Abstract
Background
Early dysregulation of local and systemic inflammatory and immune responses is implicated in the pathogenesis of fibrotic and degenerative complications after anterior cruciate ligament reconstruction (ACLR) surgery. In other surgical trauma models, ALM therapy has been shown to blunt inflammation, leading to a more permissive healing environment in injured tissues. The purpose of this study was to evaluate sex-specific effects of surgery and perioperative ALM therapy on leukocyte mobilization and activation, and systemic and joint tissue inflammation in a rat model of ACL rupture and reconstruction.
Methods
Adult male and female Sprague–Dawley rats were randomly divided into ALM (male, n = 15; female, n = 14) or Saline control (male, n = 13; female, n = 14) treatment groups. Three days after non-invasive ACL rupture, ACLR surgery was performed on the injured knee. Animals received a 1 h perioperative IV ALM or saline drip, and a 0.1 ml IA bolus of ALM or saline, and were monitored to 120 h postoperative. Hematology, leukocyte immunophenotyping, plasma and synovial inflammatory mediator concentrations, and joint tissue histopathology and gene expression of inflammatory markers were assessed.
Results
Following ACLR surgery, plasma concentrations of inflammatory cytokines IL-6, TNF-α and IL-1β peaked later and at a higher magnitude in females compared to males, with ALM dampening this systemic inflammatory response. At 1 h postoperative, ALM boosted circulating B cell numbers in males and females, and decreased neutrophil activation in females. By 72 h, numbers of circulating T cells with immunoregulatory potential were increased in all ALM-treated animals compared to Saline controls, and corresponded to a significant reduction in synovial TNF-α concentrations within the operated knees. Sex-specific treatment differences were found in inflammatory and immune profiles in the synovial fluid and joint tissues. Inflammatory cell infiltration and gene expression of markers of inflammation (Nfκb, Nlrp3), cytoprotective responses (Nrf2), and angiogenesis (Vegf) were increased in joint synovial tissue from ALM-treated males, compared to controls. In females, ALM treatment was associated with increased mononuclear cell recruitment, and expression of M2 macrophage marker (Arg1) in joint synovial tissue.
Conclusions
ALM has differential effects on the immuno-inflammatory response of males and females in the early postoperative period after ACLR surgery, with potential implications for subsequent joint tissue repair processes.
Similar content being viewed by others
Background
Anterior cruciate ligament (ACL) rupture is one of the most common and debilitating non-contact knee injuries, particularly among recreational and professional athletes [1, 2]. Annual incidence rates have risen markedly among children and adolescents and are predicted to double within the next decade, with females having a 3- to 6-times higher risk of ACL injury than males [2, 3]. Despite advancements in rehabilitation practices, and surgical techniques and biomaterials for ACL reconstruction (ACLR) surgery, more than a third of patients develop post-traumatic osteoarthritis (PTOA) within 10 to 15 years [4, 5]. Sex is an independent risk factor for PTOA predisposition following ACL injury and ACLR surgery [4].
ACL rupture is a whole joint injury that triggers a complex sequence of immune cell activation, inflammation, and neural and tissue remodelling events [6]. Surgical trauma associated with ACLR surgery has potential to exacerbate this hostile environment, with delayed resolution of inflammation a common intersect for the progressive tissue degeneration and remodeling processes associated with PTOA [7,8,9,10,11,12]. We recently showed in rat model of ACL rupture alone, that differences in the early systemic inflammatory responses of males and females were associated with contrasting joint tissue healing phenotypes at 31-days post-injury [13]. Frontline strategies to minimise sex-specific surgery-induced inflammation, and improve joint tissue healing and patient outcomes following ACLR are lacking [1]. ALM, a combination of adenosine, lidocaine and magnesium, is an emerging perioperative therapy with anti-inflammatory, immunomodulatory, chondro-protective, and anti-fibrotic properties [14,15,16,17,18,19,20]. We hypothesized that perioperative ALM therapy would dampen local and systemic inflammatory responses triggered by ACLR surgery in males and females. The aim of this study was to evaluate sex-specific effects of ALM therapy on leukocyte mobilization and activation, and systemic and joint tissue inflammation in the first 5 postoperative days in a rat model of non-invasive ACL rupture and subsequent ACLR surgery.
Methods
Study design
Conventional, 16-week male (n = 28; 410 ± 39 g) and female (n = 29; 239 ± 15 g) Sprague–Dawley rats obtained from the James Cook University Small Animal Facility were randomly divided into ALM (male, n = 15; female, n = 14) or Saline control (male, n = 13; female, n = 14) treatment groups. Three days after non-invasive ACL rupture [13], animals underwent remnant-sparing ACLR surgery using a tail tendon autograft, with post-operative assessment over 120 h (Fig. 1). Animals were housed in individually ventilated cages (Tecniplast® Australia, NSW, Australia) in a 14-10 h dark–light cycle under controlled temperature (21–22 °C) and humidity (65–75%) conditions, with access to standard rodent pellets (Specialty Feeds, WA, Australia) and water ad libitum. Animals were acclimated for at least 7 days prior to experimentation. All animal experiments followed protocols approved by the institutional Animal Ethics Committee (A2684) and the US Army Animal Care and Review Use Office (ACURO), and are reported according to the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines.
Study protocol schematic. Non-invasive ACL rupture was performed on the right knee of anesthetized male (n = 28) and female (n = 29) Sprague Dawley rats, and confirmed by a positive anterior drawer test, prior to recovery from anesthesia. Three days after ACL rupture, re-anesthetized animals underwent ACLR surgery to repair the ruptured ACL using a tail tendon autograft. Animals were randomized to Saline control (1 h 0.9% NaCl IV drip with IA bolus of 0.9% NaCl) or ALM treatment (1 h 0.9% NaCl ALM IV drip with IA bolus of 0.9% NaCl ALM) groups. Blood samples were collected the day before surgery (-24 h), at completion of the IV drip (1 h post-surgery), and at 24, 72 and 120 h post-surgery. A subset of animals (n = 5 per group) was euthanized 72 h post-surgery for terminal joint tissue analyses. IV, intravenous; IA, intra-articular
Treatment
The ALM treatment group received a 0.5 ml/kg/h IV infusion dose developed from previous rat studies (adenosine 18.7 mM, lidocaine 34.6 mM, MgSO4 41.5 mM in 0.9% NaCl) [15,16,17]. Immediately following capsule closure, and prior to skin closure, animals also received an intra-articular (IA) bolus of ALM (0.1 ml; 1 mM adenosine, 3 mM lidocaine, and 2.5 mM MgSO4 in 0.9% NaCl) [18, 19]. Saline control animals received a 0.9% NaCl drip with an IA bolus of 0.9% NaCl.
Non-invasive ACL rupture
Non-invasive ACL rupture of the right hind limb was performed on anesthetized animals as described previously [13]. ACL rupture was confirmed by anterior drawer test and by gross morphological examination at the time of ACL surgery. Analgesia (Carprieve® (Carprofen), 5 mg/kg, s.c. in 1 ml saline) was administered within 15 min of ACL rupture, prior to recovery from anesthesia.
ACLR surgical procedure
ACLR surgery was performed on animals 72 h after ACL rupture. On the day prior to surgery (-24 h), animals were anaesthetized for blood collection and hair removal from the surgical site. Surgeries were performed on 8–10 animals per surgery day, with equal numbers of animals per treatment group and randomisation of the order of animals undergoing surgery. All surgeries were performed by a single surgeon within a sterile surgical field, using aseptic techniques, sterile instruments, gowns, gloves and drapes. Anesthesia was induced with 5% isoflurane (in 100% oxygen) during the induction phase and maintained with 2.5% isoflurane during surgery, with animals breathing spontaneously. On the day of surgery, a temporary polyethylene catheter (I.D. 0.023 in) was implanted in the left femoral vein of animals to facilitate perioperative fluid infusion and blood sampling (Fig. 2A, B). The catheter was secured within the vessel, and the skin incision closed, with a 4–0 braided silk suture (DC0210D, Look™). Following a 10 min baseline stabilisation period, surgery for tail tendon harvest commenced. A 1 cm dorsal incision was made approximately 10 cm from the base, and a single tendon bundle was harvested using a sterile dissection needle (Fig. 2C,D). After cutting the distal end of the tendon, the tail incision was closed (polydioxanone (PDS II) 5–0 suture, Z463G, Ethicon) and Opsite™ dressing spray applied. The harvested tendon was bundled using a PDS II 5–0 suture, and a silk braided 5–0 suture (JJ-W580, Ethicon) was situated on the graft end as a lead (Fig. 2E). Grafts were preserved in vancomycin (5 mg/ml) until placement. For ACLR surgery, a medial parapatellar approach was used to expose the femoral condyles and tibial plateau of the knee. A mini aluminium hand drill with keyless chuck and high-speed stainless steel drill bit (1.5 mm) was used to create tunnels in the proximal tibia and distal femur using a trans-tibial approach (Fig. 2F). To assist graft placement, a 16G IV catheter was inserted through both tunnels, then under the traction of the attached suture, the tail tendon graft was passed through (Fig. 2G). The graft was positioned and secured within the distal femoral socket with stainless steel, cone point grub screw (M1.6 × 3 mm, DIN 914, A2). After graft tensioning a second screw was used to secure the graft within the tibial socket (Fig. 2H,G). The joint capsule was closed (PDS II 5–0), then an IA bolus of ALM or Saline was administered to the operated knee, prior to skin closure (MonoQ Plus, Q463, Provet). Animals remained under anesthesia until completion of the 1 h IV drip. A 0.5 ml venous blood sample was collected into a micro collection tube (K3 EDTA) prior to removal of the catheter and closure of the skin (4–0 braided silk, DC0210D, Look™). Intraoperative blood loss from vascular and ACLR surgery was measured as previously described [18]. Immediately after catheter removal and prior to recovery from anesthesia, animals received 5 mg/kg Carprieve s.c. in 1 mL saline, with analgesic administered 24 hourly thereafter, according to pain scores of individual animals. Clinical signs including body weight, temperature and weight-bearing activity were monitored daily throughout the 120 h experimental period.
ACL reconstruction surgery. A A temporary catheter was placed within the left femoral vein to facilitate perioperative fluid infusion using a B syringe pump. C Schematic of transverse section of rat tail showing site of left dorsal tail tendon bundle harvested for graft preparation (asterisk). D A 1 cm incision was made approximately 10 cm from the base of the rat tail to expose the left dorsal tendon bundle. A dissection needle was used to lever the tendon until it released proximally, then the distal end cut. E The tail tendon was bundled to prepare an ACL graft using a PDS II 5–0 suture, with a braided 5–0 suture situated on the graft end as a lead. F A medial parapatellar approach was used to expose the femoral condyles and tibial plateau, then a hand drill with a 1.5 mm drill bit was used to create femoral and tibial tunnels. G Under traction of the lead suture, the tendon graft was positioned, and H, I) tensioned under extension using M1.6 grub screws to secure within the femoral and tibial sockets
Hematology and inflammatory assessments
In addition to sampling on the day of surgery (1 h), blood (0.5 ml) was collected from the tail vein of anesthetized animals at -24 h (2 days after ACL rupture, one day before ACLR surgery), and 24, 72 and 120 h post-ACLR surgery. A complete blood cell count was performed (VetScan HM5 hematology analyser, Abaxis, CA, USA), and plasma collected and stored at -80 °C until further analysis. Inflammatory cytokines chemokines, and growth factors were measured in plasma (interleukin [IL]-6, tumor necrosis factor [TNF]-α, IL-1β, granulocyte colony-stimulating factor [G-CSF], growth regulated protein/keratinocyte chemoattractant [GRO/KC], lipopolysaccharide-inducible CXC chemokine [LIX], monocyte chemoattractant protein [MCP]-1, macrophage inflammatory protein [MIP]-1a, regulated upon activation, normal T cell expressed and presumably secreted [RANTES]), and synovial fluid (IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p70, IL-13, TNF-α, interferon [IFN]-γ, MCP-1, MIP-1α, MIP-2, RANTES, GRO/KC, LIX, fractalkine, IL-17A, IL-18, interferon-inducible protein [IP]-10, vascular endothelial growth factor [VEGF]), using custom Milliplex® Rat Cytokine/Chemokine Magnetic Bead Panels (Abacus ALS, Cannon Hill, QLD, Australia), as described previously [13]. Baseline ranges for hematology parameters and plasma inflammatory mediators were determined in healthy male (n = 8) and female (n = 8) animals.
Leukocyte profiling
Leukocyte phenotyping of peripheral blood (ALM, n = 10; Saline, n = 9 rats per sex) was performed using cell surface and intracellular markers and flow cytometry. Briefly, after blocking non-specific binding (anti-rat CD16/CD32, Rat Fc Block) leukocytes (2 × 106/ml) were stained with anti-rat fluorochrome-conjugated antibodies (Table S1, Additional file 1) using standard surface and intracellular staining protocols, and according to manufacturer’s instructions. Unstained and Fluorescence Minus One (FMO) controls were included in each batch. Cells (5 × 104 leukocytes per sample) were acquired on a FACSCanto II (BD Biosciences), with compensation using fluorescent compensation beads (OneComp eBeads, eBioscience, USA). Data was analysed with FLOWJO analysis software v10 (FlowJo LLC, Inc, Ashland, OR, USA). Percentages and absolute numbers of B cells (CD3−CD45RA+), T helper cells (Th; CD3+CD4+), T cytotoxic cells (Tc; CD3+CD8+), T regulatory cells (Tregs; CD3+CD4+CD25+Foxp3+), precursor Tregs (CD3+CD4+CD25−Foxp3+), NK cells (CD3−CD8+CD161+), neutrophils (SSCHI CD43HI), and classical (SSCLoCD43Lo) and non-classical (SSCLoCD43HI) monocytes were measured according to the gating strategy shown (Fig. S1, Additional file 6). In addition, expression of the surface activation markers CD62L and CD11b/c on neutrophils and monocytes were determined with data shown as median fluorescence intensity (MFI) on populations of interest.
Joint swelling
Joint swelling was assessed 24 h prior to surgery (2 days after ACL rupture), and 24, 72 and 120 h after ACLR surgery by measuring the diameter (medial–lateral) of ACL-ruptured (right) and ACL-intact (left) knees with digital callipers. Data show the difference in size between the injured/operated and non-injured/non-operated knee.
Joint tissue collection
At 72 h postoperative, a subgroup of animals within each treatment group (n = 5) were euthanized to assess joint pathology and tissue inflammation and pathology. Synovial wash was performed on operated (right) and non-operated (left) knees with 0.06 ml of saline, as previously described [13]. The douche fluid was snap frozen and stored at − 80 °C for subsequent analysis of inflammatory cytokines and chemokines. Samples of the ACL graft and remnant, and medial capsular tissue (surgical incision site) were collected, snap-frozen and stored at -80 °C for gene expression studies. Remaining joints were fixed in 4% paraformaldehyde (PFA) for 48 h, decalcified with 14% EDTA, processed and paraffin-embedded. Sections (4 μm) were cut in the frontal plane, stained with hematoxylin and eosin (H&E), and visualized with light microscopy (Nikon Eclipse i50; Japan).
RNA isolation and quantitative RT-PCR
Total RNA was isolated from joint tissue samples and cDNA was prepared by reverse transcription, as described previously [18]. Real-time PCR with custom-designed primers was used to assess gene expression of key markers of inflammation (chemokine ligand 6 [Cxcl6), nuclear factor kappa B subunit 1 [Nfkb1], nitric oxide synthase 2, inducible [Nos2], nucleotide-binding domain-like receptor protein 3 [Nlrp3]) and wound healing (arginase 1 [Arg1], nuclear factor erythroid 2-related factor 2 [Nrf2], vascular endothelial growth factor A [Vegfa], platelet-derived growth factor subunit A [Pdgfa], fibroblast growth factor 1 [Fgf1]) (Table S2, Additional file 2). The relative expression of each gene was calculated using the concentration-Ct-standard curve method and normalized using the average expression of the housekeeping hypoxanthine–guanine phosphoribosyl transferase (Hprt1) gene for each sample. All reactions were independently performed in duplicate to assess the repeatability of the results, and mean values for each sample used for analyses.
Statistics
Statistical analyses were performed using GraphPad Prism software (version 9.0.0). Normality assumptions and equality of variances were assessed in datasets using Shapiro-Wilks and Levene’s test, respectively. Two-way ANOVA with Tukey’s HSD test was used for between and within groups comparison. Inflammatory mediator concentrations were analysed using MILLIPLEX Analyst 5.1 software (Luminex Corporation, Austin, Texas, USA) with a 5-parametric logistic weighted curve fit. Results are expressed as mean ± standard error (SEM) unless otherwise stated, with significance set at p < 0.05.
Results
Operative metrics and post-surgery recovery
Following non-invasive ACL rupture, no adverse events occurred and there were no signs of lameness for any animal, with all animals weight-bearing immediately following recovery from anesthesia. ACL rupture was confirmed at the time of surgery, with comparable injury profiles between treatment groups and sexes (Table S3, Additional file 3). No meniscal or ligamentous defects were noted at the time of ACLR surgery. For each sex, total surgery times and blood loss was comparable for ALM-treated and Saline control groups (Table S3, Additional file 3). All animals recovered from anesthesia and were conscious and ambulant within fifteen minutes. Similar to ACL rupture, there were no adverse events after ACLR surgery, and no animals showed signs of infection. Minor weight loss occurred in males and females following ACLR surgery, however there were no statistically significant differences between treatment groups (Table S3, Additional file 3).
ALM elicits sex-specific differences in systemic inflammatory cytokine and chemoattractant profiles
Plasma concentrations of the prototypical inflammatory cytokines IL-6, TNF-α and IL-1β were compared in ALM-treated and Saline control animals to 120 h after ACLR surgery (Fig. 3A). At 1 h postoperative, plasma IL-6 increased threefold in male ALM-treated animals, and tenfold in male Saline controls compared to baseline, though these differences were not statistically significant (Fig. 3A). In contrast, plasma IL-6 concentrations peaked later in females, and were 20-fold higher than baseline in female Saline controls at 24 h postoperative (p = 0.043; Fig. 3A). ALM treatment blunted the plasma IL-6 response to ACLR surgery in females (p = 0.007 vs Saline control; Fig. 3A). In males, plasma TNF-α remained low or undetectable (< 2.4 pg/ml), and comparable to baseline for both Saline controls and ALM-treated animals to 120 h postoperative. In contrast, plasma TNF-α concentrations were significantly increased in Saline control females at 24 h (p = 0.043), with ALM treatment blunting this response (Fig. 3A). At 72 h postoperative, plasma IL-1β levels were ninefold higher in male than female Saline controls (p = 0.008), however differences between treatment groups were not statistically significant (Fig. 3A).
Systemic inflammatory cytokine and chemokine responses in male and female ALM-treated and Saline control animals after ACLR surgery. Plasma concentrations of A pro-inflammatory cytokines, interleukin (IL)-6, tumor necrosis factor alpha (TNF-α) and IL-1β, B neutrophil chemoattractants, granulocyte colony stimulating factor (G-CSF), growth-regulated oncogene/keratinocyte chemoattractant (GRO/KC) and lipopolysaccharide-induced CXC chemokine (LIX), and C monocyte and lymphocyte chemoattractants, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 alpha (MIP-1α) and regulated on activation, normal T cell expressed and secreted (RANTES) prior to (-24 h), and at 1, 24, 72 and 120 h after ACLR surgery. Data show mean ± SEM. Mixed ANOVA, Tukey post-hoc test, * p < 0.05, ALM (Female), compared to Saline Control (Female). Ψ p < 0.05 Saline (Male), compared to Saline (Female). Shaded areas in graphs show mean ± SEM for healthy baseline male (blue) and female (red) animals (n = 8 per sex). Dotted line represents commencement of ACLR surgery (time 0)
Changes in plasma levels of key mediators involved in neutrophil (G-CSF, GRO/KC, LIX), monocyte (MCP-1), and lymphocyte (MIP-1α, RANTES) mobilization were also compared to 120 h after ACLR surgery (Fig. 3B). While steady increases occurred in male Saline controls from time of surgery to 72 h (sixfold, p = 0.164), G-CSF levels in female Saline controls did not increase until 72 h. In male and female ALM-treated animals, levels remained comparable to baseline across the 120 h period (Fig. 3B). Compared to pre-surgery levels, there was a significant increase in GRO/KC in ALM-treated and Saline control males at 1 h postoperative (2 to 7-fold, p = 0.02), followed by a decrease over the remaining 120 h. In females, peak GRO/KC levels occurred at 24 h postoperative in both ALM-treated (1.4-fold, p = 0.052) and Saline controls (1.5-fold, p = 0.045), compared to baseline. At 72 h postoperative, GRO/KC concentrations tended to be lower in ALM-treated males and females compared to Saline controls, and baseline levels, though differences were not statistically significant (Fig. 3B). Changes in LIX were similar for female ALM-treated and Saline controls after surgery. In contrast, LIX levels tended to be higher in ALM-treated males compared to Saline controls at 1 h (1.4-fold), 24 h (2.4-fold) and 72 h (twofold), though differences were not statistically significant (Fig. 3B). Plasma MCP-1 concentrations peaked at 24 h postoperative in all treatment groups following surgery, however levels were lower in ALM-treated males (p = 0.059) and females (p = 0.005) compared to Saline controls (Fig. 3C). While levels remained at or below baseline levels in Saline control and ALM-treated males, MIP-1α was significantly decreased in ALM-treated females compared to Saline control animals at 24 h postoperative (p = 0.001; Fig. 3C). RANTES levels remained at or below baseline for treated and untreated males prior to, and following ACLR surgery. In contrast, RANTES concentrations were elevated in ALM-treated females compared to Saline controls at 72 h postoperative, though differences were not statistically significant (p = 0.113).
ALM modulates sex-specific peripheral blood leukocyte mobilization and activation
To further characterize the systemic cellular response to ACLR surgery, hematology and immunophenotyping of leukocyte subsets was performed to 120 h postoperative. Platelet and red blood cell numbers, hematocrit and hemoglobin levels were comparable between treatment groups for males and females throughout the experimental period (Table S4, Additional file 4). Frequencies and proportions of peripheral blood leukocyte subsets were comparable between male and female animals 48 h after ACL rupture (24 h prior to ACLR surgery). However, following surgery sex-specific differences were observed (Fig. 4A; Table S4, Additional file 4). Total circulating leukocytes decreased significantly at 1 h postoperative in males and females, with a return to pre-injury levels between 24 to 72 h, followed by increases above baseline at 120 h (Fig. 4A). Compared to female Saline controls, total leukocyte numbers were significantly higher in male Saline controls at 120 h after surgery (Table S4, Additional file 4). There were no significant differences between treatment groups for either sex.
Changes in peripheral blood leukocyte subsets in male and female ALM-treated and Saline control animals after ACLR surgery. A Average circulating lymphocytes, monocytes and neutrophils counts at baseline, prior to surgery (-24 h), and at 1, 24, 72 and 120 h after ACLR surgery. B Relative cell-surface expression of CD11b/c and CD62L on circulating neutrophils at 1 h postoperative. C Relative cell-surface expression of CD11b/c on peripheral blood monocytes to 120 h postoperative. D Total number of circulating non-classical (SSCLo CD43HI) monocytes at 120 h postoperative. Changes in the number of circulating E precursor T regulatory cells (Tregs; CD4+CD25−FoxP3+), and F conventional Tregs (CD4+CD25+FoxP3+) to 120 h postoperative. Data show mean ± SEM. Mixed ANOVA, Tukey post-hoc test. * p < 0.05, male ALM, compared to female ALM. ⁑ p < 0.05 male Saline, compared to female Saline. ^ p < 0.05 male ALM, compared to male Saline. # p < 0.05 female ALM, compared to female Saline. Shaded area in C, E and F shows mean ± SEM for healthy baseline male (blue) and female (red) animals (n = 8 per sex). MFI, median fluorescence intensity; Tx, treatment
Minor transient decreases occurred in circulating neutrophils and monocytes for both males and females at 1 h postoperative, with responses comparable between ALM-treated and Saline control animals (Table S4). Since a key step in the extravasation of circulating neutrophils and monocytes to sites of tissue injury involves CD62L downregulation, and upregulation of the integrins CD11b/c [21,22,23,24,25], we assessed changes in the surface expression of these adhesion molecules after ACLR surgery. Compared to Saline controls, expression of CD11b/c was significantly lower on neutrophils from female ALM-treated animals at 1 h postoperative (p = 0.014), and corresponded to higher CD62L expression levels (1.4-fold, n.s.; Fig. 4B; Fig. S2, Additional file 7). In contrast, neutrophil CD11b/c and CD62L expression levels remained comparable for Saline control and ALM-treated males throughout the experimental period (Fig. S2, Additional file 7). At 24 h postoperative, monocyte CD11b/c expression levels were significantly higher for female, compared to male ALM-treated animals (p = 0.018; Fig. 4C). By 72 h, circulating neutrophil and monocyte numbers were elevated above baseline and pre-surgery levels for both male and female animals, with no significant differences in CD11b/c or CD62L expression (Fig. 4C; Fig. S2, Additional file 7). However, compared to female Saline controls, neutrophils and monocytes were significantly higher in ALM-treated females at 120 h (Table S4, Additional file 4). In contrast, neutrophil (p = 0.052) and monocyte (p = 0.006) numbers were significantly lower in ALM-treated males, compared to male Saline controls (Table S4, Additional file 4). The decrease in total monocyte numbers, corresponded to a minor decrease in classical (SSCLoCD43Lo_Int; p = 0.193; Fig. S2, Additional file 7) and a significant decrease in non-classical (SSCLoCD43HI; p < 0.001; Fig. 4D) monocyte subsets in ALM-treated males compared to Saline controls at 120 h postoperative.
At 1 h following surgery, absolute numbers and percentage of lymphocytes decreased significantly in both male and female animals, corresponding to decreases in circulating B and T cell numbers (Table S4, Additional file 4). Compared to female Saline controls, the number and percentage of B cells was significantly lower in ALM-treated females at this time (p = 0.004; Table S4). The decrease in T cells at 1 h postoperative corresponded to significant reductions in Th cells, and minor decreases in Tc and Tregs cells (Table S4, Additional file 4). B and T cell numbers returned to preinjury levels between 72 to 120 h postoperative in both sexes. However, the percentage of circulating T cells remained significantly higher in ALM-treated females than ALM-treated males at 120 h (p = 0.02; Table S4, Additional file 4; Fig. S2, Additional file 7).
Male and female ALM-treated animals had significantly higher numbers of precursor Tregs in peripheral blood at 72 h postoperative, compared to Saline control animals (p = 0.021 and p = 0.026 respectively; Fig. 4E). By 120 h postoperative, absolute numbers and percentages of Treg cells were significantly elevated in peripheral blood of all animals, regardless of sex and treatment group (Fig. 4E; Table S4, Additional file 4). Despite little change in males after surgery, NK cell numbers were significantly lower in ALM-treated females at 1 h postoperative, compared to baseline (p = 0.01) and pre-surgery levels (p = 0.034), returning to baseline thereafter (Table S4, Additional file 4).
ALM alters sex-specific joint pathology and inflammatory profiles
Following ACLR surgery, knee swelling peaked at 24 h postoperative, with swelling persisting to 120 h in male and female ALM-treated animals, and in female Saline controls compared to pre-surgical levels (Fig. 5A,B). Given the significant effects of circulating cytokines and shifts in peripheral blood leukocyte subsets in the first 3 postoperative days, a subset of animals were euthanized at 72 h for assessment of joint pathology and inflammatory markers. Synovial TNF-α levels were 2.4- (p = 0.01) and 2.6-fold (p = 0.039) higher in operated than non-operated knees of male and female Saline controls, respectively. This response was blunted in ALM-treated animals of both sexes, with TNF-α levels comparable to non-operated knees (Fig. 5C). Synovial IL-1α (p = 0.057) and IL-1β (p = 0.002) levels were significantly elevated above baseline in operated knees of ALM-treated females, and 3.4- (p = 0.222) and 3-fold (p = 0.042) higher than levels in male ALM-treated knees (Fig. 5C). Synovial profiles for LIX, MIP-2 and MIP-1α followed similar patterns, with significantly elevated levels in female ALM-treated knees compared to non-operated knees (LIX, 8.5-fold, p = 0.024; MIP-2, 107-fold, p = 0.02; and MIP-1α, 184-fold, p = 0.003; Fig. 5C). While levels of IL-1α (1.6-fold), IL-1β (twofold), LIX (2.1-fold), MIP-2 (2.1-fold) and MIP-1α (1.8-fold) tended to be higher in synovial fluid of ALM-treated females than Saline controls, differences were not statistically significant (Fig. 5C). ALM treatment appeared to have contrasting effects on synovial IP-10 levels for males and females, with concentrations decreased in males (2.8-fold; p = 0.021), and increased in females (1.9-fold; n.s., p = 0.482), compared to their respective controls (Fig. 5C). No significant differences were observed in GRO/KC, IL-6, IL-10, IL-18, RANTES or VEGF levels between treatment groups for either sex (Table S5, Additional file 5). Levels of fractalkine, IFN-γ, IL-2, IL-4, IL-12p70, IL-13, and IL-17 were below the limit of detection in the synovial fluid at 72 h postoperative.
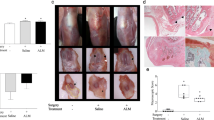
Molecular and histopathology changes in ACL graft and synovial tissue. A Representative images of operated knees, B joint swelling, and C relative concentrations (percentage change from levels in non-operated contralateral knees) of inflammatory cytokines (TNF-α, IL-1α, IL-1β) and chemokines (LIX, MIP-2, IP-10, MCP-1, MIP-1α) in synovial fluid of male and female ALM-treated and Saline control animals 72 h after ACLR surgery. D Relative expression of markers of neutrophil infiltration (chemokine ligand 6, Cxcl6), inflammation (nuclear factor kappa B, Nfκb; nucleotide-binding domain-like receptor protein 3, Nlrp3); anti-oxidative signalling (nuclear factor erythroid 2–related factor 2, Nrf2); and wound healing growth factors (vascular endothelial growth factor, Vegf; fibroblast growth factor 1, Fgf1) in the ACL graft and remnant tissue of ALM-treated and Saline control male and female animals 72 h post-surgery. Data show mean ± SEM (joint swelling, inflammatory mediators) or median ± IQR (gene expression). Mixed ANOVA, Tukey post-hoc test (joint swelling), Kruskal–Wallis test (cytokines, gene expression). Δ p < 0.05 male Saline, compared to pre-surgery. ƒ p < 0.05 male ALM, compared to pre-surgery. † p < 0.05 female Saline, compared to pre-surgery. § p < 0.05 female ALM, compared to pre-surgery
To further investigate apparent sex- and treatment differences in the inflammatory environment within the joint, gene expression levels of key inflammatory and wound healing markers were assessed in ACL graft and remnant tissue, and in joint capsular tissue of operated knees relative to non-operated, contralateral knees. Expression of Cxcl6, encoding granulocyte chemotactic protein 2 (GCP-2), was increased in ACL tissue from female knees, with no treatment differences (Fig. 5D). In contrast, Cxcl6 expression was significantly upregulated in ALM-treated males, compared to male Saline controls (p = 0.016; Fig. 5D). Similarly, while expression levels were comparable between female ALM-treated and Saline controls, expression of Nfκb (2.9-fold, p = 0.016), Nlrp3 (2.8-fold, p = 0.016) and Nos2 (2.3-fold, p = 0.064) was higher in ACL tissue from male ALM-treated knees compared to Saline controls (Fig. 5D). Arg1 expression was 3.7-fold higher in joint capsular tissue of ALM-treated females, than ALM-treated males (p = 0.004; Fig. 5D). Sex-specific differences in treatment effects were also observed for Nrf2 expression, with a 6.9-fold increase in ALM-treated males and a 2.4-fold decrease in females, compared to their respective Saline controls (p = 0.032 and p = 0.008, respectively, Fig. 5D). Vegf expression was significantly increased 3.2-fold in male ALM-treated animals compared to controls, with no treatment difference observed in females (Fig. 5D). No significant sex or treatment differences were observed for Pdgfa and Fgf1 expression in ACL tissue (Fig. 5D).
Contrasting inflammatory mediator profiles of synovial fluid and joint tissue inflammatory phenotypes of ALM-treated and control knees was supported histologically (Fig. 6). Inflammatory cell infiltrates were evident at the ACL graft-bone interface and throughout the sub-synovial tissue of the joint capsule. Cellularity was markedly decreased in joint tissues from Saline control males, compared to the other groups (Fig. 6). Cellular composition also differed between males and females, and treated and untreated animals. In males, ALM appeared to boost neutrophil, monocytes/macrophage and fibroblast numbers compared to Saline controls (Fig. 6). In contrast, ALM-treated females tended to have lower proportion of neutrophils to monocytes/macrophages, increased fibroblasts and evidence of cell proliferation compared to controls (Fig. 6). Inflammatory cell infiltration into ACL graft tissue itself was also more pronounced in females than males, with a mononuclear-dominant response in ALM-treated females (Fig. 6).
Representative hematoxylin and eosin-stained sections of ACL graft and synovial tissue in male and female ALM-treated and Saline control animals, 72 h after ACLR surgery (magnification, × 100 and × 400). Infiltrating polymorphonuclear (black arrow) and mononuclear (blue arrow) cells were observed, together with fibroblasts (yellow arrowhead) and proliferating cells (asterisk)
Discussion
Using a rat model of ACL rupture and ACLR surgery, we show perioperative ALM therapy has differential effects on early recruitment patterns of circulating leukocyte subsets and inflammatory processes in females, compared to males. This finding was based on the following results: First, ALM therapy appears to dampen the heightened systemic inflammatory cytokine response (IL-6, TNF-α, IL-1β) in females, with little or no effect in males during the early postoperative days. Second, ALM led to significantly increased synovial levels of IL-1β and MIP-1α, and Arg1 expression in joint capsular tissue in females, compared to Saline controls. Interestingly, in males ALM increased joint tissue expression of inflammatory (Nfκb, Nlrp3, Nrf2) and angiogenic (Vegf) markers compared to controls. Lastly, despite differential joint inflammatory and healing phenotypes, synovial TNF-α concentrations were significantly reduced within the operated knees of both females and males treated with ALM 72 h after ACLR surgery. These findings will now be discussed.
ALM modulates systemic inflammation and leukocyte recruitment in a sex-specific manner
The inflammatory cytokines, IL-6, TNF-α and IL-1β trigger a cascade of molecular and cellular processes involved in the acute inflammatory response, including stimulation of different components of the hypothalamo-pituitary-adrenal (HPA) axis as part of the stress response to surgery-induced tissue trauma [26, 27]. Here we show plasma concentrations of key inflammatory cytokines, IL-6 and TNF-α, peaked at a higher magnitude in females compared to males after ACLR surgery, with ALM dampening this systemic inflammatory response in the early postoperative period. Sex differences in HPA axis activation sensitivity have been described [28, 29], with females exhibiting a heightened acute stress response and a greater contribution of IL-6 than males [30, 31]. Our findings of decreased plasma inflammatory cytokines suggest ALM may limit HPA axis activation in response to surgery, although further investigations are required to determine the mechanisms underlying this protective effect.
In addition to blunting inflammatory cytokine levels, we show ALM differentially alters leukocyte chemotactic profiles in males and females in the early postoperative period. In females, ALM decreased plasma levels of monocyte and lymphocyte chemoattractants (MCP-1 and MIP-1α) 24 h after surgery, and increased levels of RANTES (CCL5), a chemoattractant for T cells and NK cells, at 72 h. In males, ALM maintained plasma concentrations of neutrophil attractants (LIX and G-CSF) at baseline levels, and decreased levels of the monocyte chemoattractant, MCP-1 at 24 h postoperative. Leukocyte recruitment to injured tissue is fundamental to the acute inflammatory process, and is enhanced by circulating inflammatory cytokines and chemokines [21,22,23,24,25]. Consistent with the disparate systemic inflammatory and chemoattractant mediator profiles, sex-specific and treatment-specific differences also occurred in circulating leukocyte subsets after ACLR surgery. In the early phase of inflammation (< 24 h), neutrophils dominate the cellular response to tissue injury, with recruitment of monocytes and lymphocytes increasing thereafter. In the present study, females demonstrated increased neutrophil and monocyte activation within the first 24 h, indicated by increased CD11b/c and decreased CD62L expression, with ALM treatment dampening the effect for neutrophils. Our findings are consistent with other studies reporting an enhanced priming of innate immune response pathways in circulating neutrophils and monocytes from females, compared to males [32, 33].
A novel finding from this study was that ALM appears to promote B cell recruitment at 1 h after ACLR surgery in both sexes (Fig. 4). Although the role of B cells in the acute inflammatory response remains poorly characterized, there is increasing recognition of the critical immunoregulatory role innate-like B cell subsets (ILBs) and regulatory B cells (Bregs) play in limiting inflammation and restoring immune homeostasis after injury and infection [34,35,36]. Interestingly, Saze et al. [37] showed that in response to increasing concentrations of extracellular adenosine triphosphate (ATP), B cells produce large amounts adenosine which, via autocrine signaling through adenosine A3 receptors (A3R), activates B cell immunosuppressive functions, including the expansion of Treg populations. In support of this, we also found significant increases in precursor Tregs in the peripheral blood of ALM-treated males and females 72 h after ACLR surgery (Fig. 4; Table S4, Additional file 4). Precursor Tregs, a subpopulation lacking CD25 expression, exert less immunosuppressive activity than conventional Tregs and may serve as a reservoir population with plasticity to differentiate into diverse T cell subsets, depending on the inflammatory signals present in the microenvironment of injured tissue [38]. While we did not assess immune cells at the injury site within 24 h postoperative, data indicate that ALM is acting as an inflammatory brake, which is consistent with our findings in other surgical models [16, 18, 39].
Importantly, ALM’s immunoregulatory effects appear to be tailored to the different innate immune responses of males and females. Further evidence of this was shown by ALM’s contrasting effects on circulating monocyte levels, with a significant decrease in non-classical ‘inflammatory’ monocytes in males, and an increase in classical monocytes in females at 120 h postoperative (Table S4, Additional file 4). Sex differences in the frequencies, phenotypes and responsiveness of monocytes to infection and injury are increasingly appreciated [40,41,42,43]. Detailed characterization of neutrophil, monocyte and T and B lymphocyte subsets using single cell genomics and multiplex immunophenotyping studies will be important to further our understanding of the mechanism underlying ALM’s sex-specific modulatory effects on leukocyte recruitment in the early postoperative period, and the implication this has on tissue repair within the operated joint.
ALM alters sex-specific inflammatory profiles within the operated joint
In our study, the contrasting peripheral blood leukocyte subset profiles between sexes and treatment groups were consistent with cellular and molecular differences within operated knees 72 h after ACLR surgery. Notably, synovial TNF-α, a central mediator of inflammation, was significantly lower in operated knees from ALM-treated animals of both sexes, compared to controls. Elevated synovial TNF-α at 3 days postoperative is associated with delayed recovery of joint function following ACLR surgery [44], with TNF-α inhibitors showing promise in affording chondroprotection following orthopedic surgery [45]. In addition to lowering TNF-α levels, ALM significantly increased synovial concentrations of IL-1β within joint tissues from female operated knees at 72 h postoperative (Fig. 5). Similar to TNF-α, IL-1β exerts time- and concentration-dependent pleiotropic functions. While both have potent pro-inflammatory roles during initiation of inflammation, they are also intricately involved in the regulation of tissue repair and transition from the inflammatory to proliferative phase of healing through their actions on immune cells, fibroblasts, vascular endothelial cells, and mesenchymal stem cells [46, 47]. For example, low concentrations of TNF-α promote fibroblast and stem cell proliferation [47], while IL-1β drives the recruitment and activation of myeloid-derived suppressor cells (MDSC), which are key contributors to the resolution of inflammation [24, 48,49,50,51]. The transition from the inflammatory to the proliferative phase of healing is marked by neutrophil removal (via apoptosis or reverse migration), polarization of macrophages to Arg1-expresssing, pro-reparative phenotypes, increased production of growth factors (VEGF, PDGF and FGF), and the migration, proliferation and differentiation of fibroblasts, MDSC and mesenchymal stem cells [52]. In line with these features, we show histopathology (increased macrophages, fibroblasts, mitotic cells), and gene (increased Arg1, Fgf1) and protein expression (low TNF-α, increased IL-1β and MIP-1α) changes in the operated joints of ALM-treated females that suggest an earlier shift to the proliferative phase of healing.
Another important finding of our study was, in contrast to females, inflammatory cell infiltration was reduced in joint tissues from males at 72 h after ACLR surgery. However, ALM treatment appeared to boost leukocyte and fibroblast infiltration. In ALM-treated males, gene expression for the neutrophil chemoattractant Cxcl6 (GCP-2) was elevated in joint tissue, with increased numbers of neutrophils in joint synovial tissue, compared to controls (Fig. 5D,E). Mononuclear cell infiltration was also more apparent in ALM-treated males than controls, albeit at a lesser extent than females. In addition, expression of markers of inflammation (Nfκb, Nlrp3), cytoprotective responses (Nrf2) and angiogenesis (Vegf) were significantly increased in joint tissues from male ALM-treated knees, with levels approximating that observed for females. Nrf2, a regulator of cellular redox status, has been shown to provide protection against inflammation and cartilage degeneration in osteoarthritis models [53], while Nlrp3 activation accelerates wound healing by promoting macrophage infiltration and proinflammatory polarization [54,55,56,57].
Together, our data show sex differences in the timing and cellular composition of the inflammatory response within the operated joint after ACLR surgery, and are consistent with recent studies suggesting accelerated resolution of acute inflammation in females, compared to males [58,59,60,61]. Despite these inherent sex differences, ALM appears to augment the healing response in females, and boost the local inflammatory response in males by switching to a ‘female-like’ response. The implications of this on joint tissue repair remain to be seen and are the focus of ongoing studies.
Limitations
There are limitations of this study, which we acknowledge. Firstly, the ACLR model used in this study involves open surgery, not arthroscopy as is typically performed. Nevertheless, the systemic and synovial inflammatory profiles reported here are consistent with the changes described clinically in the early postoperative period after ACLR surgery [62]. Although the current study highlights differences in the recruitment patterns of major leukocyte subsets, more comprehensive characterization of ALM’s effect on neutrophil, monocyte and lymphocyte subpopulations within blood, synovial fluid and joint tissues at different stages of healing after ACLR surgery are required. The restriction of the experimental period to 120 h postoperative is an additional study limitation. The implications of ALM’s early modulation of postoperative inflammatory responses on later stages of tissue repair and on prevention of long-term complications such as PTOA and arthrofibrosis is the focus of current investigations.
Clinical relevance
Dampening of early local and inflammatory responses triggered by ACL rupture and subsequent surgical reconstruction have potential to afford protection against progressive cartilage degeneration and synovial fibrosis [63, 64]. ALM is a systems-based therapy that significantly reduces local hemorrhage and blunts secondary injury processes to promote healing in various rat and pig models of surgery, trauma and infection [14, 16, 65,66,67,68,69]. Recently, ALM was shown to promote skeletal muscle regeneration following crush injury by stimulating myogenic stem cell proliferation and inhibiting apoptosis of satellite and interstitial cell between 4- and 7-days post-injury [70]. In addition, a single intra-articular bolus of ALM at the time of surgery was shown to reduce inflammation and fibrosis in the operated knee at 4 weeks postoperative in a male rat model of total knee arthroplasty [18].
The novelty of ALM therapy is that it confers multipronged protection against: 1) sterile injury [7, 66, 71], 2) infection [68, 72, 73] and 3) lipopolysaccharide (LPS) endotoxemia [74] which implies a common mechanism of action to blunt very early release of damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs), and other inflammatory signals, thereby controlling inflammatory and innate immune responses. The findings of the current study, which suggest a tailoring of ALM’s effects in a sex-specific manner in response to ACLR surgery, provide further support to this hypothesis. Improved understanding of sex-specific cellular and molecular inflammatory responses to ACL rupture, ACLR surgery, and perioperative therapies, as well as how these underpin the timing and nature of tissue reparative processes, will enable more personalized approaches for enhanced postoperative recovery.
Conclusions
In a rat model of ACL rupture and ACLR surgery, we conclude that ALM therapy modulates early systemic and local inflammatory signals differently between the sexes in the first postoperative week. Our findings suggest the possibility of personalized approaches to management of postoperative inflammation in males and females. The potential implication of ALM’s sex-specific immunomodulatory effects on subsequent joint tissue healing and functional recovery are under investigation.
Availability of data and materials
All data pertaining to this study have been included in this manuscript and additional files.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- ACLR:
-
Anterior cruciate ligament reconstruction
- ALM:
-
Adenosine, lidocaine and Mg2+
- A3R:
-
Adenosine A3 receptor
- Arg1:
-
Arginase-1
- ATP:
-
Adenosine triphosphate
- Bregs :
-
B regulatory cells
- CCL:
-
Chemokine C–C motif ligand
- CD:
-
Cluster of differentiation
- cDNA:
-
Complementary deoxyribonucleic acid
- CXCL:
-
Chemokine C-X-C motif ligand
- DAMPs:
-
Damage-associated molecular patterns
- EDTA:
-
Ethylenediaminetetraacetic acid
- Fgf1:
-
Fibroblast growth factor 1
- FMO:
-
Fluorescence minus one
- FSC:
-
Forward scatter
- G-CSF:
-
Granulocyte colony-stimulating factor
- GRO/KC:
-
Growth regulated protein/keratinocyte chemoattractant
- H&E:
-
Hematoxylin and eosin
- HPA:
-
Hypothalamo-pituitary-adrenal axis
- Hprt1:
-
Hypoxanthine–guanine phosphoribosyl transferase 1
- HSD:
-
Honestly significant difference
- IA:
-
Intra-articular
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- ILBs:
-
Innate-like B cells
- IP-10:
-
Interferon-inducible protein 10
- IV:
-
Intravenous
- LIX:
-
Lipopolysaccharide-inducible CXC chemokine
- MCP-1:
-
Monocyte chemoattractant protein
- MDSC:
-
Myeloid-derived suppressor cells
- MIP-1a:
-
Macrophage inflammatory protein-1 alpha
- NFкB:
-
Nuclear factor-kappa b
- NK:
-
Natural killer
- Nlrp3:
-
Nucleotide-binding domain-like receptor protein 3
- Nos2:
-
Nitric oxide synthase 2, inducible
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- PAMPs:
-
Pathogen-associated molecular patterns
- PCR:
-
Polymerase chain reaction
- Pdgfa:
-
Platelet-derived growth factor subunit A
- PFA:
-
Paraformaldehyde
- PTOA:
-
Post-traumatic osteoarthritis
- RANTES:
-
Regulated upon activation, normal T cell expressed and presumably secreted
- RNA:
-
Ribonucleic acid
- ROM:
-
Range of motion
- SD:
-
Standard deviation
- SEM:
-
Standard error of the mean
- SSC:
-
Side scatter
- Tc :
-
T cytotoxic cells
- Th :
-
T helper cells
- TNF-α:
-
Tumor necrosis factor alpha
- Tregs :
-
T regulatory cells
- VEGF:
-
Vascular endothelial growth factor
References
Morris JL, McEwen P, Letson HL, Dobson GP. Anterior cruciate ligament reconstruction surgery: creating a permissive healing phenotype in military personnel and civilians for faster recovery. Mil Med. 2022;usac093. https://doi.org/10.1093/milmed/usac093.
Maniar N, Verhagen E, Bryant AL, Opar DA. Trends in Australian knee injury rates: An epidemiological analysis of 228,344 knee injuries over 20 years. Lancet Reg Health West Pac. 2022;21: 100409.
Parsons JL, Coen SE, Bekker S. Anterior cruciate ligament injury: towards a gendered environmental approach. Br J Sports Med. 2021;55(17):984–90.
Wang L-J, Zeng N, Yan Z-P, Li J-T, Ni G-X. Post-traumatic osteoarthritis following ACL injury. Arthritis ResTher. 2020;22(1):57.
Webster KE, Hewett TE. Anterior cruciate ligament injury and knee osteoarthritis: an umbrella systematic review and meta-analysis. Clin J Sport Med. 2022;32(2):145–52.
Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr Cartil. 2015;23(11):1825–34.
Dobson GP. Trauma of major surgery: a global problem that is not going away. Int J Surg. 2020;81:47–54.
Han P-f, Wei L, Duan Z-q, Zhang Z-l, Chen T-y, Lu J-g, et al. Contribution of IL-1β, 6 and TNF-α to the form of post-traumatic osteoarthritis induced by “idealized” anterior cruciate ligament reconstruction in a porcine model. Int Immunopharmacol. 2018;65:212–20.
Hunt ER, Jacobs CA, Conley CE-W, Ireland ML, Johnson DL, Lattermann C. Anterior cruciate ligament reconstruction reinitiates an inflammatory and chondrodegenerative process in the knee joint. J Orthop Res. 2021;39(6):1281–8.
Kaneguchi A, Ozawa J, Minamimoto K, Yamaoka K. A rat model of arthrofibrosis developed after anterior cruciate ligament reconstruction without rigid joint immobilization. Connect Tissue Res. 2021;62(3):263–76.
O’Brien EJO, Beveridge JE, Huebner KD, Heard BJ, Tapper JE, Shrive NG, et al. Osteoarthritis develops in the operated joint of an ovine model following ACL reconstruction with immediate anatomic reattachment of the native ACL. J Orthop Res. 2013;31(1):35–43.
Heard BJ, Solbak NM, Achari Y, Chung M, Hart DA, Shrive NG, et al. Changes of early post-traumatic osteoarthritis in an ovine model of simulated ACL reconstruction are associated with transient acute post-injury synovial inflammation and tissue catabolism. Osteoarthr Cartil. 2013;21(12):1942–9.
Morris JL, Letson HL, Biros E, McEwen P, Dobson GP. Female rats have a different healing phenotype than males after ACL rupture with no intervention. Front Med. 2022; 9:976980. https://doi.org/10.3389/fmed.2022.976980.
Letson H, Dobson G. Adenosine, lidocaine and Mg2+ (ALM) fluid therapy attenuates systemic inflammation, platelet dysfunction and coagulopathy after non-compressible truncal hemorrhage. PLoS ONE. 2017;12(11): e0188144.
Letson HL, Biros E, Morris JL, Dobson GP. ALM fluid therapy shifts sympathetic hyperactivity to parasympathetic dominance in the rat model of non-compressible hemorrhagic shock. Shock. 2022;57(2).
Davenport L, Letson HL, Dobson GP. Immune-inflammatory activation after a single laparotomy in a rat model: effect of adenosine, lidocaine and Mg2+ infusion to dampen the stress response. Innate Immun. 2017;23(5):482–94.
Letson HL, Morris JL, Biros E, Dobson GP. ALM induces cellular quiescence in the surgical margin 3 days following liver resection, hemorrhage, and shock. J Surg Res. 2022;275:16–28.
Morris JL, Letson HL, McEwen P, Biros E, Dlaska C, Hazratwala K, et al. Intra-articular Adenosine, Lidocaine and Magnesium (ALM) solution decreases postoperative joint fibrosis in an experimental knee implant model. Transl Med Commun. 2021;6(1):4.
Morris JL, Letson HL, McEwen P, Biros E, Dlaska C, Hazratwala K, et al. Comparison of intra-articular administration of adenosine, lidocaine and magnesium solution and tranexamic acid for alleviating postoperative inflammation and joint fibrosis in an experimental model of knee arthroplasty. J Orthop Surg Res. 2021;16(1):726.
McCutchan A, Dobson GP, Stewart N, Letson HL, Grant AL, Jovanovic I-A, et al. Absence of cytotoxic and inflammatory effects following in vitro exposure of chondrogenically-differentiated human mesenchymal stem cells to adenosine, lidocaine and Mg2+ solution. J Exp Orthop. 2019;6(1):16.
Balamayooran G, Batra S, Cai S, Mei J, Worthen GS, Penn AL, et al. Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. Am J Respir Cell Mol Biol. 2012;47(1):104–11.
Duchene J, Lecomte F, Ahmed S, Cayla C, Pesquero J, Bader M, et al. A novel inflammatory pathway involved in leukocyte recruitment: role for the kinin B1 receptor and the chemokine CXCL5. J Immunol. 2007;179(7):4849–56.
Figueroa CD, Matus CE, Pavicic F, Sarmiento J, Hidalgo MA, Burgos RA, et al. Kinin B1 receptor regulates interactions between neutrophils and endothelial cells by modulating the levels of Mac-1, LFA-1 and intercellular adhesion molecule-1. Innate Immun. 2015;21(3):289–304.
Voronov E, Dotan S, Krelin Y, Song X, Elkabets M, Carmi Y, et al. Unique versus redundant functions of IL-1α and IL-1β in the tumor microenvironment. Front Immunol. 2013;4.
Ivetic A, Hoskins Green HL, Hart SJ. L-selectin: A major regulator of leukocyte adhesion, migration and signaling. Front Immunol. 2019;10:1068.
Margraf A, Ludwig N, Zarbock A, Rossaint J. Systemic inflammatory response syndrome after surgery: mechanisms and protection. Anesth Analg. 2020;131(6):1693–707.
Paruk F, Chausse JM. Monitoring the post surgery inflammatory host response. J Emerg Crit Care Med. 2019;3:1–13.
Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacol. 2019;44(1):45–58.
Bangasser DA, Wiersielis KR. Sex differences in stress responses: a critical role for corticotropin-releasing factor. Hormones. 2018;17(1):5–13.
Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A. 2000;97(16):9317–22.
Jankord R, Turk JR, Schadt JC, Casati J, Ganjam VK, Price EM, et al. Sex difference in link between interleukin-6 and stress. Endocrinology. 2007;148(8):3758–64.
Gupta S, Nakabo S, Blanco LP, O’Neil LJ, Wigerblad G, Goel RR, et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci. 2020;117(28):16481–91.
Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56(3):308–21.
Tsay GJ, Zouali M. The interplay between innate-like B Cells and other cell types in autoimmunity. Front Immunol. 2018;9.
Manson J, Hoffman R, Chen S, Ramadan MH, Billiar TR. Innate-like lymphocytes are immediate participants in the hyper-acute immune response to trauma and hemorrhagic shock. Front Immunol. 2019;10.
Catalán D, Mansilla MA, Ferrier A, Soto L, Oleinika K, Aguillón JC, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol. 2021;12.
Saze Z, Schuler PJ, Hong CS, Cheng D, Jackson EK, Whiteside TL. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122(1):9–18.
Zohouri M, Mehdipour F, Razmkhah M, Faghih Z, Ghaderi A. CD4+CD25-FoxP3+ T cells: a distinct subset or a heterogeneous population? Int Rev Immunol. 2021;40(4):307–16.
Dobson GP. Addressing the global burden of trauma in major surgery. Front Surg. 2015;2:43.
Merah-Mourah F, Cohen SO, Charron D, Mooney N, Haziot A. Identification of novel human monocyte subsets and evidence for phenotypic groups defined by interindividual variations of expression of adhesion molecules. Sci Rep. 2020;10(1):4397.
Becerra-Díaz M, Lerner AD, Yu DH, Thiboutot JP, Liu MC, Yarmus LB, et al. Sex differences in M2 polarization, chemokine and IL-4 receptors in monocytes and macrophages from asthmatics. Cell Immunol. 2021;360: 104252.
Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–20.
Agrawal S, Salazar J, Tran TM, Agrawal A. Sex-related differences in innate and adaptive immune responses to SARS-CoV-2. Front Immunol. 2021;12.
Inoue M, Muneta T, Ojima M, Nakamura K, Koga H, Sekiya I, et al. Inflammatory cytokine levels in synovial fluid 3, 4 days postoperatively and its correlation with early-phase functional recovery after anterior cruciate ligament reconstruction: a cohort study. J Exp Orthop. 2016;3(1):30.
Chisari E, Yaghmour KM, Khan WS. The effects of TNF-alpha inhibition on cartilage: a systematic review of preclinical studies. Osteoarthr Cartil. 2020;28(5):708–18.
Ritsu M, Kawakami K, Kanno E, Tanno H, Ishii K, Imai Y, et al. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J Dermatol Dermatol Surg. 2017;21(1):14–9.
Yan L, Zheng D, Xu RH. Critical role of tumor necrosis factor signaling in mesenchymal stem cell-based therapy for autoimmune and inflammatory diseases. Front Immunol. 2018;9:1658.
Giesbrecht K, Eberle M-E, Wölfle SJ, Sahin D, Sähr A, Oberhardt V, et al. IL-1β as mediator of resolution that reprograms human peripheral monocytes toward a suppressive phenotype. Front Immunol. 2017;8.
Jeong HJ, Lee HJ, Ko JH, Cho B-J, Park SY, Park JW, et al. Myeloid-derived suppressor cells mediate inflammation resolution in humans and mice with autoimmune uveoretinitis. J Immunol. 2018;200(4):1306–15.
Veglia F, Hashimoto A, Dweep H, Sanseviero E, De Leo A, Tcyganov E, et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J Exp Med. 2021;218(4).
Kittang AO, Kordasti S, Sand KE, Costantini B, Kramer AM, Perezabellan P, et al. Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. Oncoimmunol. 2016;5(2): e1062208.
Filep JG. Targeting neutrophils for promoting the resolution of inflammation. Front Immunol. 2022;13: 866747.
Cai D, Yin S, Yang J, Jiang Q, Cao W. Histone deacetylase inhibition activates Nrf2 and protects against osteoarthritis. Arthr Res Ther. 2015;17(1):269.
Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Rev Immunol. 2019;19(8):477–89.
Staurengo-Ferrari L, Badaro-Garcia S, Hohmann MSN, Manchope MF, Zaninelli TH, Casagrande R, et al. Contribution of Nrf2 Modulation to the mechanism of action of analgesic and anti-inflammatory drugs in pre-clinical and clinical stages. Front Pharmacol. 2019;9.
Reddy NM, Tamatam CM, Aparna A, Reddy SP. Nrf2 is required for optimal alveolar-macrophage-mediated apoptotic neutrophil clearance after oxidant injury. Antioxidants (Basel). 2022;11(2).
Ito H, Kanbe A, Sakai H, Seishima M. Activation of NLRP3 signalling accelerates skin wound healing. Exp Dermatol. 2018;27(1):80–6.
So J, Tai AK, Lichtenstein AH, Wu D, Lamon-Fava S. Sexual dimorphism of monocyte transcriptome in individuals with chronic low-grade inflammation. Biol Sex Diff. 2021;12(1):43.
Rathod K, Kapil V, Velmurugan S, Khambata R, Siddique U, Khan S, et al. Sex differences in the inflammatory response and inflammation-induced vascular dysfunction. Lancet. 2017;389:S20.
Farhat F, Amérand A, Simon B, Guegueniat N, Moisan C. Gender-dependent differences of mitochondrial function and oxidative stress in rat skeletal muscle at rest and after exercise training. Redox Rep. 2017;22(6):508–14.
Yaeger MJ, Reece SW, Kilburg-Basnyat B, Hodge MX, Pal A, Dunigan-Russell K, et al. Sex differences in pulmonary eicosanoids and specialized pro-resolving mediators in response to ozone exposure. Toxicol Sci. 2021;183(1):170–83.
Harkey MS, Luc BA, Golightly YM, Thomas AC, Driban JB, Hackney AC, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthr Cartil. 2015;23(1):1–12.
Gilbert SJ, Bonnet CS, Stadnik P, Duance VC, Mason DJ, Blain EJ. Inflammatory and degenerative phases resulting from anterior cruciate rupture in a non-invasive murine model of post-traumatic osteoarthritis. J Orthop Res. 2018;36(8):2118–27.
Mason D, Englund M, Watt FE. Prevention of posttraumatic osteoarthritis at the time of injury: Where are we now, and where are we going? J Orthop Res. 2021;39(6):1152–63.
Dobson GP, Letson HL. Far Forward Gaps in Hemorrhagic Shock and Prolonged Field Care: An Update of ALM Fluid Therapy for Field Use. J Spec Oper Med. 2020;20(3):128–34.
Dobson GP, Letson HL. Adenosine, lidocaine, and Mg2+ (ALM): from cardiac surgery to combat casualty care—teaching old drugs new tricks. J Trauma Acute Care Surg. 2016;80(1):135–45.
Letson HL, Dobson GP. Adenosine, lidocaine, and Mg2+ (ALM) resuscitation fluid protects against experimental traumatic brain injury. J Trauma Acute Care Surg. 2018;84(6):908–16.
Granfeldt A, Letson HL, Dobson GP, Shi W, Vinten-Johansen J, Tønnesen E. Adenosine, lidocaine and Mg2+improves cardiac and pulmonary function, induces reversible hypotension and exerts anti-inflammatory effects in an endotoxemic porcine model. Crit Care. 2014;18(6):682.
Griffin MJ, Letson HL, Dobson GP. Small-volume adenosine, lidocaine, and Mg2+ 4-hour infusion leads to 88% survival after 6 days of experimental sepsis in the rat without antibiotics. Clin Vacc Immunol. 2016;23(11):863–72.
Hoger NS, Mittlmeier T, Vollmar B, Stratos I, Dobson GP, Rotter R. ALM therapy promotes functional and histologic regeneration of traumatized peripheral skeletal muscle. Biology. 2023;12(6):870. https://doi.org/10.3390/biology12060870.
Dobson GP, Biros E, Letson HL, Morris JL. Living in a hostile world: inflammation, new drug development, and coronavirus. Front Immunol. 2021;11.
Griffin MJ, Letson HL, Dobson GP. Adenosine, Lidocaine and Mg2+ (ALM) induces a reversible hypotensive state, reduces lung edema and prevents coagulopathy in the rat model of polymicrobial sepsis. J Trauma Acute Care Surg. 2014;77(3):471–8.
Griffin MJ, Letson HL, Dobson GP. Small-volume Adenosine, lidocaine and Mg2+ (ALM) 4 hour infusion leads to 88% survival after 6 days of experimental sepsis in the rat without antibiotics. Clin Vaccine Immunol. 2016;23(11):863–72.
Granfeldt A, Letson HL, Dobson GP, Shi W, Vinten-Johansen J, Tønnesen E. Cardioprotective and anti-inflammatory effects of treatment with adenocaine/mg2+ in a porcine model of endotoxemia. Circulation. 2013;128(122):A195.
Acknowledgements
We are grateful to Dr Erik Biros for completion of gene expression assays, and to Ms Lindy McEwen, Ms Regina Kirk, Dr James Coulthard, Dr Aiden Sadeghilar, Dr Mac Daniel Nixon, Dr Finlea Cusack, and Dr Scott Le Rossignol for assistance with ACLR surgeries. We would like to thank the College of Medicine and Dentistry, James Cook University, the Orthopaedic Research Institute of Queensland, and the U.S. Department of Defence for their continued support.
Funding
This work was supported by US Department of Defense, Award No. W81XWH-20–1-0931. Log No. OR190008. The opinions, interpretations, and conclusions are those of the authors, and are not necessarily endorsed by the US Department of Defense.
U.S. Department of Defense,W81XWH-20-1-0931,Geoffrey P Dobson.
Author information
Authors and Affiliations
Contributions
Concept (PCM, GPD, JLM), experimental design (GPD, JLM, HLL), data collection (JLM, HLL), data analyses and interpretation (GPD, JLM), manuscript preparation (GPD, JLM) and editing (PCM, HLL).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments followed protocols approved by the James Cook University Animal Ethics Committee (A2684) and the US Army Animal Care and Review Use Office (ACURO), and were conducted and reported according to the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines.
Consent for publication
Not applicable.
Competing interests
GPD is the inventor of the ALM concept for trauma and surgery. JLL, PCM and HLL have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Flow cytometry antibodies.
Additional file 2: Table S2.
Primer sequences.
Additional file 3: Table S3.
Surgery details.
Additional file 4: Table S4.
Hematology.
Additional file 5: Table S5.
Synovial cytokine and chemokines.
Additional file 6: Figure S1.
Flow cytometry gating strategy.
Additional file 7: Figure S2.
Leukocyte activation markers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morris, J.L., McEwen, P.C., Letson, H.L. et al. Adenosine, Lidocaine and Magnesium (ALM) therapy modulates early sex-specific inflammatory and immune responses following experimental anterior cruciate ligament rupture and reconstruction. transl med commun 8, 14 (2023). https://doi.org/10.1186/s41231-023-00148-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-023-00148-6