Abstract
The idiotype network is experimentally modified to provide protective immunity against various microbial pathogens. Both internal and non-internal image-idiotype antibodies can trigger specific immune responses to antigens. The current outbreak of Severe Acute Respiratory Syndrome 2 (SARS-2) has provided a great opportunity to take advantage of idiotype / anti-idiotype antibodies as a protective regimen when no approved vaccine is available on earth. The current review identifies successful applications of idiotype/ anti-idiotype antibodies in various viral diseases and highlights their importance in COVID-19 pandemics. In the absence of vaccines and targeted therapies, polyclonal idiotype/ anti-idiotype antibodies against the viral structure may be a potential approach to the prevention and treatment of COVID-19 patients.
Similar content being viewed by others
SARS Coronavirus the escaped killer
SARS-CoV‐2, a member of the Coronaviridae family of the order Nidovirales are enveloped, positive‐sense, single‐stranded, and highly diverse RNA virus [1]. It has a crown-like shape. The genome is 26–32 Kb in length and is considered highly pathogenic [2, 3]. This family is subdivided into coronavirinae with four major generas such as alphacoronavirus, beta coronavirus, gamma coronavirus, delta coronavirus [4]. Viruses included in the alphacoronavirus and beta corona viruses affect most mammals, while the gamma coronavirus affects avian species. The delta coronavirus genus is found in both mammalian and avian hosts [5, 6]. The virus was reported in the Chinese province of Wuhan and is now classified as an pandemic. [7, 8]. They can cause infections in the gastrointestinal, respiratory, liver, and central nervous systems of humans, cattle, birds, bats, and many other wild animals [9,10,11]. Studies have shown strong evidence of close-knit among acute respiratory syndrome corona virus (SARS-CoV) and Middle East respiratory syndrome corona virus (MERS-CoV) [12,13,14,15,16].
Virus host interaction
Corona viruses are spherical as shown by Cryo-electron microscopy [17]. Corona virus particles contain four main structural proteins, including spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins. These structural proteins are necessary for virulence assembly and infection of CoVs. S proteins form spikes at the viral surface and are responsible for attachment to host receptors [18, 19]. Transmembrane domains of more prevalent viral M protein are involved in membrane curvature and budding [20, 21] It has critical role in giving shape to the virions, and binds to the nucleocapsid [22]. Viral E protein has diverse functions from assembly to the egress and interactions with host cell [23, 24]. The dynamic functions of coronavirus N proteins are involved in the formation of replication transcription complexes [25]. The initial attachment to the host cell is through interaction between the S protein and its receptor. Virion particles enter into the host cell via binding through the Angiotensin-converting enzyme 2 (ACE2). Subsequently, the coronavirus genome (ss RNA) attaches to the host’s ribosomes leading to the translation of 2 co-terminal and large polyproteins further processed by proteolytic enzymes [26, 27]. Proteolysis results in the smaller components for folding and packaging of new virions. These new virions bud through the intracellular membranes and are later released from the cells through vesicles of the secretory pathway [28, 29].
Humoral immune response to viral attack
Viral invasion leads to the development of either innate or adaptive immunity. Innate immunity is associated with the recognition of pathogen-associated molecular patterns (PAMPs) causing cytolysis, through the natural killer cells and the interferons causing the death of virus along with its resident cell. Adaptive immunity, on the other hand, plays a vital role in clearing the virus either through the cell-mediated immunity by cytotoxic T cells including CD8 + and the CD4 + subset, that kill the virus-infected cells or through the production of the B cells with antibody as a major arm [30].
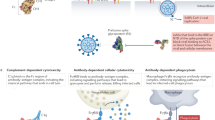
The humoral immune system gives rise to natural antibodies secreted by plasma cells upon an initial viral infection [31]. B lymphocytes are stimulated upon viral infection and differentiate into plasma cells to generate antibodies. An antibody can result in the neutralization of the virus either by hindering virus-host cell interactions or through the recognition of viral antigens on infected cells leading directly to the antibody-dependent cytotoxic cells (ADCC) or complement-mediated lysis. Mostly, IgG antibodies are responsible for the antiviral activity, while IgA is prevalent when viral infection occurs at the mucosal surfaces [32]. In vitro studies have revealed that protection through antibodies is conferred by neutralization using an infectious virus and susceptible cells [33, 34]. Neutralizing antibodies are therefore essential for the protection from viral infection [35] by targeting viral glycoproteins of enveloped viruses or the protein coat of nonenveloped viruses [36].
Antigenic mimicry of viral structure
Antigen-specific approaches in immunotherapy have gained much attention with experimental animals showing promising results upon antigen-based treatments leading to improvement in disease status [37]. The immunoglobulins are glycoprotein in nature with two identical heavy and light chains. These heavy-light chains comprise of two variable (V) immunoglobulin (Ig) domains (VL and VH) that result in a unique surface for antigen binding and these two V domains culminate the generation of the idiotype (Id) [38]. The term idiotype (Id) denotes an entire collection of idiotopes present in a single immunoglobulin (Ig) molecule and develop because of somatic mutations. Anti-idiotype (Anti-Ids) antibody, on the other hand, attaches to the idiotypic variable domains of an antibody [39]. Idiotopes can be regarded as foreign because the tiny amount of them normally present in any individual is inadequate to elicit self-tolerance and hence can be immunogenic [40]. The binding site of idiotype (Ab1) antibodies imitates the original antigen and give rise to anti-idiotype antibody (Ab2) mimicking the complete internal image of the antigen [41] as well as displays a functional activity that looks like the natural physiological activity of the antigen [42].
Idiotype are picked up by antigen-presenting cells (APCs) such as macrophages, dendritic cells, and B lymphocytes, and are then presented to T cells on MHC class II molecules. B lymphocytes continuously present Id-peptides on MHC class II molecules [43, 44]. The introduction of these identifiable peptides leads to the generation of anti-idiotypes. This presentation initiates a signal for B-lymphocytes to differentiate between antibody-producing plasma cells and secrete anti idiotype antibodies [45, 46]. B lymphocytes secrete antibodies that bind to their B cell receptor (BCR). Consequently, anti-Ids are introduced into MHC class II molecules [47]. This interaction between T and B cells produces antigen-free cooperation between T cells and B cells, which is called Id-driven TB cell cooperation, resulting in constant activity of both T and B cell in the absence of antigen [48]. This antigen-free mechanism is important for the production and upkeeping of immunological memory in the absence of antigen [49, 50]. It has been suggested that polyclonal idiotypic determinants are shared between B and T cells for the same antigen. However, monoclonal antibodies have not given these results due to the reason that BCR binds to the native epitopes whereas TCR can bind the antigens once they have been processed and presented on MHC molecules by the antigen presenting cells [51].
Current therapeutic and prophylactic approaches to COVID19
Vaccine development is a lengthy and costly process, and it takes years to develop a successful licensed vaccine [52]. Multiple techniques have been in use to prevent infectious diseases including conventional and modern vaccine pathways. DNA and RNA-based vaccines are developed rapidly as there is no prior need for the culture [53]. The development of the SARS-CoV 2 vaccine outpaced all the previous efforts resulting in over 300 projects of vaccine development in few months [54]. Currently, approved vaccines for SARS-CoV2 include inactivated (Sinopharm and SinoVac), vector-based (CanSinoBio, Sputnik V, and AstraZeneca), and mRNA-based vaccines (Pfizer BioNTech and Moderna). Inactivated SinoPharm and SinoVac vaccines both manufactured in China and studies have demonstrated that they are immunogenic and safe [55]. CanSinoBio comprises a single dose jab incorporated in an adenovirus vector and has gained much attention as the first choice for use in emergency cases including Pakistan and Mexico whereas trials are also underway in Argentina and Russia [56]. Russia’s Sputnik V vaccine carries the mRNA for spike protein of Coronavirus in two human adenoviruses vectors which are given in two different doses whereas Oxford–AstraZeneca vaccine makes use of similar material for both doses (chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19) [57, 58]. Pfizer BioNTech and Moderna vaccines make use of mRNA-based approaches which is present in liposomes and can produce antigen proteins coded by mRNA [59].
Besides vaccines, various therapeutic interventions have been tested to cure COVID 19 patients including Hydroxychloroquine, Azithromycin, and Remdesivir. Hydroxychloroquine has been found to interfere with the glycosylation of ACE2 resulting in the prevention of virus binding [60, 61]. Azithromycin in synergism with Hydroxychloroquine has suggested rapid viral clearance [62] however, this data is not sufficient to conclude any authentic results. Moreover, high doses of Hydroxychloroquine are also not suggested in severely ill COVID19 patients [63]. Remdesivir, an analog of nucleoside, can inhibit viral RNA-dependent RNA polymerase activity [64] and was successfully used in a SARS-CoV2 infected individual [65]. Its side effects are minimal resulting in decreased mortality.
Idiotypes as Pandemic savior
In human history, COVID-19 is the second major respiratory challenge after influenza. Influenza virus threatens global health despite efforts to develop an effective vaccine [66]. Being an RNA virus, replication results in mutations of SARS-CoV2 naturally. To date, 4000 known mutations have been reported in the spike protein region [67]. Antibodies are the key immune cells that confer the protective response to various pathogens [68]. The durability of antibodies depends upon the virus type as well as host and environmental factors [69, 70]. Memory B cells are involved in recall responses whereas plasma cells are only the source of circulating antibodies. Studies have revealed that memory B cells display somatic hypermutation in SARS-CoV2 indicating the continuous evolution of humoral immune response [71]. Moreover, it has been found that the immunity to seasonal coronaviruses is short-lived [72]. Reports are that newer mutations in the SARS-CoV2 may lead to immune escapes resulting in the reinfection and updating the existing vaccines. However, it has been found that the mutations in virus have not caused any vaccines upgradation making the immune system memory effective against SARS-Cov2 virus [73].
Even the development of the Covid 19 vaccine is still in its infancy, with the need to develop a universal vaccine against several antigenically different viruses, including those currently in circulation and those may come to light in the future. The development of idiotype vaccines may reduce the risk of unwanted side effects that are usually produced from the most common antigenic vaccines [74]. Idiotype vaccines are proteins in nature and can be easily handled. They are T cell-dependent antigens, therefore, effective immunogenic carriers can be used as agents to pair with the antigen. [75]. Studies have shown that immunization of BALB/c mice with pure chicken anti-H9 IgG promotes the development of anti-idiotypic antibodies and specific B-cell hybridomas. After screening for hybridomas, a monoclonal antibody was identified that was able to bind hemagglutinin and produce antibodies [76].
Antibodies may be of the IgM, IgG, and IGA types that are directed to the spike (S) protein of SARS-CoV2. Idiotype is a sequence switch of antibodies that attaches to the receptor binding domain (RBD) of the viral S protein. The initial antibody belongs to the IgM isotype which later changes to the IgG isotype. Class switching can also occur with the IgA isotype, which is produced by large-scale plasma cells in the laminar propria of mucosal surfaces. IgA antibodies play an important role in the immune defense of the virus, the entry point of the virus to the mucosal surface of the respiratory tract, related to the formation of an important immunoglobulin. IgA can neutralize SARS-CoV 2 before it reaches the epithelial cells and attaches to them. IgA may play a role in a mucosal vaccine to promote the development of idiotypic vaccines [48]. Previous research has also shown that the anti-idiotype vaccine can elicit mucosal immunity and act as an immunogenic against the coronavirus [77].
In severe SARS-CoV 2 infections, binding to different cytokines or variable domains of other antibodies (anti-idiotype antibodies) is thought to reduce the inflammatory response. Furthermore, the presence of IgG dimers may interfere with the activation of FcγR on innate immune cells [78]. It has been speculated that high doses of anti-idiotypic immunoglobulins may be helpful in severe SARS-Co-2 infections by modulating the immune system, centralizing FcγR, and lowering ADE [79].
Conclusions
The biggest challenge today is the COVID-19 pandemic. At present, more than 2.69 million people have been killed worldwide and about 122 million positive cases have been reported. Vaccine and effective treatment strategies are growing rapidly, but time is needed to make effective progress. Significant life-saving support was offered in the treatment of plasma in patients with COVID 19. Considering past and present research, it has been pointed out that neutralizing antibodies in the form of hyperimmune serum has always been valuable in saving lives. Timely implementation of controlled studies on immunoglobulins for the development of idiotype / anti-idiotype vaccines is needed.
Availability of data and materials
Not Applicable.
Abbreviations
- ADE:
-
Antibody dependent enhancement
- FcγR:
-
Fc gamma receptor
- ssRNA:
-
Single stranded RNA
References
Zumla A, Chan JF, Azhar EI, et al. Coronaviruses—drug discovery and therapeutic options. Nature reviews Drug discovery. 2016;15:327–47.
Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395:565–74.
Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet respiratory medicine. 2020;8:420–2.
Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–35.
Groot R de, Baker S, Baric R, et al. Family Coronaviridae,, Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. London: Academic Press; 2012. pp. 806–20.
Drexler JF, Corman VM, Drosten C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral research. 2014;101:45–56.
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395:497–506.
Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–207.
Wang LF, Shi Z, Zhang S, et al. Review of bats and SARS. Emerg Infect Dis. 2006;12:1834.
Ge XY, Li JL, Yang XL, et al Zhang C, Peng. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–8.
Chen Y, Guo D. Molecular mechanisms of coronavirus RNA capping and methylation. Virol Sin. 2016;31:3–11.
Gytis D, Carvalho LM, Rambaut A, et al, MERS-CoV spillover at the camel-human interface. eLife. 2018; 7.
Hu B, Ge X, Wang LF, et al. Bat origin of human coronaviruses. Virol J. 2015;12:1–10.
Leopardi S, Holmes EC, Gastaldelli M, et al. Interplay between co-divergence and cross- species transmission in the evolutionary history of bat coronaviruses. Infection Genetics Evolution. 2018;58:279–89.
Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–92.
Cauchemez S, Kerkhove MV, Riley S, et al. Transmission scenarios for Middle East Respiratory Syndrome Coronavirus (MERS- CoV) and how to tell them apart. Eurosurveillance. 2013;18:20503.
Neuman BW, Adair BD, Yoshioka C, et al. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. Journal of virology. 2006;80:7918–28.
Beniac DR, Andonov A, Grudeski E, et al. Architecture of the SARS coronavirus prefusion spike. Nat Struct Mol Biol. 2006;13:751–2.
Delmas B, Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. Journal of virology. 1990;64:5367–75.
Armstrong J, Niemann H, Smeekens S, et al. Sequence and topology of a model intracellular membrane protein, E1 glycoprotein, from a coronavirus. Nature. 1984;308:751–2.
Nal B, Chan C, Kien F, et al. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. Journal of general virology. 2005;86:1423–34.
Neuman BW, Kiss G, Kunding AH, et al. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174:11–22.
DeDiego ML, Álvarez E, Almazán F, et al. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. Journal of virology. 2007;81:1701–13.
Nieto-Torres JL, DeDiego ML, Verdiá-Báguena C, et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10:e1004077.
Chang Ck, Sue SC, Yu TH, et al. Modular organization of SARS coronavirus nucleocapsid protein. Journal of biomedical science. 2006;13:59–72.
Zhang H, Penninger JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS- CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive care medicine. 2020;46:586–90.
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.e8.
Holmes KV, Lai M. Coronaviridae: the viruses and their replication. Fields virology. 1996;1:1075–93.
Prentice E, Denison MR. The cell biology of coronavirus infection. The Nidoviruses. 2001; pp 609–614.
Zhong J, Tang J, Ye C, et al. The immunology of COVID-19: is immune modulation an option for treatment. The Lancet Rheumatology. 2020;2:E428–36.
Baumgarth N, Herman OC, Jager GC, et al., Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proceedings of the National Academy of Sciences. 1999; 96: 2250–2255.
Klimpel GR. Immune defenses, Medical Microbiology. 4th edition, University of Texas Medical Branch at Galveston, 1996.
Burton DR, Desrosiers RC, Doms RW, et al. HIV vaccine design and the neutralizing antibody problem. Nature immunology. 2004;5:233–6.
Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262.
Klasse P. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol. 2014;2014:157895.
Corti DA. Lanzavecchia. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705–42.
Sabatos-Peyton CA, Verhagen J, Wraith DC. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr Opin Immunol. 2010;22:609–15.
López-Requena A, Burrone OR, Cesco-Gaspere M. Idiotypes as immunogens: facing the challenge of inducing strong therapeutic immune responses against the variable region of immunoglobulins. Frontiers in oncology. 2012;2:159.
Naveed A, Rahman SU, Arshad MI. Recapitulation of the anti-idiotype antibodies as vaccine candidate. Translational Medicine Communications. 2018;3:1–7.
Bendandi M. Role of anti-idiotype vaccines in the modern treatment of human follicular lymphoma. Expert Rev Anticancer Ther. 2001;1:65–72.
Garcia KC, Ronco PM, Verroust PJ, et al. Three-dimensional structure of an angiotensin II- Fab complex at 3 A: hormone recognition by an anti-idiotypic antibody. Science. 1992;257:502–7.
Taub R, Greene MI. Functional validation of ligand mimicry by anti-receptor antibodies: structural and therapeutic implications. Biochemistry. 1992;31:7431–5.
King C, Wills M, Hamblin T, et al. Idiotypic IgM on a B-cell surface requires processing for recognition by anti-idiotypic T cells. Cellular immunology. 1993;147:411–24.
Weiss S, Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proceedings of the National Academy of Sciences. 1989; 86: 282–286.
Janeway CA, Sakato N, Eisen HN. Recognition of immunoglobulin idiotypes by thymus- derived lymphocytes. Proceedings of the National Academy of Sciences. 1975; 72: 2357–2360.
Reitan SK, Hannestad K. The primary IgM antibody repertoire: a source of potent idiotype immunogens. Eur J Immunol. 2001;31:2143–53.
Jacobsen JT, Lunde E, Sundvold-Gjerstad V, et al, et al. The cellular mechanism by which complementary Id + and anti‐Id antibodies communicate: T cells integrated into idiotypic regulation. Immunology cell biology. 2010;88:515–22.
Munthe LA, Corthay A, Os A, et al. Systemic autoimmune disease caused by autoreactive B cells that receive chronic help from Ig V region-specific T cells. J Immunol. 2005;175:2391–400.
Nayak R, Mitra-Kaushik S, Shaila M. Perpetuation of immunological memory: a relay hypothesis. Immunology. 2001;102:387–95.
Vani J, Nayak R, Shaila M. Maintenance of antigen-specific immunological memory through variable regions of heavy and light chains of anti‐idiotypic antibody. Immunology. 2007;120:486–96.
Uner A, Gavalchin J, Idiotypes. eLS. 2006.
Gouglas D, Le TT, Henderson K, Kaloudis A, Danielsen T, et al. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. The Lancet Global Health. 2018;6:e1386–96.
Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969–73.
Forni G, Mantovani A. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differentiation. 2021;28:626–39.
Wu Z, Hu Y, Xu M, Chen Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021. https://doi.org/10.1016/S1473-3099(20)30987-7.
Baraniuk C, What do we know about China’s covid-19 vaccines?, bmj. 2021;373.
Jones I, Roy P, Sputnik V. COVID-19 vaccine candidate appears safe and effective. The Lancet. 2021;397:642–3.
Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. The Lancet. 2021;397:72–4.
Vincent MJ, Bergeron E, Benjannet S, Erickson BR, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:1–10.
Wang M, Cao R, Zhang L, Yang X, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research. 2020;30:269–71.
Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949.
Borba MGS, Val FFA, Sampaio VS, et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(4):e208857. doi:https://doi.org/10.1001/jamanetworkopen.2020.8857.
Gordon CJ, Tchesnokov EP, Feng JY, et al. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773–9.
Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. New England Journal of Medicine. N Engl J Med. 2020;382:929–36.
Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: Immunology and treatment options. Clinical Immunology. 2020;215:108448.
Nickol ME, Kindrachuk J. A year of terror and a century of reflection: perspectives on the great influenza pandemic of 1918–1919. BMC Infect Dis. 2019;19:117.
Wise J. Covid-19: New coronavirus variant is identified in UK. BMJ. 2020;16:371:m4857. doi:https://doi.org/10.1136/bmj.m4857.
Chen Y, Zuiani A, Fischinger S, et al., Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell. 2020; 183: 1496–507. e1416.
Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15.
Hagan T, Cortese M, Rouphael N, et al., Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178: 1313–28. e1313.
Gaebler C, Wang Z, Lorenzi JC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–44.
Edridge AW, Kaczorowska J, Hoste AC, et al. Seasonal coronavirus protective immunity is short-lasting. Nature medicine. 2020;26:1691–3.
Kupferschmidt K. New mutations raise specter of ‘immune escape’. Science. 2021; 22:329–330. doi: https://doi.org/10.1126/science.371.6527.329. PMID: 33479129.
Ma J, Zhou L, Wang D. Functional mimicry of an anti-idiotypic antibody to nominal antigen on cellular response. Japanese journal of cancer research. 2002;93:78–84.
Fredriksen AB, Sandlie I, Bogen B. Targeted DNA vaccines for enhanced induction of idiotype-specific B and T cells. Frontiers in oncology. 2012;2:154.
Li B, Peng J, Niu Z, et al. Preparation of anti-idiotypic antibody against avian influenza virus subtype H9. Cellular Molecular Immunology. 2005;2:155–7.
Sune C, Smerdou C, Anton I, et al. A conserved coronavirus epitope, critical in virus neutralization, mimicked by internal-image monoclonal anti-idiotypic antibodies. Journal of virology. 1991;65:6979–84.
Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system. Nat Rev Immunol. 2013;13:176–89.
Nguyen AA, Habiballah SB, Platt CD, et al. Immunoglobulins in the treatment of COVID- 19 infection: Proceed with caution! Clinical Immunology. 2020;216:108459.
Acknowledgements
Not Applicable.
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
Naveed generated the idea and Naz and Naveed wrote the original article while Rahman critically review the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
No competing interests exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naveed, A., Naz, D. & Rahman, S.u. Idiotype/anti-idiotype antibodies: as a glorious savior in COVID-19 pandemics. transl med commun 6, 18 (2021). https://doi.org/10.1186/s41231-021-00097-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-021-00097-y