Abstract
Background
Prophylactic platelet transfusion is given to patients when the platelet count is less than ten thousand to prevent clinically significant bleeding till platelet engraftment is documented. Despite a very low platelet count, if platelet engraftment is confidently predicted, then platelet transfusion can be avoided in an otherwise stable patient.
Objective
To determine the role of post-transplant day + 14 immature reticulocyte fraction (IRF) and immature platelet fraction (IPF) as surrogate markers for early prediction of platelet engraftment in pediatric hematopoietic stem cell transplant patients.
Material and methods
This prospective study was done at the National Institute of Blood Diseases and Bone Marrow Transplantation between January 2017 and December 2020. A total of 56 and 31 patients were enrolled in the deviation and validation cohorts respectively. IPF and IRF were tested on a Sysmex XN-1000 hematology analyzer on days + 14 and + 21 of the bone marrow transplant. Platelet count on day + 14 and the day of engraftment was documented. Spearman correlation analysis and receiver operating characteristic curve (ROC) calculation were done using the statistical package STATA version 12, to determine IRF and IPF cut-off values to predict a median platelet engraftment day.
Results
The derivation and validation cohorts were statistically comparable. The area under the receiver operating characteristic curve (ROC) for IPF and IRF was 0.53 (95% CI: 0.37 – 0.68, p = 0.750) and 0.74 (95% CI: 0.61 – 0.89, p = 0.001) respectively. A weak inverse correlation (rs0.36, p = 0.007) between IRF and platelet engraftment day was found. The ROC demonstrated that the cut-off value for Day + 14 IRF of 13% has a sensitivity and specificity of 92.9% and 37% respectively. This finding was confirmed in the validation group with sensitivity and specificity of 88.2% and 45.2% respectively.
Conclusion
This study found that Day + 14 IRF but not IPF value can reliably predict platelet engraftment by day + 17 post-transplant.
Similar content being viewed by others
Introduction
The average time required for bone marrow engraftment is two to three weeks [1]. Neutrophils are engrafted around 4 to 7 days earlier than platelets [2]. Prevention and management of febrile episodes and prophylactic blood transfusions are the mainstays of patient care during the pre-engraftment period [3]. In a hemodynamically stable child, there is a low threshold to transfuse platelets with a cutoff value of 10,000 [4,5,6]. During this critical period, the transplant team faces the risk of transfusion-transmitted infection, transfusion reaction, cost, difficulty in getting desired platelet components due to peculiar blood group issues, HLA-matched platelet, and CMV negative donor availability [7,8,9]. Bacterial sepsis, CMV reactivation or infection, acute graft versus host disease, hepatic sinusoidal obstruction syndrome, and hemorrhagic cystitis have severe consequences on bone marrow engraftment. In the absence of these complications, engraftment occurs between 14 to 21 days after the transplant [10].
The presence of immature reticulocyte fraction in peripheral blood preemptively indicates marrow engraftment [11,12,13]. Similarly, immature platelet fraction serves as a surrogate marker of platelet engraftment [14,15,16].
In this regard, the relationship between IRF and IPF with neutrophil and platelet engraftment respectively has been studied multiple times. Adult patients with malignant disorders were selected in these studies [17,18,19]. Previously IRF has been used to predict neutrophil engraftment. This was keeping in view the fact that all the cell lineages have a common progenitor. However, the engraftment day for the proposed IRF cutoff values was not established [18, 20, 21]. There is a paucity of data on pediatric bone marrow transplant patients with benign disorders. However, to the best of our knowledge, there is a single study in which immature reticulocyte fraction was assessed as an early predictor of marrow engraftment of both neutrophils and platelets in pediatric patients, and IRF cutoff values were also validated. The IRF value was more than 5% after 11.1 ± 3.6 days following HSCT. The predicted cut-off value of IRF was 3.5% at 8 days after HSCT with an area under the curve of 0.879 (95% CI: 0.759–0.999). Sensitivity and specificity were 86.7% and 82.8%, respectively [22]. Both IRF and IPF have clinical implications in hematopoietic bone marrow transplant setup. Primary graft failure can be preemptively managed by using the cutoff values of IRF and IPF. Similarly delayed platelet engraftment can be very challenging for transplant physicians. It can also be anticipated by utilizing these values at different time points. In the context of poor graft function, IRF and IPF can serve as ancillary markers in establishing the diagnosis. Platelet refractoriness is a very common phenomenon especially encountered in the pre-engraftment period of thalassemia and aplastic anemia children [23]. The cutoff values of IRF and IPF in post-transplant pediatric patients with benign hematological disorders will immensely benefit them and prevent the unjudicial use of platelet transfusion. The clinical utility of IRF and IPF in the fore mentioned areas of bone marrow transplant needs to be explored and established. Ultimately transfusions will be cost-effective and the incidence of reactions will be minimized.
With this background, we conducted this study to analyze the relationship of platelet engraftment with D + 14 IPF and IRF in pediatric patients undergoing stem cell transplants for beta-thalassemia and aplastic anemia.
Material and methods
Study design and setting
This prospective cohort study was targeted to determine the cutoff value of IRF and IPF to predict platelet engraftment day with a platelet count of ≥ 20 × 109/L. This was followed by the prospective validation of the establishment of the IRF/IPF cutoff. This study was done at the National Institute of Blood Diseases & Bone Marrow Transplant from January 2017 to December 2020 after approval from the Ethics Review Committee of NIBD & BMT.
Transplant characteristics
Pediatric patients less than 18 years of age with benign hematological disorders (beta-thalassemia and Aplastic anemia) were recruited in the study after obtaining informed written consent from their parents. All patients in the derivation and validation cohort underwent matched-related donor transplants. Myeloablative and reduced intensity conditioning was used according to the disease type. Bone marrow was infused in 36 and 20 patients, whereas peripheral blood stem cells were given to 20 and 11 patients in the derivative and validation cohorts respectively. Prophylaxis against graft versus host disease consisted mainly of methotrexate at a dose of 15 mg/m2 on day + 1 and 10 mg/m2 on days + 3, + 6, + 11 along with cyclosporine A intravenous starting from day -2 at a dose of 3.5 mg/kg changing to an oral dose of 10 mg/kg at day + 14 and continued for an year after transplant. All patients received granulocyte colony-stimulating factor at a dose of 5 mcg/kg daily starting four days after graft infusion until the day they achieve an absolute neutrophil count of 500/ ul for three consecutive days. After stem cell infusion patients were monitored for any febrile episode, packed cell transfusion (Hb < 8 gm/dl, any febrile episode or sign of heart failure), platelet transfusion (platelet less than 10,000 or any febrile episode or bleeding symptom).
On days + 14 and + 21, 3 ml of whole blood was collected in an EDTA tube to perform IRF and IPF on the Sysmex hematology XN 1000 analyzer. IRF and IPF were not recorded daily as the study aim was to assess two points at which the maximum benefit of IPF/IRF could be ascertained in the context of platelet engraftment.
Principle of Sysmex hematology XN-1000 analyser
Reticulocyte and platelet analysis was performed on a Sysmex-hematology XN-1000 analyzer. This system contains specific lysing solutions that penetrate the cell membrane and makes them fluorescent. Then the o-polymethine dye enters the nuclear membrane and binds to the nucleic acid. Similarly, it also enters the membrane of the cell organelles and binds with the proteins. Oxadine-based fluorescent dye also stains platelets and immature platelet fractions. SFL (scattered side fluorescence) light gives information on the DNA/RNA content of the cell. The same principle applies in measuring the immature reticulocyte fraction (IRF). Morkis et al., evaluated normal cutoff values of IRF and IPF in healthy subjects [23].
Statistical analysis
Frequency and percentages were computed to present categorical variables. Numerical variables were summarized as median with inter-quartile range (IQR) due to violation of normality assumption. The Shaprio-wilk test was used to assess the assumption of normality. Patients' characteristics were compared among the two study cohorts using the chi-square test and Mann–Whitney U test for categorical and numerical variables respectively. The receiver operating characteristic curve (ROC) was constructed to determine the predictive ability of two biomarkers by the computing area under the curve (AUC). AUC indicates excellent, good, fair, poor, and no discriminating ability between case positive and case negative for the following ranges; 1 – 0.9, 0.8 – < 0.9, 0.7 – < 0.8, and 0.6 – < 0.7, 50 – < 0.6 respectively [24]. The threshold value was determined for the test that was found to be significantly predicting the variable of interest. A threshold value was determined where the curve was closed to the top axis and the rate of true positive was maximized because the screening tests must have maximum sensitivity while optimizing specificity [25]. STATA version 12 was used to perform statistical analysis of the data. A two-tailed p-value less than or equal to 0.05 was taken as statistically significant.
Results
Comparison of patients’ characteristics in derivation and validation cohort
Fifty-six and thirty-one patients were enrolled in the derivation and validation cohort respectively whose characteristics are depicted in Table 1. Patients in the two study cohorts were similar in terms of age (p = 0.130), gender (p = 0.911), blood group (p = 0.406), disease (p = 0.991), day + 14 platelet count (p = 0.555) and the days of platelet engraftment (p = 0.835).
Reliability of IPF and IRF as a screening marker for platelet engraftment
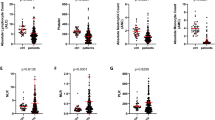
The median platelet engraftment day was 17.5 in the derivation cohort. Therefore, we used 14th-day IPF and IRF values to predict platelet engraftment within 17 days of the bone marrow transplant. Out of 56 patients, half of the patients achieved platelet engraftment within 17 days. The area under the ROC curve for IPF and IRF was 0.53 (95% CI: 0.37 – 0.68, p = 0.750) and 0.74 (95% CI: 0.61 – 0.89, p = 0.001) respectively (Fig. 1). The cut-off value of 13 or above is determined which demonstrated a sensitivity of 92.9% and specificity of 37%.On day 21, out of 56 patients, 12(21.43%) patients were discharged and ROC analysis done for day 21 + IRF showed an AUC of 0.54 (95% CI: 0.36 – 0.72, p = 0.647).
Correlation between IRF, IPF and platelet engraftment day
A significantly negative weak correlation was observed between IRF and engraftment days (rs = -0.36, p = 0.007) (Fig. 2). There was no statistically significant correlation between IPF and engraftment days (rs = -0.086, p = 0.53) (Fig. 3) and IRF and IPF (rs = 0.001, p = 0.99) (Fig. 4).
Validation of IRF cut-off value
Out of 31 patients in the validation cohort, 17(54.8%) achieved platelet engraftment within 17 days of the transplant. 15 (88.2%) patients out of 17 were correctly identified for the achievement of platelet engraftment within 17 days using an IRF threshold of 13 or above which is the sensitivity of the marker. Overall IRF ≥ 13% was observed in 27 (87.1%) patients on day + 14. Whereas out of 14 (45.2%) patients who did not achieve platelet engraftment within 17 days, 2 (14.3%) were correctly classified as not achieving platelet engraftment which shows specificity of the test for IRF threshold of 13 or above. This cut-off yield a PPV of 55.6% and an NPV of 50%.
Discussion
Hematology analyzer Sysmex XN 1000 has provided us with parameters like immature fractions of reticulocytes and platelets that indicate early regenerating hematopoietic cells before entering circulation [26]. Despite the fact that IRF and IPF are not directly involved in the clinical decision-making process they may be considered surrogate markers of early engraftment. Multiple studies demonstrated the usefulness of these indices in Hematopoietic Cell Transplants for transfusion assessment and anticipation of successful engraftment [27,28,29]. Measurement of IRF as a harbinger of platelet recovery is useful in two aspects, firstly to curtail the platelet transfusion in the period of post-transplant marrow suppression and secondly delayed platelet engraftment. Previous studies have shown a correlation between IRF and IPF with neutrophil and platelet engraftment respectively [29,30,31].
To the best of our knowledge, the present study is the first one to ascertain the importance of IRF as an early screening marker in a pediatric hematopoietic stem cell transplant setting. Literature documents that rising IRF, expressed in terms of percentage of total reticulocyte count, is the first hematologic recovery following allogeneic bone marrow transplantation and peripheral blood stem cell transplantation [31]. In the present study, there was a significantly weak negative correlation of IRF with platelet engraftment days which means that IRF values will be higher in those achieving early platelet engraftment. According to some researchers, the first IRF value > 10% is considered as recovery criteria in BMT patients. In the present study, the ability of day 14 IRF to indicate platelet recovery was fair with a threshold value of 13% or above that showed a sensitivity and specificity of 92.9% and 37% respectively. However day 21 IRF failed to discriminate against patients achieving platelet engraftment on day 17. The mechanistic evidence involved in the association of IRF with platelet is the fact that begins from the pluripotent hematopoietic stem cells that produce all lineages of blood cells including Megakaryocyte-Erythroid Progenitor (MEP) cells that are differentiated into megakaryocytes and erythroid cells. More than 30 genes are involved in platelet biogenesis from which 7 genes are transcription factors [32, 33] GATA1 gene is highly expressed on MEP and its mutation can affect both megakaryopoiesis and erythropoiesis [34]. Thus having a common progeny is also reflected later on by the correlation of IRF with early platelet engraftment. Morkis and coworkers used a Sysmex XE-5000™ analyzer and found that the IRF reference range was 1.6–12% [8]. We devised an IRF cutoff of ≥ 13% in our patients who engrafted earlier reflecting that this value is very close to the normal value of IRF indicating marrow engraftment and normal marrow recovery.
In the present study, IPF measured on the 14th post-transplantation day was not found to be significantly discriminating patients achieving platelet engraftment within 17 days from those who achieved platelet engraftment after 17 days. Contrary to our findings, a study conducted in China on pediatric patients who had undergone successful hematopoietic stem cell transplantation intending to assess IPF as a predictor of platelet engraftment following hematopoietic stem cell transplant, concluded that there was a role of IPF in dynamic predicting the platelet engraftment. However, the study suffers from serious methodological drawbacks such as inadequate sample size and inappropriate data analysis [23].
Molina et al., did a daily estimation of IRF values to establish a cutoff value of > 10% in predicting neutrophil engraftment within 3 days. This is comparable to our study with a day + 14 IRF cutoff value of 13 but it is predicting platelet engraftment [29].
Diseases with low platelet count generally have suppressed bone marrow activity and their IPF is also low [35]. A weak negative correlation of IPF with platelet engraftment was also observed in our study but it was not statistically significant. It is also noticeable that there was no correlation between IPF and IRF in our study. In contrast to this, it was found in another Japanese study that IPF and IPF equivalently contributed to predicting platelet and RBC engraftment in patients undergoing bone marrow transplants or cord blood transplants [31].
The limitation of this study is that it is a single-center study and the conclusions obtained needs to be further verified at other centers. Secondly, with a small sample size, we cannot exclude bias in the results of the analysis. Further in this study we included beta-thalesemia and aplastic anemia cases and sub-group analysis was not run for these two disease categories because of smaller sample. Hence, it requires a larger validation cohort, and further studies should be done in the future to implement this cutoff and document its positive impact.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CMV:
-
Cytomegalovirus
- HLA:
-
Human Leukocyte Antigen
- RNA:
-
Ribonucleic Acid
- IRF:
-
Immature Reticulocyte Fraction
- IPF:
-
Immature Platelet Fraction
- BMT:
-
Bone Marrow Transplantation
- EDTA:
-
Ethyl enediamine tetraacetic Acid
- DNA:
-
Deoxyribonucleic Acid
- SFL:
-
Scattered Side-Fluorescence
- IQR:
-
Interquartile Range
- ROC:
-
Receiver Operator Characteristic Curve
- AUC:
-
Area under the Curve
- PPV:
-
Positive Predictive Value
- NPV:
-
Negative Predictive Value
References
Mahadeo KM, Weinberg KI, Abdel-Azim H, Miklos DB, Killen R, Kohn D, Crooks GM, et al. A reduced-toxicity regimen is associated with durable engraftment and clinical cure of nonmalignant genetic diseases among children undergoing blood and marrow transplantation with an HLA-matched related donor. Biol Blood Marrow Transplant. 2015;21(3):440–4.
Pihusch M. Bleeding complications after hematopoietic stem cell transplantation. Semin Hematol. 2004;41(1 Suppl 1):93–100.
Slichter SJ. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfus Med Rev. 2004;18(3):153–67.
Estcourt LJ, Stanworth S, Murphy M. Platelet transfusions for patients with hematological malignancies: who needs them? Br J Haematol. 2011;154(4):425–40.
Schiffer CA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1519–38.
British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br J Med 2003;122:10–23.
Philip J, Kumar S, Chatterjee T, Mallhi R. Prevalence of alloimmunization to human platelet antigen glycoproteins and human leucocyte antigen class I in β thalassemia major patients in Western India. Indian J Hematol Blood Transfus. 2014;30(4):309–12.
Mahesar A, Mazari MA, Faizan SM, Farzana T, Bukhari JN, Shamsi TS. Platelet Refractoriness during Bone Marrow Transplantation, Comparison in Aplastic anemia and Beta-thalassemia Major Patients. An Experience of Public Sector BMT Unit in Pakistan. Biol Blood Marrow Transplant. 2020;26(3):S210–1.
Marktel S, Napolitano S, Zino E, Cappelli B, Chiesa R, Poli F, et al. Platelet transfusion refractoriness in highly immunized beta thalassemia children undergoing stem cell transplantation. Pediatr Transplant. 2010;14(3):393–401.
Shinjo K, Takeshita A, Nakamura S, Naitoh K, Yanagi M, Tobita T, et al. Serum thrombopoietin levels in patients correlate inversely with platelet counts during chemotherapy-induced thrombocytopenia. Leukemia. 1998;12(3):295–300.
Davies SV, Cavill I, Bentley N, Fegan CD, Poynton CH, Whittaker JA. Evaluation of erythropoiesis after bone marrow transplantation: quantitative reticulocyte counting. Br J Haematol. 1992;81(1):12–7.
Van Hove L, Goossens W, Van Duppen V, Verwilghen RL. Reticulocyte count using thiazole orange. A flow cytometry method. Clin Lab Haematol. 1990;12(3):287–99.
Riley RS, Ben-Ezra JM, Tidwell A, Romagnoli G. Reticulocyte analysis by flow cytometry and other techniques. Hematol Oncol Clin. 2002;16(2):373–420.
Torres A, Sánchez J, Lakomsky D, Serrano J, Alvarez MA, Martín C, et al. Assessment of hematologic progenitor engraftment by complete reticulocyte maturation parameters after autologous and allogeneic hematopoietic stem cell transplantation. Haematologica. 2001;86(1):24–9.
Noronha JF, De Souza CA, Vigorito AC, Aranha FJ, Zulli R, Miranda EC, et al. Immature reticulocytes as an early predictor of engraftment in autologous and allogeneic bone marrow transplantation. Clin Lab Haematol. 2003;25(1):47–54.
Dalal BI, Stockford GR, Naiman SC, Spinelli JJ, Phillips GL. Criteria for marrow engraftment: comparison of reticulocyte maturity index with conventional parameters. Bone Marrow Transplant. 1996;17(1):91–2.
Kanold J, Bezou MJ, Coulet M, Quainon F, Malpuech G, Travade P, et al. Evaluation of erythropoietic/hematopoietic reconstitution after BMT by highly fluorescent reticulocyte counts compares favorably with traditional peripheral blood cell counting. Bone Marrow Transplant. 1993;11(4):313–8.
Greinix HT, Linkesch W, Keil F, Kalhs P, Schwarzinger I, Schneider B, et al. Early detection of hematopoietic engraftment after bone marrow and peripheral blood stem cell transplantation by highly fluorescent reticulocyte counts. Bone Marrow Transplant. 1994;14(2):307–13.
Sica S, Salutari P, Laurenti L, Ortu La Barbera E, Zini G, d’Onofrio G, et al. Highly fluorescent reticulocytes after CD34+ peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 1998;21(4):361–4.
Grotto HZ, Vigoritto AC, Noronha JF, Lima GA. Immature reticulocyte fraction as a criterion for marrow engraftment. Evaluation of a semi-automated reticulocyte counting method. Clin Lab Haematol. 1999;21(4):285–7.
Grazziutti ML, Dong L, Miceli MH, Cottler-Fox M, Krishna SG, Fassas A, et al. Recovery from neutropenia can be predicted by the immature reticulocyte fraction several days before neutrophil recovery in autologous stem cell transplant recipients. Bone Marrow Transplant. 2006;37(4):403–9.
Kim NH, Chun BC, Kim H, Lee JW, Hong KT, Kim MS, et al. Assessment of immature reticulocyte fraction as an early predictor of marrow engraftment after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(2):S235.
Morkis IV, Farias MG, Scotti L. Determination of reference ranges for immature platelet and reticulocyte fractions and reticulocyte hemoglobin equivalent. Rev Bras Hematol Hemmeter. 2016;38(4):310–3.
Li F, He H. Assessing the accuracy of diagnostic tests. Shanghai Arch Psychiatry. 2018;30(3):207–12.
Barton B, Peat J. Medical statistics: A guide to SPSS, data analysis and critical appraisal. John Wiley & Sons; 2014.
Piva E, Brugnara C, Chiandetti L, Plebani M. Automated reticulocyte counting: state of the art and clinical applications in the evaluation of erythropoiesis. Clin Chem Lab Med. 2010;48(10):1369–80.
Briggs C, Kunka S, Hart D, Oguni S, Machin SJ. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol. 2004;126(1):93–9.
Gonçalo A, Barbosa I, Campilho F, Campos A, Mendes C. Predictive value of immature reticulocyte and platelet fractions in hematopoietic recovery of allograft patients. Transplant Proc. 2011;43(1):241–3.
Molina JR, Sanchez-Garcia J, Torres A, Alvarez MA, Serrano J, Casaño J, et al. Reticulocyte maturation parameters are reliable early predictors of hematopoietic engraftment after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(2):172–82.
van der Linden N, Klinkenberg LJ, Meex SJ, Beckers EA, de Wit NC, Prinzen L, et al. Immature platelet fraction measured on the Sysmex XN hemocytometer predicts thrombopoiesis recovery after autologous stem cell transplantation. Eur J Haematol. 2014;93(2):150–6.
Takami A, Shibayama M, Orito M, Omote M, Okumura H, Yamashita T, et al. Immature platelet fraction for prediction of platelet engraftment after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39(8):501–7.
Noris P, Pecci A. Hereditary thrombocytopenias: a growing list of disorders. Hematology Am Soc Hematol Educ Program. 2017;1:385–99.
Almazni I, Stapley R, Morgan NV. Inherited thrombocytopenia: update on genes and genetic variants which may be associated with bleeding. Front Cardiovasc Med. 2019;6:80.
Freson K, Wijgaerts A, Van Geet C. GATA1 gene variants associated with thrombocytopenia and anemia. Platelets. 2017;28(7):731–4.
Jung H, Jeon HK, Kim H-J, Kim S-H. Immature platelet fraction: establishment of a reference interval and diagnostic measure for thrombocytopenia. Korean J Lab Med. 2010;30(5):451–9.
Acknowledgements
The authors thank the staff of the Hematology-Oncology and Stem Cell Transplantation Research Center, National Institute Of Blood Disease for their kind assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ZG-Contributed to the conceptualization and design of the study and manuscript writing.UZ- Shared her valuable comments and reviewed the manuscript. MB- Reviewed and did final editing which was subsequently reviewed by UZ, SAS, and TSS. ND performed data analysis. MF and SZ- organized, integrated, and maintained the data. AJ- Reviewed the final version of the paper; TSS- Revised the manuscript critically for important intellectual content and approved the final submitted version. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of The National Institute of Blood Diseases (NIBD), IRB number; NIBD/RD-176/04–2017, and written informed consent was taken from the study participants.
Consent for publication
Not applicable.
Competing interests
No conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghias, Z., Zaidi, U., Borhany, M. et al. Is post-transplant day + 14 immature reticulocyte fraction (IRF) a reliable surrogate marker for predicting early platelet engraftment in pediatric hematopoietic stem cell transplant?. transl med commun 8, 6 (2023). https://doi.org/10.1186/s41231-023-00138-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-023-00138-8