Abstract
The BRAF and MEK inhibitors combined strategies have dramatically changed the outcome of BRAF-mutated metastatic melanoma patients. However, despite the initial promising results, the onset of primary or acquired resistance occurs in nearly half of the patients at about one year from the diagnosis. Understanding the mechanisms of resistance to these inhibitors is therefore critical for planning more effective therapeutic strategies able to improve patient outcomes. To this aim we generated BRAF and MEK inhibitors resistant melanoma cells starting from the SAN and A375 lines, both harboring the most common BRAF-V600 mutation and sensitive to these drugs. The obtained double-resistant cell lines were characterized by MTT cell proliferation, migration, invasion assays, phosphoarray and western blot analysis. Here we report that the overexpression of several Tyrosine Kinase Receptors (TKRs), such as EphA2 and DDRs, drives the resistance to these drugs and that this resistance can be overcome by treatment with ALW‑II‑41‑27 multikinase inhibitor. ALW‑II‑41‑27 blocks not only TKRs expression, but also the related downstream AKT and MAPK signaling pathways and its efficacy is documented by decreased cell viability and reduced cell invasion/migration of the resistant cells. Our results can delineate a novel promising therapeutic approach to overcoming the drug resistance occurring in BRAF-mutated metastatic melanoma.
Similar content being viewed by others
Introduction
The incidence of melanoma, a highly aggressive skin cancer that arises from melanocytes, has dramatically increased over the past few years worldwide [1]. Although this tumor represents approximately 1% of all skin malignant diseases, once it becomes metastatic the prognosis is very poor [2,3,4]. Activating mutations in the BRAF oncogene occur in nearly 50% of patients with advanced or metastatic melanoma and these mutations cause constitutive activation of the downstream mitogen-activated protein kinase (MAPK) pathway [5, 6]. The BRAF most common causative mutations, V600E (~ 80%) and V600K (~ 16%), lead respectively to the substitution of valine (V) with glutamate (E) or with lysine (K) at codon 600; both events result in constitutive kinase activity and unregulated cell growth [7, 8]. Other V600 mutations (e.g. V600D or V600R) are very rare (~ 6%) [9, 10]. Targeted therapies based on inhibitors against the mutated BRAF protein have revolutionized the treatment of this disease. Initially, the treatment of metastatic BRAF mutated melanoma patients with BRAF inhibitor alone, vemurafenib and dabrafenib, have improved progression-free survival (PFS) and overall survival (OS) compared to chemotherapy [11,12,13,14]. Unfortunately, these tumors are often intrinsically resistant and many of them, that initially respond, develop resistance during treatment. To overcome the acquired resistance, the combined blockade of both BRAF and MEK has been proposed as a useful strategy [15, 16]. In particular, preclinical and clinical data suggest that the dual inhibition of both BRAF (e.g. vemurafenib, dabrafenib and encorafenib) and MEK (e.g. cobimetinib, trametinib and binimetinib) proteins result in a greater initial tumor response, decrease the severity of toxic events and delay the onset of resistant mechanisms [17,18,19,20,21,22,23,24,25,26]. However, despite the initial promising results obtained with BRAF and MEK inhibitors combination, the prognosis of metastatic melanoma patients remains poor [27]. This obstacle might be overcome by the identification and characterization of new therapeutic targets that might lead to develop new strategies able to change the landscape of metastatic melanoma treatment.
In this scenario, several activated tyrosine kinases receptor (TKRs) have been implicated as key drivers in cancer tumorogenesis and in the onset of drug resistance. Among these, erythropoietin-producing hepatocellular (EPH) receptors and discoidin domain receptors (DDRs) are known tact as crucial mediators in different tumor types. EPHA2, a member of the EPH family of TKR (that is classified in two subfamilies, EPHA and EPHB), is known to contribute to the essential process responsible for carcinogenesis and progression of several cancer types, including breast, lung and melanoma. Moreover, high EphA2 expression in the malignant cells is correlated to a poor prognosis [28,29,30,31,32]. On the other hand, the DDRs belong to another important family of TKRs (composed of the two members DDR1 and DDR2) which undergone activation upon binding to collagen fibers [33]. These receptors are major mediators of the crosstalk between tumor cells and extracellular matrix components and play critical roles in regulating essential cellular process, including proliferation, differentiation, adhesion, migration, and matrix remodeling [34]. As a result, DDRs dysregulation is attributed to a variety of human cancer disorders, such as non-small cell lung cancer, ovarian cancer, breast cancer, and melanoma [35, 36]. Hence, given their role in tumor survival, growth factor signaling and drug resistance in several cancer types, both EPHA2 and DDRs could be strong candidates for mediating the resistance of melanoma cells to BRAF and MEK inhibitors. With the aim to investigate this aspect, we have generated vemurafenib and cobimetinb-resistant melanoma cell lines in vitro, starting from SAN and A375 cells, both harboring BRAF V600E mutation and sensitive to these drugs. Cells resistant to single drugs (SAN-VR, SAN-CR, A375-VR and A375-CR) or to combination of both BRAF and MEK inhibitors (SAN-VRCR and A375-VRCR) were generated by treating parental cells with continuously increasing concentration (see Materials and Methods) for one year before to start the experiments with the newly obtained clones. We found that EPHA2 and DDRs are both overexpressed in BRAF and MEK inhibitors resistant cells compared to the parental cell lines. Thus, we evaluated the efficacy of ALW-II-41-27, a multikinase inhibitor, in overcoming resistance to vemurafenib and cobimetinib combined treatment. This small ATP-competitive molecule is able to inhibit different targets involved in key oncogenic pathways, including DDR1, DDR2 and several EPH members, such as EPHA2 [37]. We show here that the ALW-II-41-27 treatment decreased cell viability and reduced cell invasion and migration in both the obtained double-resistant melanoma lines. Hence, we conclude that the blockade of multiple drivers signaling pathways by means of this inhibitor can provide a rationale for planning a more efficient therapeutic treatment of BRAF-mutated melanoma patients.
Materials and methods
Cell lines
Human SAN malignant melanoma cell line harboring BRAFV600E mutation, was kindly provided by Prof. F. Romano (Department of Molecular Medicine and Medical Biotechnology, University of Naples Federico II, Italy), whereas human A375 malignant melanoma cell line harboring BRAFV600E mutation, was purchased by ATCC. The SAN vemurafenib-resistant (SAN-VR), SAN cobimetinib-resistant (SAN-CR), SAN vemurafenib plus cobimetinib-resistant (SAN-VRCR) and the A375 vemurafenib-resistant (A375-VR), A375 cobimetinib-resistant (A375-CR), and A375 vemurafenib plus cobimetinib-resistant (A375-VRCR) cells were generated by treating parental melanoma cell lines (SAN and A375 cells), with continuous increasing concentration of vemurafenib and/or cobimetinib (BRAF and MEK inhibitors, respectively). Specifically, the concentration used ranged from 0,001 to 5 µM. The started dose was that causing the inhibition of 50% of parental cancer cell growth (IC50) for each drug. Parental and resistant human SAN cell lines were grown in RPMI-1640 medium (Merck) supplemented with 10% fetal bovine serum (FBS, Merck), 100 U/ml penicillin and 100 mg/ml streptomycin. Parental and resistant human A375 cell lines were cultured in DMEM high glucose (Merck) supplemented with 10% fetal bovine serum (Merck), 100 U/ml penicillin and 100 mg/ml streptomycin. All cells were maintained in a humidified controlled atmosphere with 95–5% ratio of CO2, at 37 °C and the medium changed every 2–3 days.
Cell viability assay
All cell lines were seeded in 48-well plates at the density of 8 × 103 cell per well, then exposed to increasing concentrations of vemurafenib and/or cobimetinib (range, 0.001-25 µM) for 72 h (hrs). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide (MTT) solution (Merck) was used to measure cell viability, according to the manufacturer’s instructions. The IC50 value was determined by interpolation from the dose-response curves. The results represent the median of three separate experiments, each performed in duplicate.
Western blot assay
Protein concentration of all cell lines, seeded into 100 mm3 dishes, was determined using a Bradford assay (Bio-Rad). Equal amounts of proteins were firstly separated by SDS-PAGE gel and then transferred to nitrocellulose membranes (Bio-Rad) to be incubated with the following primary polyclonal antibodies: anti-BRAF, anti-phospho-BRAF (Ser445), anti-MAPK, anti-phospho-44/42 MAPK (Thr202/Tyr204), anti-MEK, anti-phospho-MEK 1/2 (Ser217/221), anti-AKT, anti-phopho-AKT (Ser 473), E-cadherin, N-cadherin, vimentin, SNAIL, SLUG, anti-EphA2, anti-phospho-EphA2 (Tyr772), anti-DDR1 and DDR2. All antibodies were purchased from Cell Signaling. The monoclonal anti-tubulin antibody (Merck) was used as loading control antibody. The primary antibodies were diluted in a blocking solution, according to the specifications on the product analysis certificate. The secondary antibodies were diluted in a blocking solution of 5% w/v nonfat dry milk. After incubation with the secondary antibody, membranes were analysed by an enhanced chemi-luminescence (ECL) detection system (BioRad). Whole blot showing the bands of Western blot sections in the following figures are reported in the Figures S3–S6.
Confocal microscopy for cytoskeleton staining
Cells were fixed with 4% paraformaldehyde for 20 min, permeabilized in 0.1% TritonX-100 for 10 min and blocked-in phosphate-buffered saline buffer (PBS) supplemented with 0.5% bovine serum albumin (BSA) for 30 min. After each step, cells were rinsed in PBS, incubated for 1 h (hr) at room temperature (RT) with the primary antibody anti-tubulin (Thermo-Fisher) and then with the Alexa Fluor 488 goat anti-mouse IgG (Thermo-Fisher) secondary antibody for 30 min at RT. Cell nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Samples were analysed by fluorescence confocal microscope system (Zeiss LSM 700) with 40X and 63X oil immersion objective, and images acquired with a 1024 × 1024-pixel resolution; in case of z-stacking, the thickness of each z-slice was set to 1.5 μm.
Invasion assay
Transwell chambers (6,5 mm diameter, 8 μm pore size polycarbonate membrane, Corning) were used to evaluate the invasion capability of cells in basal conditions and upon treatment (ALW‑II‑41‑27 inhibitor). 5 × 104 cells were plated in the top chamber pre-coated with Matrigel (BD Bioscience) in medium without serum, while the medium containing 10% FBS was added in the lower chamber. After 48 h, cells were fixed in 4% paraformaldehyde for 20 min and stained with 0.5% crystal violet solution for 30 min. Cells not invading through the pores were carefully wiped out with cotton wool. After staining, cells were photographed using the camera-attached light microscope (magnification, 10X), dissolved in lysis solution containing Isopropanol-HCl and shaking for 10 min. The eluent was transferred to 96-well clear microplate (Corning) and the absorbance at 590 nm was measured using a spectrophotometer.
Wound healing assay
1 × 105 cells per well were seeded in 6-well plate to assess cell migration capability of parental and resistance cell lines in basal conditions and after ALW‑II‑41‑27 treatment. Cells were wounded by scratching with a sterile pipette tip after reached 90% confluence, subsequently, washed with PBS to eliminate the impaired cells. Cells were photographed using the camera-attached light microscope (magnification, 10X) at point 0 and after 48 h. The captured image was opened by Image-J software (NIH, Bethesda, MD, USA) in order to identify the wound area containing cells. The percentage wound closure was calculated by the software.
Phospho-TKR array kit
To analyze the phosphorylation profile of 48 TKRs, the Human Phospho-TKR Array kit has been performed according to the manufacturer’s protocol (R&D Systems). Briefly, melanoma cells were seeded in 100 mm3 dishes in basal conditions, rinsed with PBS and solubilized with Lysis Buffer 6 (provided by the kit) at 1 × 107 cells/ml. Samples were rocked for 30 min at 4 °C and centrifuged for 5 min at 14,000 x g at 4 °C. The supernatants were then collected, and protein concentration determined by the BioRad Reagent (BioRad). Protein lysates (~ 300 µg) were incubated overnight with the human phospho-kinase array, and the levels of phosphorylated proteins analyzed by quantification of the pixel density of each spot by using the ImageLab software (BioRad), according to the manufacturer’s recommendations.
Statistical analysis
The statistical analyses of in vitro data were carried out using Prism 4.02 (GraphPad Software, Inc). Quantitative data are reported as mean ± standard deviation (SD) in all cases. The one-way analysis of variance (ANOVA) was used to evaluate the statistical significance of the results. P values < 0.05 and < 0.0001 were considered statistically significant and highly significant, respectively.
Results
Development and characterization of vemurafenib and/or cobimetinib-resistant melanoma cell lines harboring BRAFV600E mutation
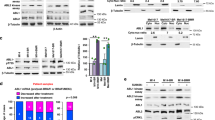
To identify molecular mechanisms of acquired resistance to vemurafenib and/or cobimetinib, selective BRAF and MEK inhibitors, respectively, we have generated melanoma cell lines resistant to vemurafenib (SAN-VR and A375-VR), cobimetinib (SAN-CR and A375-CR) alone and in combination (SAN-VRCR and A375-VRCR). Firstly, we have evaluated the sensitivity of parental SAN and A375 cell lines to both selective inhibitors by cytotoxicity assay. As shown in Figure S1, the combined treatment caused a more evident anti-proliferative effect compared to single agent in both parental cell lines. Subsequently, SAN and A375 cell lines were continuously exposed to increasing concentration of BRAF and MEK inhibitors, either as single agents or in combination, until resistant clones were generated. As reported in Fig. 1A, IC50 of vemurafenib was 10 and 100-fold higher for SAN-VR and A375-VR cells, respectively, and IC50 of cobimetinib was 10 to 500-fold higher for SAN-CR and A375-CR as compared to parental cell lines. Moreover, also the fold change for drug combination (vemurafenib plus cobimetinib) was increased in resistant cell lines (SAN-VRCR and A375-VRCR) ranging from to 500 and 5000-fold compared to SAN and A375 cells. To investigate molecular pathway implicated in the development of acquired resistant mechanisms, Western Blot assay was performed. All resistant cell lines showed a persistent upregulation of BRAF and MEK phosphorylation. Furthermore, an upregulation of AKT phosphorylation was observed in the SAN-VR, SAN-CR and SAN-VRCR lines compared to the parental cells (SAN), suggesting an activation of the AKT pathway (Fig. 1B). On the other hand, increased MAPK phosphorylation was observed in the A375-VR, A375-CR and A375-VRCR lines compared to the parental cells (A375).
Development and characterization of vemurafenib and/or cobimetinib-resistant melanoma cell lines. A Parental and resistant cell lines were treated with increasing concentrations of vemurafenib, cobimetinib and their combination (0.001–5 µg/mL) for 72 h and then evaluated for proliferation by MTT staining. The table summarizes IC50 values and the relative fold-change in resistant versus parental cell lines. B Protein expression and phosphorylation levels of parental and resistant cell lines determined by western blot analysis in basal condition. C Confocal images of parental and resistant cell lines showing different tubulin organization. Different colors are applied to improve visualization: nuclei (DAPI) are in blue and microtubules in green. Scale bar for 40X objective: 20 μm; for 63X: 10 μm; maximum projection of Z-stack sections. D Expression level of proteins involved in epithelial-mesenchymal transition was detected by Western blot
Morphological and molecular changes in of vemurafenib and/or cobimetinib-resistant melanoma cell lines
The development of acquired resistance phenotype included a substantial number of changes in cellular and molecular morphology. Firstly, we observed a different morphology in resistant cell lines compared to parental ones. To better examine this feature, we employed immunofluorescent staining (Fig. 1C). Cancer cells were stained to visualize the nucleus (blue), while the cytoskeleton was marked by tubulin (green), to visualize the microtubules organization. We observed that resistant cell lines were more spread-out, and that the cytoskeleton organization was more highly definite. Specifically, an evident increase in microtubules assembly was observed, a feature that plays a pivotal role in epithelial and mesenchymal (EMT) transition [38]. This phenotype was particularly evident for double resistant SAN-VRCR and A375-VRCR cell lines compared to single agent resistant (SAN-VR, SAN-CR and A375-VR and A375-CR) and parental cell lines. These data suggest that, during the achievement of acquired resistance to targeted therapies, cancer cells show a transition from epithelial to mesenchymal phenotype. To further investigated this aspect, protein expression of different epithelial and mesenchymal markers was investigated by Western Blot assay. As shown in Fig. 1D, the resistant cells showed a strong increase of several mesenchymal markers, such as N-cadherin, vimentin, SLUG and SNAIL. This effect was more evident in double resistant cells compared to single agent resistant cells. On the other hand, a down regulation of E-cadherin, a common epithelial marker, has been observed in resistant cells compared to parental ones.
Migration and invasion properties in vemurafenib and/or cobimetinib-resistant melanoma cell lines
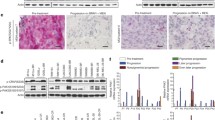
To further evaluate the ability of BRAF and MEK inhibitors resistant melanoma cells in cancer progression, cell invasion and migration assays were performed. For the invasion assays, we have seeded cells on the top of transwell membranes pre-coated with matrigel. As depicted in Fig. 2A-B all cell lines resistant to vemurafenib and/or cobimetinib showed an increased invasion capability compared to each parental cell line. In addition, we observed that SAN-VRCR and A375-VRCR cells exhibited an increased invasion rate compared to SAN-VR, SAN-CR and A375-VR, A375-CR cells. To better characterize the resistant phenotype of established cell lines, wound healing experiments were also performed (Figure S2A-B). As shown in Fig. 2C-D, migration wound ability was remarkably higher in SAN-VRCR and A375-VRCR compared to both parental and single agent resistant cell lines. Moreover, double resistant cells migrated faster than parental cell lines. SAN-VRCR and A375-VRCR cell lines showed the greatest increase in migration rate, reaching a complete covering of the wound. On the contrary, parental, and single agent resistant cell lines were not able to cover the wound even after 48 h.
Characterization of vemurafenib and/or cobimetinib-resistant melanoma cell lines. A Cell invasion ability of SAN, SAN-VR, SAN-CR and SAN-VRCR cell lines evaluated by Transwell invasion assay after 48 h in basal condition. B Cell invasion ability of A375, A375-VR, A375-CR and A375-VRCR cell lines evaluated by Transwell invasion after 48 h in basal condition. C, D Wound healing assays performed to detect migration ability of C SAN, SAN-VR, SAN-CR and SAN-VRCR cell lines and D A375, A375-VR, A375-CR and A375-VRCR cell lines after 48 h of culture. * <0,05. **: P < 0.001; ****: P < 0,0001
Receptor tyrosine kinases activation in double vemurafenib and cobimetinib-resistant melanoma cell lines
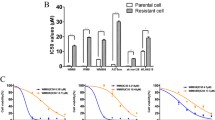
Activation of alternative TKRs signaling pathway plays a key role in the development of acquired resistance to target agents. To identify possible mechanisms related to the resistance to combined treatment of vemurafenib and cobimetinib, basal activation of several TKRs has been evaluated through phospho-TKR kit. As shown in Fig. 3A, we observed that SAN-VRCR and A375-VRCR cells displayed an elevated activation level of several TKRs involved in cancer development, such as EphA2, EphB2, DDR1 and DDR2. Next, to assess whether elevated activation of these TKRs is recapitulated at the protein level, we performed a Western Blot analysis (Fig. 3B). As shown in Fig. 3B, despite a variability in the EphA2 expression level was observed between SAN and A375 parental cell lines, in both resistant lines the expression level of EphA2, DDR1 and DDR2 was found significantly increased. The blockade of multiple receptor pathways could be necessary to overcome resistance to BRAF and MEK inhibitors in melanoma. Based on these results, we evaluated in resistant cell lines the efficacy of ALW-II-41-27, a multikinases inhibitor able to inhibit several targets such as DDR1, DDR2 and several EPH members (including EPHA2). Firstly, we examined the viability of all our cell lines. ALW-II-41-27 drastically decreased the viability of SAN-VRCR and A375-VRCR cells compared to parental ones (Fig. 3C). To further investigate the role of ALW-II-41-27 inhibitor in overcoming BRAF and MEK inhibitors resistance, the activation status of up and downstream signaling effectors pathway has been evaluated by Western Blot assay. As shown in Fig. 3D, ALW-II-41-27 treatment in SAN-VRCR cells substantially inhibited phosphorylation of EphA2, DDR1 and DDR2, while in A375-VRCR cells it reduces phosphorylation of EphA2 and DDR2 (Figure S7-S8); no modification was observed in parental cells. Furthermore, the treatment strongly decreases the phosphorylation of AKT, MAPK and MEK in SAN-VRCR cells, while it reduces the phosphorylation of AKT and MEK in A375-VRCR cells (Figure S7-S8). Again, ALW-II-41-27 treatment induced no modifications in both parental cell lines. All these findings suggested that ALW-II-41-27 treatment could overcome resistance to vemurafenib and cobimetinib treatment by inhibiting AKT and MAPK pathways.
Expression and activation of receptor tyrosine kinases (RTK) and sensitivity to ALW‑II‑41‑27inhibitor in parental and resistant melanoma cell lines. A Cell lysates of SAN, SAN-VRCR, A375 and A375-VRCR cell lines were analyzed using the TKR array kit. The graph represents quantification of mean pixel density using ImageJ program. B Western blot analysis of EphA2, DDR1 and DDR2 proteins in basal condition of melanoma cancer cell lines. Tubulin was used as a loading control. C Cell viability was evaluated by MTT staining on parental (SAN and A375) and resistant (SAN-VRCR and A375-VRCR) cell lines following treatment for 72 h with increasing concentrations of ALW‑II‑41‑27inhibitor (range 0,01–10 µM). D Western blot analysis of EphA2, DDR1, DDR2, AKT, MAPK and MEK proteins in all cell lines untreated and treated with ALW‑II‑41‑27 inhibitor (0,5 µM)
Effects of ALW-II-41-27 inhibitor on migration and invasion ability of vemurafenib and cobimetinib-resistant melanoma cell lines
Subsequently, we evaluated the role of ALW-II-41-27 inhibitor on migration and invasion ability of melanoma resistant cell lines. Interestingly, in the absence of treatment, both these properties were substantially unchanged in SAN-VRCR and A375-VRCR cell lines compared to their parental cells (Fig. 4A-B). However, while ALW-II-41-27 treatment after 48 h drastically reduced the invasion capability of SAN-VRCR and A375-VRCR cells, no differences have been observed in parental cell lines (Fig. 4A-B).
We then performed a wound healing assay to better evaluate the effect of ALW-II-41-27 on migration ability. The treatment induced a drastic reduction in the migration capability of both SAN-VRCR and A375-VRCR cell lines, no variation on migration in parental cells was observed (Fig. 4C-D).
Effects of ALW‑II‑41‑27 treatment on invasion and migration in vemurafenib and/or cobimetinib-resistant melanoma cell lines. Parental and resistant cell lines were treated with 0,5 µM of ALW‑II‑41‑27for 48 h. A Cell invasion ability was evaluated by the Transwell invasion assay. B Graph bar showing the invasion capability of melanoma cancer cell lines untreated and after treatment. C Cell migration capability was measured by the wound-healing assay. D Graph bar showing the migration capability of melanoma cancer cell lines untreated and after treatment. **: P < 0.001; ***: P < 0,0005
Discussion
The development of more effective therapies for advanced melanoma is currently attracting great interest, mainly due on the increasing incidence and high mortality rates of this aggressive form of skin tumor. In recent years, advances in targeted therapy and immunotherapy have changed the treatment algorithm of BRAF-V600E mutated metastatic melanoma patients [39]. The target therapy, mainly based on the combination of BRAF and MEK inhibitors, is in clinical practice the standard of care for BRAF-mutated advanced melanoma patients. Vemurafenib plus cobimetinib, dabrafenib plus trametinib, and encorafenib plus binimetinib are the three drug combinations so far approved for the treatment of metastatic melanoma patients harboring the BRAF-V600E mutation [18,19,20,21,22,23,24,25,26]. However, although these combinations are highly active, the duration of response is limited due to the onset of adaptive resistance mechanisms responsible for the progression of the disease in most of the patients [27]. Different mechanisms of resistance enable the expansion of distinct cell subpopulations in the presence of BRAF and MEK inhibitors, supporting survival and proliferation of the cancer cells that develop an adaptive response by activating feedback loops that can affect cell phenotype and melanoma progression [40, 41]. Consistent evidence indicates that a multicellular heterogenous ecosystem of melanoma cells can change in a stepwise manner during the development of resistance, and many alterations accompanying this process contributes to a high plasticity of melanoma cells [42, 43]. In this scenario, a better understanding of mechanisms of drug resistance and the development of new strategies able to prevent and/or overcome it, represents a challenge that needs to be pursued. Several mechanisms, including secondary mutations, bypass signaling and activation of other compensatory downstream effectors, are known to be responsible for the development of acquired resistance [44]. TKRs overexpression or activation have been shown to be able to bypass the BRAF and MEK blockade as a mechanism of resistance [45]. TKRs may in fact act as upstream activators of MAPK/AKT signaling pathways, and their increased expression in BRAF-resistant cells has been described in multiple studies [46]. Based on these data, to better understand mechanisms underlying BRAF and MEK inhibitors resistance, we generated two cell lines (SAN-VRCR and A375-VRCR) with acquired resistance to vemurafenib and cobimetinib, alone and in combination. These lines were obtained from parental SAN and A375 lines, both harboring BRAFV600E mutation and sensitive to treatment with these drugs. Moving forward vemurafenib and cobimetinib resistance, we assisted to morphological changes in microtubule organization, increased cell invasion, mobility capacity and high expression of EMT markers. All these aspects could be associated with acquired drug resistance after long-term exposure to BRAF and MEK inhibitor treatment [47]. Interestingly, in both SAN-VRCR and A375-VRCR lines the MAPK/AKT pathways were also upregulated, suggesting that the reactivation of these signaling cascades might be involved in the development of acquired resistance. Several TKRs whose activation has generally been associated with invasive behavior -such as EhpA2, DDR1 and DDR2- were highly expressed and activated in our resistant cell lines compared to parental ones. Both DDRs are known to modulate cell invasion and migration; in fact, DDRs are RTKs can target fibrillar collagens, specifically types II and III, that are the major components of extracellular matrix (ECM) [48]. The role of DDRs in promoting cell invasion is also due to their ability to modulate the expression and activity of metalloproteinases (MMPs), a group of the enzymes that degrades ECM components. Specifically, DDR1 has been shown to control melanoma cell invasion and survival and its high expression has been correlated with poor prognosis in melanoma lesions [49]. Other studies have reported that DDR2 depletion in melanoma cell lines reduced the invasive and metastatic abilities [50, 51]. To note, the emerging general role of DDR family receptors as attractive targets in anti-cancer therapies has been highlighted by several studies [33,34,35,36], and EphA2 activation has been shown to be directly involved in BRAF inhibitor resistant melanoma cell lines [52]. In particular, the inhibition of DDR1 or DDR2 with nilotinib, dasatinib, or other still not-approved inhibitors, has been shown to decrease invasion and metastatic capability of several types of carcinomas [53,54,55].
Based on these data, we treated SAN-VRCR and A375-VRCR resistant cell lines with ALW-II-27-41, a multikinases inhibitor able to inhibit several targets, including DDR1, DDR2 and several EPH members. Strikingly, once resistant cell lines were exposed to this inhibitor, a significant reduction of expression and activation of EphA2, DDR1 and DDR2 was observed. Moreover, the ALW‑II‑41‑27 treatment of both SAN-VRCR and A375-VRCR resistant cell lines substantially inhibited phosphorylation of AKT, MEK and MAPK downstream pathways. Importantly, the multikinase inhibitor treatment was also able to reduce the invasion and migration abilities of vemurafenib and cobimetinib-resistant cells compared to the parental ones. Collectively, these data demonstrated that inhibition of multiple TKRs with ALW‑II‑41‑27 treatment might represent a promising therapeutic approach to overcome the limitations of targeting individual growth pathways. We suggest that the ALW‑II‑41‑27inhibitor, by simultaneous targeting multiple TRKs, might provide an effective therapy to overcome acquired resistance to BRAF and MEK inhibitors, possibly improving both prognosis and survival of BRAF-V600E melanoma patients.
Conclusion
This study indicates that blockade of multiple TRKs and related downstream pathways by using ALW‑II‑41‑27multikinase inhibitor can provide a novel strategy to overcome the resistance to BRAF and MEK inhibitors occurring in BRAF-V600E melanoma patients. This therapeutic approach, once validated in in vivo models, might be fruitfully included in the clinical practice to prolong the survival of melanoma patients.
Availability of data and materials
The data generated in this manuscript are available from the corresponding author on reasonable request.
References
Liu D, Liu X, Xing M. Activities of multiple cancer-related pathways are associated with BRAF mutation and predict the resistance to BRAF/MEK inhibitors in melanoma cells. Cell Cycle. 2014;13(2):208–19. https://doi.org/10.4161/cc.26971.
Domingue B, Lopes JM, Soares P, Pópulo H. Melanoma treatment in review. Immunotargets Ther. 2018;7:35–49. https://doi.org/10.2147/ITT.S134842 eCollection 2018.
Ronchi A, Montella M, Marino FZ, Caraglia M, Grimaldi A, Argenziano G, et al. Predictive evaluation on cytological sample of metastatic melanoma: the role of BRAF immunocytochemistry in the molecular era. Diagnostics (Basel). 2021;11(6):1110. https://doi.org/10.3390/diagnostics11061110.
Troiani T, De Falco V, Napolitano S, Trojaniello C, Ascierto PA. How we treat locoregional melanoma. ESMO Open. 2021;6(3):100136. https://doi.org/10.1016/j.esmoop.2021.100136 Epub 2021 Apr 27.
Wang L, Leite R, de Oliveira SH, Bosdriesz E, Pencheva N, DiedeBrunen AB, et al. An acquired vulnerability of drug-resistant melanoma with therapeutic potential. Cell. 2018;173(6):1413–1425.e14. https://doi.org/10.1016/j.cell.2018.04.012 Epub 2018 May 10.
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47. https://doi.org/10.1056/NEJMoa050092.
Ascierto PA, Kirkwood JM, Grob J-J, Simeone E, Grimaldi AM, Maio M, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. https://doi.org/10.1186/1479-5876-10-85.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54. https://doi.org/10.1038/nature00766 Epub 2002 Jun 9.
Proietti I, NevenaSkroza NB, Tolino E, Balduzzi V, Marchesiello A, Michelini S, et al. Mechanisms of acquired BRAF inhibitor resistance in melanoma: a systematic review. Cancers (Basel). 2020;12(10):2801. https://doi.org/10.3390/cancers12102801.
Rossi A, Roberto M, Panebianco M, Botticelli A, Mazzuca F, Marchetti P. Drug resistance of BRAF-mutant melanoma: review of up-to-date mechanisms of action and promising targeted agents. Eur J Pharmacol. 2019;862:172621. https://doi.org/10.1016/j.ejphar.2019.172621.
Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–14. https://doi.org/10.1056/NEJMoa1112302.
Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. NatRevDrugDiscov. 2012;11:873–86. https://doi.org/10.1038/nrd3847 PMID:23060265.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. https://doi.org/10.1056/NEJMoa1103782.
Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. https://doi.org/10.1016/S0140-6736(12)60868-X.
Cohen JV, Sullivan RJ. Developments in the space of new MAPK pathway inhibitors for BRAF-mutant melanoma. Clin Cancer Res. 2019;25(19):5735–42. https://doi.org/10.1158/1078-0432.CCR-18-0836 Epub 2019 Apr 16.
Savoia P, Fava P, Casoni F, Cremona O. Targeting the ERK signaling pathway in melanoma. Int J Mol Sci. 2019;20(6):1483. https://doi.org/10.3390/ijms20061483.
Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–88. https://doi.org/10.1056/NEJMoa1406037.
Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–76. https://doi.org/10.1056/NEJMoa1408868 Epub 2014 Sep 29.
Long GV, Weber JS, Infante JR, Kim KB, Daud A, Gonzalez R, et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving Dabrafenib combined with Trametinib. J Clin Oncol. 2016;34:871–8. https://doi.org/10.1200/JCO.2015.62.9345.
Long GV, AxelHauschild MS, Atkinson V, Mandalà M, Chiarion-Sileni V, Larkin J, et al. Adjuvant Dabrafenib plus Trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–23. https://doi.org/10.1056/NEJMoa1708539 Epub 2017 Sep 10.
Giunta EF, De Falco V, Napolitano S, Argenziano G, Brancaccio G, Moscarella E, et al. Optimal treatment strategy for metastatic melanoma patients harboring BRAF-V600 mutations. Ther Adv Med Oncol. 2020;12:1758835920925219. https://doi.org/10.1177/1758835920925219 eCollection 2020.
Eroglu Z, Ribas A. Combination therapy with BRAF and MEK inhibitors for melanoma: latest evidence and place in therapy. Ther Adv Med Oncol. 2016;8(1):48–56. https://doi.org/10.1177/1758834015616934.
Dréno B, Ribas A, Larkin J, Ascierto PA, Hauschild A, Thomas L, et al. Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study. Ann Oncol. 2017;28(5):1137–44. https://doi.org/10.1093/annonc/mdx040.
Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248–60. https://doi.org/10.1016/S1470-2045(16)30122-X Epub 2016 Jul 30.
Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(10):1315–27. https://doi.org/10.1016/S1470-2045(18)30497-2 Epub 2018 Sep 12.
Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–15. https://doi.org/10.1016/S1470-2045(18)30142-6.
Welsh SJ, Rizos H, Scolyer RA, Long GV. Resistance to combination BRAF and MEK inhibition in metastatic melanoma: where to next? Eur J Cancer. 2016;62:76–85. https://doi.org/10.1016/j.ejca.2016.04.005 Epub 2016 May 24.
Wilson K, Shiuan E, Brantley-Sieders DM. Oncogenic functions and therapeutic targeting of EphA2 in cancer. Oncogene. 2021;40(14):2483–95. https://doi.org/10.1038/s41388-021-01714-8 Epub 2021 Mar 8.
Miao B, Ji Z, Tan L, Taylor M, Zhang J, Choi HG, et al. EPHA2 is a mediator of vemurafenib resistance and a novel therapeutic target in melanoma. Cancer Discov. 2015;5(3):274–87. https://doi.org/10.1158/2159-8290.CD-14-0295.
Xiao T, Xiao Y, Wang W, Tang YY, Xiao Z, Min S. Targeting EphA2 in cancer. J Hematol Oncol. 2020;13:Article number: 114.
Zhao P, Jiang D, Huang Y, Chen C. EphA2: a promising therapeutic target in breast cancer. J Genet Genomics. 2021;48(4):261–7. https://doi.org/10.1016/j.jgg.
Martini G, Cardone C, Vitiello PP, Belli V, Napolitano S, Troiani T, et al. EPHA2 is a predictive biomarker of resistance and a potential therapeutic target for improving Antiepidermal growth factor receptor therapy in colorectal cancer. Mol Cancer Ther. 2019;18(4):845–55. https://doi.org/10.1158/1535-7163.MCT-18-0539 Epub 2019 Mar 1.
Gao Y, Zhou J, Li J. Discoidin domain receptors orchestrate cancer progression: a focus on cancer therapies. Cancer Sci. 2021;112(3):962–9. https://doi.org/10.1111/cas.14789 Epub 2021 Jan 27.
Elkamhawy A, Qili L, Nada H, Woo J, GuofengQuan KL. The journey of DDR1 and DDR2 kinase inhibitors as rising stars in the fight against cancer. Int J Mol Sci. 2021;22(12):6535. https://doi.org/10.3390/ijms22126535.
Rammal H, Saby C, Magnien K, Van-Gulick L, Garnotel R, Buache E, et al. Discoidin domain receptors: potential actors and targets in cancer. Front Pharmacol. 2016;14(7):55. https://doi.org/10.3389/fphar.2016.00055.
Reger C, de Moura M, Battistella AS, Caudron A, Feugeas JP, Podgorniak M-P, et al. Discoidin domain receptors: a promising target in melanoma. Pigment Cell Melanoma Res. 2019;32(5):697–707. https://doi.org/10.1111/pcmr.12809.
Amato KR, Wang S, Hastings AK, Youngblood VM, Santapuram PR, Chen H, et al. Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC. J Clin Invest. 2014;124(5):2037–49. https://doi.org/10.1172/JCI72522 Epub 2014 Apr 8.
Datta A, Deng S, Gopal V, Yap KC-H, Halim CE, Lye ML, et al. Cytoskeletal dynamics in epithelial-mesenchymal transition: insights into therapeutic targets for cancer metastasis. Cancers (Basel). 2021;13(8):1882. https://doi.org/10.3390/cancers13081882.
Ernst M, Giubellino A. The current state of treatment and future directions in cutaneous malignant melanoma. Biomedicines. 2022;10(4):822. https://doi.org/10.3390/biomedicines10040822.
Pópulo H, Domingues B, Sampaio C, Lopes JM, Soares P. Combinatorial therapies to overcome BRAF/MEK inhibitors resistance in melanoma cells: an in vitro study. J Exp Pharmacol. 2021;13:521–35. https://doi.org/10.2147/JEP.S297831.
Wang B, Zhang W, Gao Z, Kwong L, Lu H, Tan J, et al. Targeting mTOR signaling overcomes acquired resistance to combined BRAF and MEK inhibition in BRAF-mutant melanoma. Oncogene. 2021;40(37):5590–9. https://doi.org/10.1038/s41388-021-01911-5 Epub 2021 Jul 24.
Hartman ML, Sztiller-Sikorska M, Gajos-Michniewicz A, Czyz M. Dissecting mechanisms of melanoma resistance to BRAF and MEK inhibitors revealed genetic and non-genetic patient- and drug-specific alterations and remarkable phenotypic plasticity. Cells. 2020;9(1):142. https://doi.org/10.3390/cells9010142.
Arozarena I, Wellbrock C. Phenotype plasticity as enabler of melanoma progression and therapy resistance. Nat Rev Cancer. 2019;19(7):377–91. https://doi.org/10.1038/s41568-019-0154-4 Epub 2019 Jun 17.
Luebker SA, Koepsell SA. Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front Oncol. 2019;9:268. https://doi.org/10.3389/fonc.2019.00268 eCollection 2019.
Tian Y, Guo W. A review of the molecular pathways involved in resistance to BRAF inhibitors in patients with advanced-stage melanoma. Med Sci Monit. 2020;26:e920957. https://doi.org/10.12659/MSM.920957.
Tangella LP, Clark ME, Gray ES. Resistance mechanisms to targeted therapy in BRAF-mutant melanoma - a mini review. Biochim Biophys Acta Gen Subj. 2021;1865(1):129736. https://doi.org/10.1016/j.bbagen.2020.129736 Epub 2020 Sep 18.
Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141–60. https://doi.org/10.20517/cdr.2019.10 Epub 2019 Jun 19.
Berestjuk I, Lecacheur M, Carminati A, Diazzi S, Rovera C, Prod’homme V, et al. Targeting Discoidin domain receptors DDR1 and DDR2 overcomes matrix‐mediated tumor cell adaptation and tolerance to BRAF‐targeted therapy in melanoma. EMBO Mol Med. 2022;14(2):e11814. https://doi.org/10.15252/emmm.201911814.
Reger de Moura C, Battistella M, Sohail A, Caudron A, Feugeas JP, Podgorniak M-P, et al. Discoidin domain receptors: a promising target in melanoma. Pigment Cell Melanoma Res. 2019;32:697–707.
Badiola I, Villace P, Basaldua I, Olaso E. Downregulation of discoidin domain receptor 2 in A375 human melanoma cells reduces its experimental liver metastasis ability. Oncol Rep. 2011;26:971–8.
Poudel B, Lee YM, Kim DK. DDR2 inhibition reduces migration and invasion of murine metastatic melanoma cells by suppressing MMP2/9 expression through ERK/NF-kappaB pathway. Acta Biochim Biophys Sin Shanghai. 2015;47:292–8.
Udayakumar D, Zhang G, Ji Z, Njauw C-N, Mroz P, Tsao H. EphA2 is a critical oncogene in melanoma. Oncogene. 2011;30(50):4921–9. https://doi.org/10.1038/onc.2011.210 Epub 2011 Jun 13.
Jeitany M, Leroy C, Tosti P, Lafitte M, Le Guet J, Simon V, et al. Inhibition of DDR1-BCR signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol Med. 2018;10(4):e7918. https://doi.org/10.15252/emmm.201707918.
von Mässenhausen A, Sanders C, Brägelmann J, Konantz M, Queisser A, Vogel W, et al. Claudia Lengerke 5, Sven Perner. Targeting DDR2 in head and neck squamous cell carcinoma with dasatinib. Int J Cancer. 2016;139(10):2359–69. https://doi.org/10.1002/ijc.30279 Epub 2016 Jul 30.
Grither WR, Longmore GD. Inhibition of tumor-microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of DDR2 extracellular domain. Proc Natl Acad Sci U S A. 2018;115(33):E7786–94. https://doi.org/10.1073/pnas.1805020115 Epub 2018 Jul 30.
Acknowledgements
Not applicable.
Funding
I-CURE research project: “Identification, characterization, and mining of Colorectal tumorigenesis: cause, prevention & cure (ICURE)”. Roche Foundation Medicine: Roche S.p.A., Monza, Italy.
Author information
Authors and Affiliations
Contributions
Conceptualization, VB and TT; methodology, VB; formal analysis, VDF, GS and AP; investigation, VB, MT.; data curation, GM, CMD, DC, LPG; writing—original draft preparation, VB, SN and TT; writing-review and editing, VB, SN and TT; visualization, EM, FM and GA; supervision, MF, SN, TT and FC; funding acquisition, TT and FC. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Evaluation of cell proliferation of parental SAN and A375 BRAF mutated human melanoma cell lines following treatment with BRAF (vemurafenib) and/or MEK (cobimetinib) inhibitors. Cell viability of SAN and A375 cell lines was evaluated by MTT staining after treatment with increasing concentration of BRAF and MEK inhibitors (range 0,001–25 μM) for 72 hrs. The half-maximal inhibitory concentration (IC50) was reported in the tables. Fig. S2. (A) Wound healing assay performed to detect migration ability of SAN, SAN-VR, SAN-CR and SAN-VRCR cell lines after 48 hrs of culture. (B) Wound healing assay was performed to detect migration ability of A375, A375-VR, A375-CR and A375-VRCR cell lines after 48 hrs of culture. Fig. S3. Western blots showing all the bands and molecular weight of sections reported in Fig. 1B. Fig. S4. Western blots showing all the bands and molecular weight of sections reported in Fig. 1D. Fig. S5. Western blots showing all the bands and molecular weight of sections reported in Fig. 3B. Fig. S6. Western blots showing all the bands and molecular weight of sections reported in Fig. 3D. Fig. S7. The figure shows the densitometry readings/intensity ratios of the various markers reported in Fig. 3D (western blots analysis of SAN and SAN-VRCR cells after ALW-II-41-27 treatment) normalized against tubulin levels. Fig. S8. The figure shows the densitometry readings/intensity ratios of the various markers reported in Fig. 3D (western blots analysis of A375 and A375-VRCR cells after ALW-II-41-27 treatment) normalized against tubulin levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Belli, V., Napolitano, S., De Falco, V. et al. Targeting EphA2 and DDR signaling can overcome the BRAF and MEK inhibitors acquired resistance in melanoma cell lines. transl med commun 8, 3 (2023). https://doi.org/10.1186/s41231-022-00133-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-022-00133-5