a higher number of piRNAs expressed in tumors compared to normal tissues. The patterns of size distribution between normal and tumor tissues are also different, with an enrichment of 32 nt long-piRNAs across all tumor types. PiRNA expression patterns showed their uniqueness to malignancies and clinical categories. While some piRNA exhibited similar expression pattern across all cancer types, some showed subgroup-specific expression pattern and relevance to certain clinical features that belong to each individual tumor type [41].
PiRNA microarray data from human gastric cancer showed that piR-651 level was closely correlated with tumor-node-metastasis (TNM) stage. High levels of piR-651 were found in those patients that have poorly differential tumors. Further exploration revealed that the level of piR-651 was also increased in many other types of cancers such as hepatic carcinoma, breast cancer, stomach cancer, cervical cancer, lung cancer, and so on [42]. In the meantime, the same group discovered that, in contract to piR-651, piR-823 is less expressed in gastric cancer tissue than in the control tissue. Data from the xenograft mice model indicated that piR-823 plays an antagonistic role in gastric cancer development [43]. A tentative study tried using piRNAs as biomarkers to detect circulating cancer cells in the blood from gastric cancer cases. Researchers detected that the levels of piR-823 and piR-651 in the peripheral blood from gastric cancer patients were markedly lower than the control samples. The level of piR-823 is positively correlated with TNM stage as well as distant metastasis [44]. The results above provided evidence for using certain piRNAs as biomarkers for detecting circulating gastric cancer cells.
Another intensively studied type of cancer in which piRNAs are involved is breast cancer (BC). It was found that the key biogenic components as well as the effectors are present in human BC cells and tumor biopsies [45]. Around 40% of the BC piRNAs are located in the protein coding/small non-coding RNA genomic regions, which suggest that the corresponding transcript might serve as piRNA precursors that subsequently leading to the regulation of their host genes. The hypothesis is in agreement with the finding in this study that the putative target mRNAs regulated by the 8 piRNAs found deregulated in BC tissues encode proteins involved in key cancer cell function [45]. To explore the expression status of piRNAs in human BC clinical samples, Huang et al. (2012) did deep piRNA sequencing on 4 tumor tissues and their corresponding normal tissues. Four piRNAs (piR-20365, piR-4987, piR-20582 and piR-20485) were up-regulated in 50 breast cancer cases. PiR-4987 expression level was correlated with lymph node metastasis [46]. Zhang et al. (2013) did a piRNA microarray analysis on ten cases of BC including cancer stem cells induced to Epithelial Mesenchymal Transition (EMT) status using TGF-β. They found that piR-932, which forms a complex with PIWIL2, displayed markedly higher expression in EMT cancer stem cells. Based on the fact that the expression of tumor suppressor protein Latexin is decreased due to the hypermethylation in its promoter region in cancer cells, the authors speculated that piR-932 might be a stimulator of the BC cell EMT process by stimulating the methylation of Latexin promoter region [47]. The clinical-oriented studies above are challenged with limited sample sizes. Later on, Krishnan et al. investigated the piRNA profile from a larger clinical sample collection including 104 breast cancer samples using next generation sequencing. PiRNAs as well as PIWI genes were evaluated for their prognostic significance from clinical features of Overall Survival (OS) and Recurrence Free Survival (RFS). Totally 8 piRNAs were identified as potential markers for breast cancer prognosis. Four and six piRNAs were discovered to be linked with OS and RFS respectively, among which 2 piRNAs correlate with both OS and RFS [48]. Single theragnosis system utilizing a piRNA molecular beacon (MB) enabled visualization of specific piRNA expression in cancer cells and molecular activation for tumor suppression. By using this technology, Lee and colleagues (2016) directly visualized the expression of piR-360269 in MCF7 cells. Being able to hybridize with endogenous piR-360269, piR-36026 MB is able to inhibit the function of piR-360269, which result in cellular death through caspase-3 mediated signaling. Bioinformatics study revealed SERPINA1 and LRAT could be the downstream targets of piR-360269. To further confirm this regulatory cascade, the authors did a mutiplex fluorescence analysis by simultaneously introduce piR-36026 MB, GFP-SERPINA1, GFP-LRAT, caspase-3 function fluorescent probe, Hoechst and PI into single MCF7 cell. Results indicated that piR-360269 could directly target SERPINA1 and LRAT, which protect MCF7 cells from caspase-3 mediated cell apoptosis. What is more, the MCF7 cells from above were further injected into the nude mice. In vivo assay results also showed the protective role of piR-360269 in BC cell apoptosis. When piR-360269 expression was inhibited by piR-360269 MB, clear activation signals of caspase-3, LRAT and SERPINA1 were visualized. Hematoxylin and eosin (H&E) stain results displayed that tissue isolated from injection site contains numerous apoptotic tumor cells [49]. As we know, triple-negative breast cancer (TNBC) is an aggressive, poorly-prognostic cancer. Koduru and colleagues (2016) analyzed the publicly available small RNA sequencing data from 24 TNBC and 14 corresponding normal tissue samples. They found that more than 139 piRNAs were differentially expressed compare to the normal tissue control, out of which 103 piRNAs and 36 piRNAs were up- and down-regulated respectively. A stage-wise differential expression analysis results showed there are 46 piRNAs were common for stages I & II, 3 piRNAs were common for stage II & III, while no common piRNAs for stage I & III. Eight piRNAs were differentially expressed in all three stages [50]. Another study in breast cancer deals with piR-0211285. PiR-0211285 decreases breast cancer risk through increasing the methylation level on genomic region of a number of breast cancer-related genes, which leads to the decreased expression of the protein coded by those genes. For example, piR-0211285 could inhibit the expression of ARHGAP11A, a known tumor suppressing factor, which subsequently suppress the invasiveness of colon cancer cells in vivo [51].
Several independent research teams explored the role of piRNAs in renal cell carcinomas (RCC). Totally 19 piRNAs were differentially expressed in normal kidney tissue and metastatic clear cell RCCs, and 46 piRNAs present in the samples are associated with metastasis. It is worth noticing that 3 piRNAs which are linked with metastasis locate in the same piRNA cluster in the chromosome 17. Clinical analysis indicted that the up-regulation of these three piRNAs are highly linked with RCC metastasis, poor cancer-specific survival as well as late clinical stage [52]. Another research team did a similar research on RCC with a different sample cohort. Microarray data showed the expression of 235 piRNAs were up-regulated while 369 piRNAs were down-regulated. They picked piR-30924, piR-57125 and piR-38756 for further investigation. All three piRNAs were verified by quantitative PCR to be down-regulated in non-metastatic RCCs compared to normal tissue and all three piRNAs were shown to be linked with OS in both non-metastatic and metastatic RCC patients. Together with tumor grade, piR-38756 is an independent marker for recurrence and survival prognosis in non-metastatic RCC patients [53]. Specifically, one study comprehensively analyzed the expression of piR-823 in various biological samples (tumor tissue, normal renal parenchyma, blood serum and urine) from patients underwent nephrectomy for RCC. There is a prominent down-regulation of piR-823 in tumor biopsies, while the expressions of piR-823 in blood serum and urine are up-regulated. Higher piR-823 levels in serum are correlated with advance clinical stages of RCC, indicating piR-823 would be used as a diagnostic marker for RCC [54].
In human bladder cancer, data from three cancer biopsies and their adjacent normal tissues showed the up- and down-regulated expression of 106 piRNAs and 91 piRNAs respectively. Further investigation on piR-60152, which displayed the highest level of down-regulation in cancerous bladder tissue (fold change = 31.63), showed that the mRNA of TNFSF4 is a downstream target of piR-60152. The piR-60152/TNFSF4 signaling axis was further confirmed by the result showing that TNFSF4 mRNA levels were significantly suppressed in 25 bladder cancer biopsies [55].
Single nucleotide polymorphisms (SNPs) affect the risk of in the colorectal cancer (CRC) research field worldwide. In a Chinese case-control study, Chu and colleagues (2015) assessed the links between CRC risk and 7 piRNA SNPs. Disappointedly, the authors did not find significant protective role of piR-015551/rs11776042 SNP on the risk of CRC, nor did the authors detect any expression level change of piR-015551 in CRC tissue. However, the rs11776042 SNP in piR-015551 changed the secondary structure energy of the piR-015551. This energy change would subsequently affect the role of piR-015551 on CRC development, which would be an interesting future research direction [56]. Another research group analyzed the piRNA expression profile among three different groups of CRC (benign group, tumor group and metastasis group). They pointed out that four piRNAs were enriched by comparing tumor group with benign group, with the up-regulation of piR-25447 and piR-23992, down-regulation of piR-28876. Twenty-seven piRNAs were enriched by comparing metastasis group with benign group, with the up-regulation of piR-22842, piR-23317, piR-26131, piR-23210.1 and piR-25447 (top 5 out of 22), and down-regulation of piR-27729, piR-7193.1, piR-7193.2, piR-27729.1 and piR-27730.1 [57]. Yin et al. reported that piR-823 is significantly up-regulated in CRC. The biological function of piR-823 in CRC is to promote cell proliferation and inhibit apoptosis. Inhibition of piR-823 arrests the cell cycle in the G1 phase within HCT116 and DLD-1 cells. Intriguingly, inhibition of piR-823 leads to the decrease of HTRA, IGFBP5, HSP27, HSP60 and HSP70 levels in CRC cell line. Deeper mechanistic study showed that piR-823 influences the transcriptional activity but not the expression of HSF1, the common transcription factor of HSPs. PiR-823 is able to bind directly to HSF1 and promote its phosphorylation at Ser326 [58]. Recently, Weng and colleagues (2018) conducted an intense clinical study for the piRNAs in CRC. A big sample collection which contains 771 CRC patients from three independent cohorts was utilized. It was reported that only piR-1245 is differentially expressed in all three cohorts. High expression of piR-1245 correlated with advanced disease, metastasis and poor prognosis in CRC. Functional studies exhibited that a number of tumor suppressor genes might be targets of piR-1245, which contain ATF3, BTG1, DUSP1, FAS, NFKBIA, UPP1, SESN2, TP53INP1 and MDX1. It was also validated that the protein expression above is inversely correlated with piR-1245 in CRC [59].
Given the facts that human DLK1-DIO3 locus at 14q32.1-14q32.31 is aberrantly hyper-methylated and that piRNA/PIWI complex could repress gene transcription through inducing DNA methylation, Enfield and colleagues (2016) checked the piRNA expression level in the lung cancer tissues. They found that 4 piRNAs (DQ596225, DQ596306, DQ596309, and DQ596354) are overexpressed in lung adenocarcinoma while one piRNA (DQ596309) is overexpressed in lung squamous cell carcinoma. Combined with miRNA signature, the newly discovered piRNAs are good prognostic factors for the overall survival of lung adenocarcinoma and lung squamous cell carcinoma patients, as well as the recurrence-free survival [60]. In a particular case, the expression of piR-55490 was found to be suppressed in human lung cancer. Deeper characterization of piR-55490 showed that piR-55490 is a good prognostic marker for lung cancer. Mechanistically, piR-55490 suppresses cell growth in both cell and mouse xenograft model via inhibiting Akt/mTOR signaling. PiR-55490 could bind to mTOR mRNA at 3’UTR and promote its degradation [61]. RASSF1C is an intensively studied protein which is known to be able to promote lung cancer cell growth and migration. Reeved and colleagues (2015) did a piRNA microarray study using H1229 cell line over-expressing RASSF1C (H1229:RASSF1C) and control. They discovered that piR-52200 and piR-34871 were up-regulated while piR-46545 and piR-35127 were down-regulated in H1229:RASSF1C. Notably, there was an inverse correlation between the expression of piR-35127 and RASSF1C in ten out of twelve lung cancer biopsies. Forced expression of 2 piRNAs (piR-35127 and piR-46545) and knock-down of 2 piRNAs (piR-52200 and piR-34871) at the same time reduced normal lung epithelial cell proliferation and colony formation in lung cancer cell lines [62]. It is well known that resistance to chemotherapy in lung squamous cell carcinoma (LSCC) is very common, while the underlying molecular mechanism still remains elusive. A study on LSCC revealed that piRNA-like (piR-L) small RNA piR-L-138 was up-regulated following cisplatin (CDDP)-based chemotherapy both in vitro and in vivo. Suppression of piR-L-138 could promote CDDP induced apoptosis in vivo. Mechanistically, piR-L-138 is shown to be able to bind directly to p60-MDM2 protein to influence apoptosis [63]. A recent research characterized piR-651 in the carcinogenesis of non-small cell lung cancer (NSCLC). Using NSCLC A549 and HCC827 cell lines as models, the authors demonstrated that piR-651 could regulate tumorigenesis via inhibiting cell migration invasion, and proliferation while promoting apoptosis [64].
PiRNAs have entered the stage of hepatocellular carcinoma (HCC) as well. It was discovered that there are more than 700 known piRNAs and 900 novel piRNA-likes expressed in 14 cirrhotic and 20 corresponding HCC samples. Liver piRNA expression patterns were analyzed in various liver pathological stages such as cirrhotic nodules, early HCC, advanced HCC, etc. A total of 125 piRNA expression signature of HCC were identified, which is correlated to microvascular invasion in HCC. Predicted downstream targets of these aberrantly regulated piRNAs are involved in key signaling cascades such as telomerase activity, cell cycle regulation, apoptosis and so on which all correlated to hepato-carcinogenesis and HCC progression. The piRNAs discovered in the study above are likely to represent a new class of mediators in HCC [65].
Silencing of tumor-suppressor genes (TSGs) by altering the DNA methylation status at their promoter regions have long been documented in multiple myeloma (MM). Yan and colleagues (2015) found that expression of piR-823 was correlated to de novo DNA methyltransferases, DNM3A and 3B. PiRNAs are able to promote vascular endothelial growth factor section followed by a promotion in angiogenesis in MM. Mechanistically, piR-823 may affect p16INK4A/ cyclin D1/CDK4/Rb pathway through changing DNA methylation status of p16INK4A [66].
In the human head and neck squamous cell carcinoma (HNSCC) research field, one group of researchers found the correlation between piRNA expression and nodal metastasis [41]. Further TCGA data mining of 43 tumor-normal small RNA-seq datasets and Level 3 gene expression analyses discovered 61 piRNAs were markedly dysregulated in HNSCCs. It is worth to note that comparison of the HNSCC-dysregulated piRNAs to some previous studies of their expression in other types of cancer only yielded little overlapping indicating different regulatory mechanisms of piRNAs in different cancer types [67]. Since that Human papillomavirus (HPV)-positive HNSCC patients have a better prognosis while a prognostic biomarker is still missing, the same research team went on to analyze the association of the expression of some piRNAs with survival as well as HPV infection status. Of the total 87 piRNAs that specifically expressed in tumor samples, 41 of them showed significant connection with HPV infection status. Moreover, five piRNAs expression in HPV positive HNSCC cancer samples was correlated with worse OS [68]. Using 77 RNA-sequencing datasets from TCGA, another similar research on HPV-induced HNSCC examined the expression of piRNAs between HPV16 (+) HNSCC and normal controls. A total of 30 piRNAs were dysregulated in HPV16 (+) HNSCC with the protein PIWIL4 and RTL family of retrotransposon-like genes been their potential targets. Three differential expressed piRNAs were further validated in vitro [69]. It was also reported that a collection of 13 piRNAs was found in HNSCC related to smoking. Among those 13 piRNAs, 2 piRNAs are shown to be linked with tumor stage while one piRNA (NONHSAT067200) is shown to be a potential indicator of patient survival rate [70].
The function of piRNAs in either testicular germ cell tumors (TGCTs) or germ cell neoplasia in situ (GCNIS) is a little different. PIWI/piRNA signaling and biogenesis is found to be missing in GCNIS and TGCT cells while piRNA biogenesis in the health testis tissue adjacent to TGCTs remains intact. This result suggests that piRNAs is unlikely to be oncogenic factors for the TGCT development. It is also suggested that piRNA could perform an inhibitory role in GCNIS and TGCT [71].
In endometrium cancer field, small-RNA sequencing and microarray data using normal, hyperplastic and neoplastic endometrium tissues indicated that 2 piRNAs are under-expressed and 8 piRNAs are over-expressed in cancerous tissue compared to normal ones. It appears that there are a total of 1526 putative mRNA target for the piRNAs identified above among which 170 were found to be aberrantly expressed in hyperplastic and/or tumor tissues. The protein encoded by those mRNAs take parts in various carcinogenetic related process such as cell death, growth and survival, 38 of which have been documented to be related to endometrial cancer [72].
By doing a genome-wide association study (GWAS) and functional analysis on a total of 4241 (1840 cases and 2401 controls) glioma samples, Jacobs and colleagues found that four piRNAs, which are expressed in glial cell lines, harbor glioma-associated germline variants. Functional studies on one of these piRNA, piR-598, indicated that piR-598 could mediate cell death and survival and suppress glioma cell viability as well as colony formation. On the other hand, variant rs147061479 of piR-598 counteracts the tumor inhibitory function of piR-598, which subsequently increases the risk of glioma [73]. As we know, the blood-tumor barrier (BTB) is a big limitation for the delivery of drugs into the glioma microenvironment. A latest research demonstrated that piR-593109 was overexpressed in glioma endothelial cells (GECs). The permeability of BTB could be increased via knock-down of PIWIL1 or piR-593109. Deeper mechanistic study revealed that piR-593109 affects BTB in glioma through a MEG3/miR-330-5p/RUNX3 signaling cascade in which piR-593109 could regulate MEG3 in a sequence-specific manner [74].
Recently, Roy and colleagues (2018) started to look at the role of piRNAs in human neuroblastoma (NB). By using next generation sequencing, the authors identified a common pool of 525 piRNAs in two different NB cell lines. Further bioinformatics analysis showed that the 589 putative target mRNAs, which are the key regulators of signaling pathways and biological processes related to NB, are involved in 185 biological functions relevant to tumorigenesis. The authors confirmed the expression of key piRNAs and their targets enriched in biological processes which are proposed to be important player in neoplastic event of NB. Although the piRNA targets still need to be better experimentally characterized in the future, the study opened new avenue for piRNA-mediated therapeutics for NB [75].
A research was conducted to unravel the altered expression profile of all the small noncoding RNAs in six pancreatic ductal adenocarcinoma (PDAC) patients compared to five normal pancreatic tissue samples. It was found that one piRNA (piR-017061) was significantly down-regulated in the PDAC samples [76]. It would be exciting to explore its downstream targets for the future research.
PiRNAs in other types of diseases
PiRNAs have long been famous for their roles played in silencing retrotransposons in germline cells. It was found not long ago that the expressions of piRNAs are also present in the mammalian brain. In Mili/piRNA-null mice, hypomethylation of intergenic regions as well as LINE1 promoter area in the brain genomic DNA was detected. Mili null mice showed hyperactivity and reduced anxiety. The results above indicated that brain piRNAs are likely to be involved in suppressing retrotransposons which play important roles in brain pathology [77]. Indeed, a recent research proved the function of piRNAs in the brain. Joy and colleagues (2007) investigated the piRNA profiles of normal and Alzheimer disease (AD) affected brain. The authors found 1923 mRNAs were significantly down-regulated in AD, all of which were the putative targets of 125 up-regulated piRNAs. Pathway study results showed that four genes (LIN7C, RAB11A, CYCS and KPNA6) in the AD-associated pathways are putative targets of four piRNAs. The inverse correlation between the three out of four piRNAs and their corresponding target genes were further confirmed by real-time PCR [78]. Another similar research utilized prefrontal cortical tissues of six AD patients and six controls. Meanwhile, the samples were also genotyped for 17 significant and replicated risk SNPs. In this study, a total of 9453 piRNAs were identified in human brains with 103 piRNAs showed altered expression in AD cases versus controls. What is more, most of the 103 piRNAs correlate with genome-wide significant risk SNPs indicating that piRNAs would be promising risk biomarkers of AD [79]. Interestingly, one research of the piRNAome on transient focal ischemia suggested that a total of 105 piRNAs showed differential expression in ischemic rat brain, although the function for the changes in those piRNA expressions still remains elusive, it was predicted that the role of altered piRNAome is to control mutagenesis through suppressing the aberrant transposon activity in the ischemic brain [80].
Rett syndrome (RTT), a genetic neurodevelopmental disorder that happens in females, is mostly characterized by the mutation in MECP2 gene. Knockout of Mecp2 in mouse brain results in a 1.6-fold increase in transposon sequences such as LINE-1. Since piRNA is famous for its role in transposon silencing, Saxena and colleagues (2012) explored the expression level of piRNAs in the Mecp2 null brain. Results showed that while majority (81%) of the piRNAs found in the cerebellum has increased expression in Mecp2 null brain, 59% piRNAs displayed over 1.5-fold difference between Mecp2 null brain and controls. Meanwhile, there are 1.9-fold increases in the number of total piRNAs in Mecp2 null brain [81]. It would be exciting for the future research to dissect the underlying regulatory mechanism of those piRNAs in Rett syndrome.
PiRNAs are also expressed abundantly in cardiomyocytes. Bioinformatics analysis showed that piRNAs were aberrantly expressed in cardiac hypertrophy with an increase of piRNA reads in hypertrophied heart (9.7%) compared to control hearts (5%). The expressions of a total of 22 piRNAs were found to be significantly altered in hypertrophied heart, which was further validated by RNA immunoprecipitation as well as qPCR. Specifically, it was found that the expression of piR-2106027 was increased in myocardial infracted patients, which suggest that piR-2106027 could be a promising diagnostic marker for myocardial infraction [82].
Since discovery, piRNAs are the most famous for the role they played in spermatogenesis [83,84,85]. One study looked at the connection between SNPs of several key proteins involved in piRNA signaling pathway and idiopathic non-obstructive azoospermia (NOA) using a sample collection of Iranian infertile males with NOA. It turns out that rs508485 polymorphism in HIWI is correlated with the increased risk if azoospermia in the studied population [86]. If the research above showed an indirect relationship between piRNA and NOA, another recent research demonstrated the relationship more directly. A total of 18,324 piRNAs were found to exist in NOA patient testicular biopsies, among which 951 piRNAs were down-regulated and 8 piRNAs were up-regulated in samples from unsuccessful sperm retrieval (USR) groups compared to the samples from successful sperm retrieval (SSR) groups. Intriguingly, 553 piRNAs which were highly expressed in SSR were absent in USR. The presence of 20 piRNAs in NOA biopsies were further validated via qPCR. Pathway enrichment study of the putative piRNA target genes showed that the altered piRNAs take parts in numerous biological pathways such as cell proliferation, apoptosis and differentiation [87].
Recently, the regulatory roles of piRNAs have stepped into the diabetes-related field as well. Around 12,000 piRNAs were detected in rat pancreatic islets, some of which showed differentiated expression pattern throughout islet postnatal development. Pathologically, several piRNAs showed differentiated expression profile in the islets of Goto-Kakizaki (GK) rats. Over-expression of 2 piRNAs (DQ732700 and DQ746748), which were found to be up-regulated in the islets of GK rats, in the islets of normoglycaemic rats led to a defect in insulin secretion following glucose stimulation without affecting cellular insulin content and potassium stimulated insulin secretion. Furthermore, forced expression of the piRNAs above could not influence cell survival with or without using a mixture of proinflammatory cytokines. Target hunting for the two piRNAs above indicated that the predicted targets of these piRNAs were enriched for genes that play critical roles in insulin secretion and function [88].
Uterine leiomyoma (UL) are the common benign neoplastic disease among women worldwide. Screening the RNA-sequencing data with the sncRNA database leaded to the findings that 24 piRNAs were differentially expressed by more than 1.5 fold in UL compared to adjacent normal myometrium [89]. For future study, it would be interesting to determine their molecular functions in the UL.
Conclusions
Since initial discovery, researches on piRNA have made tremendous progress in the past decade. It is now known that piRNAs could be found in various animal species from protozoans to humans and the expressions of piRNAs are present in both germline cells and somatic cells [90,91,92]. As discussed in this review, piRNAs are aberrantly regulated in numerous types of diseases (Fig. 3) (Table 1). They represent a novel class of molecules which are shown to be potential diagnostic and prognostic markers. However, in vast majority of the cases, researchers only checked and confirmed the misregulation of the piRNA species, analyzed the correlation between and misregulated piRNAs with some of the clinical features but did not investigate the underlying molecular mechanisms. Lots of questions still remain to be elucidated, such as how the expression of those differentially expressed piRNAs been regulated? If a protein is found to be the upstream of a piRNA, does it influence the piRNA itself or the PIWI protein? What is/are the downstream target(s) for the misregulated piRNAs in each specific pathological condition? How, mechanistically, does the piRNAs regulate their targets in each given case, through promoting the decay of mRNA or through affecting the methylation status on the promoter region of the genes or from decay of pre-mRNA, a mechanism been reported previously [93]. Experiments on cellular level and animal level would be needed to for elucidating the questions above. Answering the question above will enable us to find the drug targets for each disease more precisely. It is worth to mention that, similar to miRNA, piRNA has been found to present in body fluid such as blood, urine and saliva [54, 94]. Importantly, it was found that most of piRNAs exist in the exosome, while certain types of piRNAs within saliva are not associated with exosome [94]. These interesting findings could give us the following thoughts: 1, piRNA levels in the body fluid would potentially be used as prognostic markers for certain diseases; 2, like miRNAs, piRNAs secreted from one site may also influence the distant target site via exosomes.
Much evidence has suggested that PIWI/piRNA could have great therapeutic value in the clinic. Most expression of PIWI is restricted to stem cells and germline cells. In human, the PIWI ortholog HIWI is present in hematopoietic stem cell but is absent in the stem cell-derived differentiated cells [95]. The critical role of PIWI played in stem cell self-renewal has been well established in various organisms [96]. The facts above give us hint that there could be potential link between PIWI and the field of cancer stem cell. Indeed, several research teams have already provided evidence which supporting the idea of targeting PIWI as a potential approach in cancer therapy [97,98,99,100]. Positive correlation between PIWI proteins and cancer stem cell markers has been identified in colorectal cancer [97]. It is worth to note that for one species of PIWI protein, HILI, it is upregulated in some types of cancer while downregulated in other studies on bladder and colon cancer. Furthermore, different research groups reported contradictive results in terms of HILI expression level in colon cancer. The author reasoned that reciprocal regulation of different PIWI species is also important [97, 101]. These findings suggest that when targeting PIWI in the cancer therapy, case-specific treatment should take into consideration. Also, a full spectrum of PIWI family protein expression profile is needed before using PIWI as drug target. On the other hand, the detailed molecular mechanisms of how PIWI protein regulates cancer cell stemness are largely unknown. Knowing how PIWI protein contributes to the stemness of different cancer cell, especially whether they function independently or together with piRNA, will greatly help us in the drug designing. As we know, the way of function between miRNA and piRNA has several differences. For instance, piRNA could inhibit target mRNA in a similar way as miRNA without the need of Drosha and Dicer. Through ping-pong cycle, piRNA could not only amplify itself, but also piRNA could complex with PIWI. The PIWI-piRNA complex, which contains piRNA with the sequence complimentary to the mRNA-inhibiting piRNA, could go into the nuclear and affect the methylation status of its target genomic DNA through binding to a nascent transcript of that specific DNA [102]. These lead to the thinking of using either synthetic piRNA alone or in combination with miRNA to silence cancer-related protein expression, especially for those cases where miRNA therapy alone could not achieve satisfactory results. A more exciting direction would be use piRNA to directly silence the transcription of a specific gene/several specific genes through epigenetic modification. In order to achieve this goal, further intensive study on how exactly piRNA recognizes its target in the genome and whether there are any off-target effects would be necessary.
Another point that makes the mechanistic study on piRNAs more challenging is that many piRNAs could be generated from the same genomic locus known as piRNA cluster [103]. In one extreme case, a chromosomal location with the length of merely 32 nt could generate three different piRNAs, which causes the piRNAs generated all have highly overlapped sequence [52]. It would be necessary to explore the combined biological function of several differentially expressed piRNAs simultaneously when those piRNAs are located very closely in the genome.
In short, the area of utilizing piRNAs clinically is still at its infancy compared to miRNAs. However, given the all the evidence that have been collected in the field of piRNA during the past 12 years as well as the arrival of precision medicine age, it should not be long before the real application of piRNAs in the prognostic, diagnostic as well as therapeutic health care.
Abbreviations
- 3’UTR:
-
3′ untranslated region
- AD:
-
Alzheimer disease
- BC:
-
Breast cancer
- BTB:
-
Blood-tumor barrier
- CRC:
-
Colorectal cancer
- FISH:
-
Fluorescence in situ hybridization
- GCNIS:
-
Germ cell neoplasia in situ
- GK:
-
Goto-Kakizaki
- GWAS:
-
Genome-wide association study
- H&E:
-
Hematoxylin and eosin
- HCC:
-
Hepatocellular carcinoma
- HNSCC:
-
Human head and neck squamous cell carcinoma
- HP1A:
-
Heterochromatin Protein 1A
- HPV:
-
Human papillomavirus
- IHC:
-
Immunohistochemistry
- LSCC:
-
Lung squamous cell carcinoma
- miRNA:
-
micoRNA
- MM:
-
Multiple myeloma
- NB:
-
Neuroblastoma
- NOA:
-
Non-obstructive azoospermia
- NSCLC:
-
Non-small cell lung cancer
- nt:
-
Nucleotides
- OS:
-
Overall Survival
- PDAC:
-
Pancreatic ductal adenocarcinoma
- piRNA:
-
PIWI-interacting RNA
- qPCR:
-
Quantitative polymerase chain reaction
- RFS:
-
Recurrence Free Survival
- siRNA:
-
Short interference RNA
- snoRNA:
-
Small non-coding RNA
- SNPs:
-
Single nucleotide polymorphisms
- SSR:
-
Successful sperm retrieval
- TDRD9:
-
Tudor domain containing protein 9
- TGCTs:
-
Testicular germ cell tumors
- TNBC:
-
Triple-negative breast cancer
- TNM:
-
Tumor-node-metastasis
- UL:
-
Uterine leiomyoma
- USR:
-
Unsuccessful sperm retrieval
References
Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Mol Cell. 2007;26:611–23.
Azlan A, Dzaki N, Azzam G. Argonaute: the executor of small RNA function. J Genet Genomics. 2016;43:481–94.
Hock J, Meister G. The Argonaute protein family. Genome Biol. 2008;9:210.
Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32.
Grivna ST, Beyret E, Wang Z, Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20:1709–14.
Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–7.
Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202.
Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, et al. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–7.
Grillari J, Grillari-Voglauer R. Novel modulators of senescence, aging, and longevity: small non-coding RNAs enter the stage. Exp Gerontol. 2010;45:302–11.
Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development. 2008;135:3–9.
Hartig JV, Tomari Y, Forstemann K. piRNAs--the ancient hunters of genome invaders. Genes Dev. 2007;21:1707–13.
Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell. 2007;129:69–82.
Lin H, Yin H. A novel epigenetic mechanism in Drosophila somatic cells mediated by Piwi and piRNAs. Cold Spring Harb Symp Quant Biol. 2008;73:273–81.
Lee EJ, Banerjee S, Zhou H, Jammalamadaka A, Arcila M, Manjunath BS, et al. Identification of piRNAs in the central nervous system. RNA. 2011;17:1090–9.
Hirakata S, Siomi MC. piRNA biogenesis in the germline: from transcription of piRNA genomic sources to piRNA maturation. Biochim Biophys Acta. 2016;1859:82–92.
Thomson T, Lin H. The biogenesis and function of PIWI proteins and piRNAs: progress and prospect. Annu Rev Cell Dev Biol. 2009;25:355–76.
Iwasaki YW, Siomi MC, Siomi H. PIWI-Interacting RNA: Its Biogenesis and functions. Annu Rev Biochem. 2015;84:405–33.
Gan H, Lin X, Zhang Z, Zhang W, Liao S, Wang L, et al. piRNA profiling during specific stages of mouse spermatogenesis. RNA. 2011;17:1191–203.
Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–103.
Frank F, Sonenberg N, Nagar B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature. 2010;465:818–22.
Kawaoka S, Izumi N, Katsuma S, Tomari Y. 3′ end formation of PIWI-interacting RNAs in vitro. Mol Cell. 2011;43:1015–22.
Aravin AA, Sachidanandam R, Bourc'his D, Schaefer C, Pezic D, Toth KF, et al. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell. 2008;31:785–99.
Gou LT, Dai P, Yang JH, Xue Y, Hu YP, Zhou Y, et al. Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Res. 2014;24:680–700.
Zheng K, Xiol J, Reuter M, Eckardt S, Leu NA, McLaughlin KJ, et al. Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc Natl Acad Sci U S A. 2010;107:11841–6.
Watanabe T, Tomizawa S, Mitsuya K, Totoki Y, Yamamoto Y, Kuramochi-Miyagawa S, et al. Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse Rasgrf1 locus. Science. 2011;332:848–52.
Liu X, Sun Y, Guo J, Ma H, Li J, Dong B, et al. Expression of hiwi gene in human gastric cancer was associated with proliferation of cancer cells. Int J Cancer. 2006;118:1922–9.
Wang Y, Liu J, Wu G, Yang F. Manipulations in HIWI level exerts influence on the proliferation of human non-small cell lung cancer cells. Exp Ther Med. 2016;11:1971–6.
Grochola LF, Greither T, Taubert H, Moller P, Knippschild U, Udelnow A, et al. The stem cell-associated Hiwi gene in human adenocarcinoma of the pancreas: expression and risk of tumour-related death. Br J Cancer. 2008;99:1083–8.
Yang L, Bi L, Liu Q, Zhao M, Cao B, Li D, et al. Hiwi promotes the proliferation of colorectal Cancer cells via upregulating global DNA methylation. Dis Markers. 2015;2015:383056.
Liang D, Dong M, Hu LJ, Fang ZH, Xu X, Shi EH, et al. Hiwi knockdown inhibits the growth of lung cancer in nude mice. Asian Pac J Cancer Prev. 2013;14:1067–72.
Zeng Y, Qu LK, Meng L, Liu CY, Dong B, Xing XF, et al. HIWI expression profile in cancer cells and its prognostic value for patients with colorectal cancer. Chin Med J (Engl). 2011;124:2144–9.
Wang DW, Wang ZH, Wang LL, Song Y, Zhang GZ. Overexpression of hiwi promotes growth of human breast cancer cells. Asian Pac J Cancer Prev. 2014;15:7553–8.
Lu L, Katsaros D, Risch HA, Canuto EM, Biglia N, Yu H. MicroRNA let-7a modifies the effect of self-renewal gene HIWI on patient survival of epithelial ovarian cancer. Mol Carcinog. 2016;55:357–65.
Xie Y, Yang Y, Ji D, Zhang D, Yao X, Zhang X. Hiwi downregulation, mediated by shRNA, reduces the proliferation and migration of human hepatocellular carcinoma cells. Mol Med Rep. 2015;11:1455–61.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74.
Liu JJ, Shen R, Chen L, Ye Y, He G, Hua K, et al. Piwil2 is expressed in various stages of breast cancers and has the potential to be used as a novel biomarker. Int J Clin Exp Pathol. 2010;3:328–37.
He G, Chen L, Ye Y, Xiao Y, Hua K, Jarjoura D, et al. Piwil2 expressed in various stages of cervical neoplasia is a potential complementary marker for p16. Am J Transl Res. 2010;2:156–69.
Lee JH, Schutte D, Wulf G, Fuzesi L, Radzun HJ, Schweyer S, et al. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 2006;15:201–11.
Guijo M, Ceballos-Chavez M, Gomez-Marin E, Basurto-Cayuela L, Reyes JC. Expression of TDRD9 in a subset of lung carcinomas by CpG island hypomethylation protects from DNA damage. Oncotarget. 2018;9:9618–31.
Lu Y, Li C, Zhang K, Sun H, Tao D, Liu Y, et al. Identification of piRNAs in Hela cells by massive parallel sequencing. BMB Rep. 2010;43:635–41.
Martinez VD, Vucic EA, Thu KL, Hubaux R, Enfield KS, Pikor LA, et al. Unique somatic and malignant expression patterns implicate PIWI-interacting RNAs in cancer-type specific biology. Sci Rep. 2015;5:10423.
Cheng J, Guo JM, Xiao BX, Miao Y, Jiang Z, Zhou H, et al. piRNA, the new non-coding RNA, is aberrantly expressed in human cancer cells. Clin Chim Acta. 2011;412:1621–5.
Cheng J, Deng H, Xiao B, Zhou H, Zhou F, Shen Z, et al. piR-823, a novel non-coding small RNA, demonstrates in vitro and in vivo tumor suppressive activity in human gastric cancer cells. Cancer Lett. 2012;315:12–7.
Cui L, Lou Y, Zhang X, Zhou H, Deng H, Song H, et al. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using piRNAs as markers. Clin Biochem. 2011;44:1050–7.
Hashim A, Rizzo F, Marchese G, Ravo M, Tarallo R, Nassa G, et al. RNA sequencing identifies specific PIWI-interacting small non-coding RNA expression patterns in breast cancer. Oncotarget. 2014;5:9901–10.
Huang G, Hu H, Xue X, Shen S, Gao E, Guo G, et al. Altered expression of piRNAs and their relation with clinicopathologic features of breast cancer. Clin Transl Oncol. 2013;15:563–8.
Zhang H, Ren Y, Xu H, Pang D, Duan C, Liu C. The expression of stem cell protein Piwil2 and piR-932 in breast cancer. Surg Oncol. 2013;22:217–23.
Krishnan P, Ghosh S, Graham K, Mackey JR, Kovalchuk O, Damaraju S. Piwi-interacting RNAs and PIWI genes as novel prognostic markers for breast cancer. Oncotarget. 2016;7:37944–56.
Lee YJ, Moon SU, Park MG, Jung WY, Park YK, Song SK, et al. Multiplex bioimaging of piRNA molecular pathway-regulated theragnostic effects in a single breast cancer cell using a piRNA molecular beacon. Biomaterials. 2016;101:143–55.
Koduru SV, Tiwari AK, Leberfinger A, Hazard SW, Kawasawa YI, Mahajan M, et al. A comprehensive NGS data analysis of differentially regulated miRNAs, piRNAs, lncRNAs and sn/snoRNAs in triple negative breast Cancer. J Cancer. 2017;8:578–96.
Fu A, Jacobs DI, Hoffman AE, Zheng T, Zhu Y. PIWI-interacting RNA 021285 is involved in breast tumorigenesis possibly by remodeling the cancer epigenome. Carcinogenesis. 2015;36:1094–102.
Li Y, Wu X, Gao H, Jin JM, Li AX, Kim YS, et al. Piwi-interacting RNAs (piRNAs) are dysregulated in renal cell carcinoma and associated with tumor metastasis and Cancer-specific survival. Mol Med. 2015;21:381–8.
Busch J, Ralla B, Jung M, Wotschofsky Z, Trujillo-Arribas E, Schwabe P, et al. Piwi-interacting RNAs as novel prognostic markers in clear cell renal cell carcinomas. J Exp Clin Cancer Res. 2015;34:61.
Iliev R, Fedorko M, Machackova T, Mlcochova H, Svoboda M, Pacik D, et al. Expression levels of PIWI-interacting RNA, piR-823, are deregulated in tumor tissue, blood serum and urine of patients with renal cell carcinoma. Anticancer Res. 2016;36:6419–23.
Chu H, Hui G, Yuan L, Shi D, Wang Y, Du M, et al. Identification of novel piRNAs in bladder cancer. Cancer Lett. 2015;356:561–7.
Chu H, Xia L, Qiu X, Gu D, Zhu L, Jin J, et al. Genetic variants in noncoding PIWI-interacting RNA and colorectal cancer risk. Cancer. 2015;121:2044–52.
Koduru SV, Tiwari AK, Hazard SW, Mahajan M, Ravnic DJ. Exploration of small RNA-seq data for small non-coding RNAs in human colorectal Cancer. J Genomics. 2017;5:16–31.
Yin J, Jiang XY, Qi W, Ji CG, Xie XL, Zhang DX, et al. piR-823 contributes to colorectal tumorigenesis by enhancing the transcriptional activity of HSF1. Cancer Sci. 2017;108:1746–56.
Weng W, Liu N, Toiyama Y, Kusunoki M, Nagasaka T, Fujiwara T, et al. Novel evidence for a PIWI-interacting RNA (piRNA) as an oncogenic mediator of disease progression, and a potential prognostic biomarker in colorectal cancer. Mol Cancer. 2018;17:16.
Enfield KS, Martinez VD, Marshall EA, Stewart GL, Kung SH, Enterina JR, et al. Deregulation of small non-coding RNAs at the DLK1-DIO3 imprinted locus predicts lung cancer patient outcome. Oncotarget. 2016;7:80957–66.
Peng L, Song L, Liu C, Lv X, Li X, Jie J, et al. piR-55490 inhibits the growth of lung carcinoma by suppressing mTOR signaling. Tumour Biol. 2016;37:2749–56.
Reeves ME, Firek M, Jliedi A, Amaar YG. Identification and characterization of RASSF1C piRNA target genes in lung cancer cells. Oncotarget. 2017;8:34268–82.
Wang Y, Gable T, Ma MZ, Clark D, Zhao J, Zhang Y, et al. A piRNA-like small RNA induces Chemoresistance to cisplatin-based therapy by inhibiting apoptosis in lung squamous cell carcinoma. Mol Ther Nucleic Acids. 2017;6:269–78.
Zhang SJ, Yao J, Shen BZ, Li GB, Kong SS, Bi DD, et al. Role of piwi-interacting RNA-651 in the carcinogenesis of non-small cell lung cancer. Oncol Lett. 2018;15:940–6.
Rizzo F, Rinaldi A, Marchese G, Coviello E, Sellitto A, Cordella A, et al. Specific patterns of PIWI-interacting small noncoding RNA expression in dysplastic liver nodules and hepatocellular carcinoma. Oncotarget. 2016;7:54650–61.
Yan H, Wu QL, Sun CY, Ai LS, Deng J, Zhang L, et al. piRNA-823 contributes to tumorigenesis by regulating de novo DNA methylation and angiogenesis in multiple myeloma. Leukemia. 2015;29:196–206.
Zou AE, Zheng H, Saad MA, Rahimy M, Ku J, Kuo SZ, et al. The non-coding landscape of head and neck squamous cell carcinoma. Oncotarget. 2016;7:51211–22.
Firmino N, Martinez VD, Rowbotham DA, Enfield KSS, Bennewith KL, Lam WL. HPV status is associated with altered PIWI-interacting RNA expression pattern in head and neck cancer. Oral Oncol. 2016;55:43–8.
Krishnan AR, Qu Y, Li PX, Zou AE, Califano JA, Wang-Rodriguez J, et al. Computational methods reveal novel functionalities of PIWI-interacting RNAs in human papillomavirus-induced head and neck squamous cell carcinoma. Oncotarget. 2018;9:4614–24.
Krishnan AR, Korrapati A, Zou AE, Qu Y, Wang XQ, Califano JA, et al. Smoking status regulates a novel panel of PIWI-interacting RNAs in head and neck squamous cell carcinoma. Oral Oncol. 2017;65:68–75.
Gainetdinov IV, Skvortsova YV, Kondratieva SA, Klimov A, Tryakin AA, Azhikina TL. Assessment of piRNA biogenesis and function in testicular germ cell tumors and their precursor germ cell neoplasia in situ. BMC Cancer. 2018;18:20.
Ravo M, Cordella A, Rinaldi A, Bruno G, Alexandrova E, Saggese P, et al. Small non-coding RNA deregulation in endometrial carcinogenesis. Oncotarget. 2015;6:4677–91.
Jacobs DI, Qin Q, Lerro MC, Fu A, Dubrow R, Claus EB, et al. PIWI-interacting RNAs in Gliomagenesis: evidence from post-GWAS and functional analyses. Cancer Epidemiol Biomark Prev. 2016;25:1073–80.
Shen S, Yu H, Liu X, Liu Y, Zheng J, Wang P, et al. PIWIL1/piRNA-DQ593109 regulates the permeability of the blood-tumor barrier via the MEG3/miR-330-5p/RUNX3 Axis. Mol Ther Nucleic Acids. 2018;10:412–25.
Roy J, Mallick B. Investigating piwi-interacting RNA regulome in human neuroblastoma. Genes Chromosomes Cancer. 2018;57:339–49.
Muller S, Raulefs S, Bruns P, Afonso-Grunz F, Plotner A, Thermann R, et al. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol Cancer. 2015;14:94.
Nandi S, Chandramohan D, Fioriti L, Melnick AM, Hebert JM, Mason CE, et al. Roles for small noncoding RNAs in silencing of retrotransposons in the mammalian brain. Proc Natl Acad Sci U S A. 2016;113:12697–702.
Roy J, Sarkar A, Parida S, Ghosh Z, Mallick B. Small RNA sequencing revealed dysregulated piRNAs in Alzheimer's disease and their probable role in pathogenesis. Mol BioSyst. 2017;13:565–76.
Qiu W, Guo X, Lin X, Yang Q, Zhang W, Zhang Y, et al. Transcriptome-wide piRNA profiling in human brains of Alzheimer's disease. Neurobiol Aging. 2017;57:170–7.
Dharap A, Nakka VP, Vemuganti R. Altered expression of PIWI RNA in the rat brain after transient focal ischemia. Stroke. 2011;42:1105–9.
Saxena A, Tang D, Carninci P. piRNAs warrant investigation in Rett syndrome: an omics perspective. Dis Markers. 2012;33:261–75.
Rajan KS, Velmurugan G, Gopal P, Ramprasath T, Babu DD, Krithika S, et al. Abundant and altered expression of PIWI-interacting RNAs during cardiac hypertrophy. Heart Lung Circ. 2016;25:1013–20.
Holt JE, Stanger SJ, Nixon B, McLaughlin EA. Non-coding RNA in spermatogenesis and Epididymal maturation. Adv Exp Med Biol. 2016;886:95–120.
Zhu X, Zhi E, Li Z. MOV10L1 in piRNA processing and gene silencing of retrotransposons during spermatogenesis. Reproduction. 2015;149:R229–35.
Yuan ZH, Zhao YM. The regulatory functions of piRNA/PIWI in spermatogenesis. Yi Chuan. 2017;39:683–91.
Kamaliyan Z, Pouriamanesh S, Soosanabadi M, Gholami M, Mirfakhraie R. Investigation of piwi-interacting RNA pathway genes role in idiopathic non-obstructive azoospermia. Sci Rep. 2018;8:142.
Cao C, Wen Y, Wang X, Fang N, Yuan S, Huang X. Testicular piRNA profile comparison between successful and unsuccessful micro-TESE retrieval in NOA patients. J Assist Reprod Genet. 2018;35:801–8.
Henaoui IS, Jacovetti C, Guerra Mollet I, Guay C, Sobel J, Eliasson L, et al. PIWI-interacting RNAs as novel regulators of pancreatic beta cell function. Diabetologia. 2017;60:1977–86.
Chuang TD, Xie Y, Yan W, Khorram O. Next-generation sequencing reveals differentially expressed small noncoding RNAs in uterine leiomyoma. Fertil Steril. 2018;109:919–29.
Sarkar A, Volff JN, Vaury C. piRNAs and their diverse roles: a transposable element-driven tactic for gene regulation? FASEB J. 2017;31:436–46.
Zamore PD. Somatic piRNA biogenesis. EMBO J. 2010;29:3219–21.
Li C, Vagin VV, Lee S, Xu J, Ma S, Xi H, et al. Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell. 2009;137:509–21.
Zhong F, Zhou N, Wu K, Guo Y, Tan W, Zhang H, et al. A SnoRNA-derived piRNA interacts with human interleukin-4 pre-mRNA and induces its decay in nuclear exosomes. Nucleic Acids Res. 2015;43:10474–91.
Ogawa Y, Taketomi Y, Murakami M, Tsujimoto M, Yanoshita R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol Pharm Bull. 2013;36:66–75.
Sharma AK, Nelson MC, Brandt JE, Wessman M, Mahmud N, Weller KP, et al. Human CD34(+) stem cells express the hiwi gene, a human homologue of the Drosophila gene piwi. Blood. 2001;97:426–34.
Juliano C, Wang J, Lin H. Uniting germline and stem cells: the function of Piwi proteins and the piRNA pathway in diverse organisms. Annu Rev Genet. 2011;45:447–69.
Litwin M, Dubis J, Arczynska K, Piotrowska A, Frydlewicz A, Karczewski M, et al. Correlation of HIWI and HILI expression with Cancer stem cell markers in colorectal Cancer. Anticancer Res. 2015;35:3317–24.
Liu W, Gao Q, Chen K, Xue X, Li M, Chen Q, et al. Hiwi facilitates chemoresistance as a cancer stem cell marker in cervical cancer. Oncol Rep. 2014;32:1853–60.
Liang D, Yang Y, Liu Y. The role Hiwi gene in the maintenance of lung cancer stem cell populations. Neoplasma. 2013;22. https://doi.org/10.4149/neo_2014_022.
Liang D, Fang Z, Dong M, Liang C, Xing C, Zhao J, et al. Effect of RNA interference-related HiWi gene expression on the proliferation and apoptosis of lung cancer stem cells. Oncol Lett. 2012;4:146–50.
Nikpour P, Forouzandeh-Moghaddam M, Ziaee SA, Dokun OY, Schulz WA, Mowla SJ. Absence of PIWIL2 (HILI) expression in human bladder cancer cell lines and tissues. Cancer Epidemiol. 2009;33:271–5.
Ross RJ, Weiner MM, Lin H. PIWI proteins and PIWI-interacting RNAs in the soma. Nature. 2014;505:353–9.
Rosenkranz D. piRNA cluster database: a web resource for piRNA producing loci. Nucleic Acids Res. 2016;44:D223–30.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81702882), Science and Technology Department of Jiangsu Province (No. BK20171056) and Education Department of Jiangsu Province (No. 16KJB310005).
Availability of data and materials
All data reported in this study are included in this published article.
Author information
Authors and Affiliations
Contributions
TS collected the information in the text and wrote the manuscript. XH did critical reading and gave valuable suggestion for the paper. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed on the publication of the paper.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sun, T., Han, X. The disease-related biological functions of PIWI-interacting RNAs (piRNAs) and underlying molecular mechanisms. ExRNA 1, 21 (2019). https://doi.org/10.1186/s41544-019-0021-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41544-019-0021-1