Abstract
Background
Hepatitis B virus (HBV) infection in Africa is mostly acquired before the age of 5 years through vertical or horizontal routes. While all the countries in the World Health Organization African region have introduced HBV vaccination into their national immunization programs, the rate of protective immune response to HBV vaccine among children in Africa has not been systematically synthesized. In this study, we estimated the HBV vaccine seroprotection rate (defined as anti-HBs titer ≥ 10 IU/L) and the associated factors among under-five children who completed a primary series of HBV vaccination in Africa.
Methods
We systematically searched PubMed, Web Science, and Scopus databases from inception to May 2022 for potentially eligible studies. The pooled seroprotection rate was estimated using a random-effects model with Freeman–Tukey double arcsine transformation and the associated factors were examined using odds ratio estimated by the DerSimonian and Laird method.
Results
From the 1063 records identified, 29 studies with a total sample size of 9167 under-five children were included in the meta-analysis. The pooled seroprotection rate was 89.23% (95% CI 85.68–92.33%, I2 = 95.96%, p < 0.001). In the subgroup analyses, there was a significant difference in the rate by the assay method, vaccine dose, and vaccine combination. HIV-positive children had lower odds of achieving seroprotection when compared with HIV-negative children (OR = 0.22, 95%CI 0.12–0.40).
Conclusions
The majority of under-five children in Africa achieved seroprotection after completing three or four doses of HBV vaccine. However, the rate was lower among children living with HIV. This calls for interventions to timely identify and address nonresponse to HBV vaccine, particularly among immunosuppressed children.
Similar content being viewed by others
Main text
Background
Despite the decline in the burden of hepatitis B virus (HBV) infection, it remains a major global public health problem [1, 2]. Globally, there were approximately 1.5 million new HBV infections and 820,000 HBV-related deaths in 2019 [3]. Africa continues to be disproportionately affected by HBV infection, accounting for about 67% of the new infections in 2019 [3]. HBV infection in Africa is mostly acquired before the age of 5 years through vertical or horizontal routes [4,5,6,7]. Compared with HBV infection acquired in adulthood which leads to chronic hepatitis in less than 5% of cases, about 80–90% of persons infected in the first year of life and 30% of those infected before the age of 6 years develop chronic hepatitis [8]. Thus reducing new infections among children is one of the global targets of the ongoing efforts aimed at eliminating HBV infections as a major public health threat in Africa [9].
The availability of safe and effective HBV vaccines [10,11,12,13] has been pivotal to the prevention and control of HBV infection, globally [14,15,16]. HBV vaccine, which can be plasma-derived (although no longer in use) or recombinant DNA-derived, is available as a monovalent vaccine or in combination with other vaccines [17, 18]. Given the benefits, the World Health Organization (WHO) recommends a hepatitis B vaccine birth dose (HepB-BD) for newborns, followed by two or three doses given at least four weeks apart to complete the primary series [4, 18]. HBV vaccination is also recommended for persons at increased risk such as HIV-infected persons, men who have sex with men, healthcare workers, persons with multiple sexual partners, injecting drug users, and persons who frequently require blood or blood products [18]. As recommended, all 47 countries in the WHO African region have introduced HBV vaccination into their national immunization programs [19], with the coverage of 3 doses of HBV vaccine estimated at 72% in 2022 [20]. However, as of 2022, only 15 (32%) of the African countries had introduced HepB-BD vaccine into their national immunization programs [21], with an estimated coverage of 18% in the region [20].
Notwithstanding the efficacy of the HBV vaccine [10,11,12,13], the ability to mount a protective immune response (defined as a hepatitis B surface antibody [anti-HBs] titer ≥ 10 IU/L) [22] following vaccination varies among vaccinees. Approximately 5–10% of healthy persons (referred to as non-responders) do not develop seroprotective anti-HBs level after completing HBV vaccine primary series [23, 24]. Studies have shown that factors such as age, sex, body mass index, vaccination schedule, site, dose, route of administration, and the brand of vaccine affect the immune response to HBV vaccine [24,25,26,27,28,29,30,31]. Evidence also indicates that the immune response to HBV vaccine is lower in individuals with immunosuppressive or chronic diseases, such as HIV, chronic renal failure, diabetes, celiac disease, or chronic liver disease [25, 32,33,34,35]. For those at high risk of HBV infection, post-vaccination serologic testing (PVST) within one to two months after the final dose of vaccine is recommended to identify non-responders and evaluate the need for revaccination [18]. Strategies to improve immune response among non-responders include increased vaccination dose, additional vaccination cycles, an alternative route of administration, or the use of adjuvants [35,36,37,38].
An increasing number of studies have reported on the immune response to HBV vaccine among children in Africa; however, the proportion that achieves seroprotective level has not been systematically synthesized. Previous meta-analytic studies on the immune response to HBV vaccine [24,25,26,27,28, 32,33,34, 39,40,41,42] did not focus on children or include studies from Africa, limiting the evidence on this vulnerable population who are at increased risk of developing chronic HBV infection. In a literature review of recombinant HBV vaccine among infants, the immune response rate ranged from 50% to 100% with a median of 98%. However, the review only considered trials, monovalent vaccines, and infants in the first 30 days of life [43]. Insight into the immune response to HBV vaccine among African children can inform strategies to improve its delivery or monitoring for effectiveness and maximum impact in the high-burden continent.
The objective of this review was to estimate the seroprotection rate (i.e., anti-HBs titer ≥ 10 IU/L) and the associated sociodemographic and clinical factors among under-five children who completed a primary series of HBV vaccination in Africa.
Methods
Design
This systematic review and meta-analysis was performed and reported using the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [44]. This review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration no: CRD42022299988).
Information sources and search strategy
We systematically searched PubMed, Web Science, and Scopus databases from inception to May 2022 for potentially eligible articles using search terms relating to hepatitis B; vaccination; immunogenicity; children; and African countries (see Additional file 1). No language restriction was applied. We also searched the bibliographies of the identified articles for other potentially eligible articles.
Inclusion and exclusion criteria
Articles were eligible for inclusion if: (i) they included under-five children; (ii) the participants completed a three-dose schedule of HBV vaccine primary series or birth dose vaccine plus two or three doses; (iii) they reported anti-HBs titer following the last dose of the vaccine series; and (iv) the study design was experimental or observational. We excluded studies that: (i) were not conducted in Africa, (ii) the anti-HBs were not quantified or the cutoff of ≥ 10UI/L was not reported; (iii) used the same data (we retained the one with more information regarding the inclusion criteria); or (iv) the participants did not receive HBV-specific vaccine, did not complete the HBV vaccine series, or received HBV vaccine booster dose after the completion of primary series. We also excluded conference abstracts and studies where the full articles or anti-HBs level data from figures could not be retrieved.
Study selection and abstraction
Two authors (BOO and OAO) first independently screened the title and abstracts of the articles and the full articles of those deemed eligible were retrieved and screened for inclusion. Articles were only retrieved and included if there was an agreement between the two authors. Disagreements between the two authors were resolved by a third author (DAA). Three authors (BOO, TO, and OAO) extracted data from the articles using a pretested tool that included Information such as the first author’s surname, publication year, study location, study design, study population, number of participants, participants' age, vaccine schedule, vaccine dose, vaccine type, vaccine combination, and assay method. The number of participants that had anti-HBs ≥ 10 IU/L was extracted or calculated from the included studies. Disaggregated anti-HBs ≥ 10 IU/L by sociodemographic and clinical factors were also abstracted. We grouped the study locations into regions (North; Central and West; and East and Southern Africa). In the description of the study population, we classified participants according to their reported health conditions or disease exposure. We classified participants as “healthy” if they were not primarily recruited based on or described by any specific health condition or exposure to a disease. For studies that assayed anti-HBs at multiple timepoints, we used the first timepoint.
Quality assessment
The quality of the articles included in the study was assessed using the Effective Public Health Practice Project (EPHPP) Quality Assessment tool [45]. The tool evaluates and rates the quality of quantitative studies under the following categories: study design, analysis, withdrawals and dropouts, data collection practices, selection bias, invention integrity, blinding as part of a controlled trial, and confounders. The articles were rated “strong,” “moderate,” and “weak” per the EPHPP guide for component and global rating. However, it was decided a priori not to exclude any study based on the quality rating.
Analysis
The meta-analysis for the pooled seroprotection rate was conducted using the procedure for binomial data [46]. Due to the statistical heterogeneity, the pooled rate was estimated using a random-effects meta-analysis model with Freeman–Tukey double arcsine transformation [47]. Sociodemographic and clinical factors reported by at least two articles were included in the meta-analysis and their associations with seroprotection were examined using odds ratio (OR) estimated by the DerSimonian and Laird method [48]. Statistical heterogeneity between the studies was assessed using Cochran’s Q statistic, with a p value < 0.1 set as the level of statistical significance [49]. I2 was also used to quantify the heterogeneity. We considered I2 statistic values of 50% or more as substantial heterogeneity [49]. Subgroup analyses were performed for group comparisons. The studies were grouped by region, assay method, vaccine dose, vaccine type, and vaccine combination. Meta-regression was performed to assess the effect of continuous characteristics (study sample size and publication year) on the seroprotection rate [50]. It was also used to assess the proportion of between-study variance explained by vaccine dose, vaccine type, assay method, region, vaccine combination, sample size, and publication year. Leave-one-out meta-analysis was used to investigate the influence of each study on the overall effect-size estimate and to identify influential studies. Publication bias was assessed using a funnel plot and Egger’s test [51]. The meta-analysis was conducted using STATA V.17.0 for Windows (Stata Statistical Software: Release 17. College Station, TX: StataCorp).
Search results
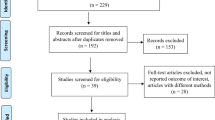
The PRISMA flow diagram for the study selection is shown in Table 1. A total of 1063 records were identified through three databases. Following the removal of 374 duplicates, the titles and abstracts of 689 records were screened and 555 were deemed ineligible. The full−text articles of 134 reports were retrieved and assessed for eligibility. Twenty−nine reports were included in the study and 98 articles were excluded with reasons illustrated in Fig. 1.
Characteristics of the included studies
All the articles were published in English. The publication year of the studies ranged from 1991 to 2021 as shown in Table 1. The studies included had a total of 9167 under-five children. Eighteen of the 29 studies were cross-sectional studies, four studies were longitudinal, and seven studies were clinical trials (see Table 1). The studies were conducted in the following countries: Benin (n=1), Burkina Faso (n=1), Cameroun (n=2), Democratic Republic of Congo (n=1), Egypt (n=3), Gambia (n=2), Ghana (n=3), Ivory Coast or Côte d'Ivoire (n=1), Kenya (n=1), Malawi (n=2), Senegal (n=1), South Africa (n=8), Tanzania (n=1), Burkina Faso and Ghana (n=1), and Cameroun and Senegal (n=1) (see Fig. 2). The population in the studies included healthy, HIV−exposed, HIV−infected, HIV−exposed uninfected, and acutely/chronically ill children. In 24 studies, the participants completed 3 doses, whereas in 1 study it was 4 doses of HBV vaccine. In 4 studies, the participants completed either 3 or 4 doses of HBV vaccine. The types of combination vaccines reported in the included studies were: monovalent (n=8), pentavalent (n=9), pentavalent and monovalent (n=1), hexavalent and monovalent (n=1), hexavalent (n=1), tetravalent (n=1), and pentavalent and heptavalent (n=1). The vaccine combination was not reported in 7 studies. Recombinant vaccine was used in 19 studies, plasma−derived vaccine in 4 studies, both recombinant and plasma−derived vaccine in 1 study. Five studies did not report the vaccine type. The vaccine schedule varied across the studies, with the 6, 10, and 14−week schedule mostly reported in the studies (n=19). A total of 20 studies used enzyme immunoassays for the quantification of the antibody response to HBV vaccine. Most of the studies were rated weak (n=15), largely because of their cross−sectional nature.
Seroprotection rate
The seroprotection rates after HBV vaccination in the included studies ranged from 45% to 100% (Fig. 3). The pooled rate was 89.23% (95% confidence interval [CI] 85.69–92.33%). The homogeneity test indicated the presence of heterogeneity in the data (I2 = 95.96%, p <0.001).
Subgroup analysis
Table 2 (Additional file 2: Fig. S1) shows the subgroup analysis of the seroprotection rate by region, assay method, vaccine dose, vaccine type, and vaccine combination. The subgroup differences by region and vaccine type were not statistically significantly different. However, there was a significant difference in the assay method, ranging from 97.61% (95%CI 94.30–99.60%) in studies that used chemiluminescence assay to 85.07% (95%CI 81.20–88.58%) in studies that used enzyme immunoassay. The subgroup analysis showed a significant difference between three vaccine doses (89.00% [95%CI 85.37–92.17%]) and four vaccine doses (97.17% [95%CI 93.29–99.62%]). There was also a significant subgroup difference by vaccine combination, ranging from 95.55% (95%CI 92.71–97.76%) in studies that used hexavalent vaccine to 71.68% (95%CI 66.13–76.65%) in one study that used tetravalent vaccine.
Meta‐regression analysis
The meta-regression model showed no statistically significant association between the sample size and seroprotection rate (p = 0.703) (Additional file 3: Fig. S2). Similarly, the association between the publication year and seroprotection rate was not statistically significant (p = 0.368) (Additional file 3: Fig. S2). Both the sample size and publication year did not account for any percentage in the between-study variance. Furthermore, the meta-regression showed that approximately 0.81%, 27.75%, 6.68%, 17.84%, and 0% of the between-study variance was explained by region, assay method, vaccine dose, vaccine combination, and vaccine type respectively.
Leave-one-out analysis
The seroprotection rate did not markedly change with the omission of each study in turn, indicating no strong influential studies. When compared with other studies, the omission of Nlend, 2016 (90.24% 95%CI 86.97–93.09%) and Madhi, 2011a (88.53%; 95% = 84.99–91.65%) had a relatively larger positive and negative influence on the pooled rate, respectively.
Publication bias
The funnel plot of the studies included in the review suggests no publication bias (Fig. 4). The absence of publication bias was further confirmed by Egger’s test (p = 0.727).
Factors associated with seroprotection after HBV vaccination
Sex, age, and HIV status were the only factors reported by at least two studies for under-five children. There was no significant difference in the seroprotection rate between males and females (OR = 0.32, 95%CI 0.03–3.22) (Fig. 5A). Similarly, the seroprotection rate was not significantly different between children less than 12 months and children ≥ 12 months (OR = 2.56, 95%CI 0.98–6.67) (Fig. 5B). However, HIV-positive children had lower odds of achieving seroprotection when compared with HIV-negative children (OR = 0.22, 95%CI 0.12–0.40) (Fig. 5C).
Discussion
In this study, we estimated the seroprotection rate among children under 5 years who completed three or four doses of HBV vaccine in Africa. Our results indicated that about 89% of children achieved seroprotection after HBV vaccination. This finding indicates a high immunogenicity of HBV vaccine among children and further supports the recommendation for country implementation for HBV infection prevention and control [18].
While we did not find similar studies at the regional level for comparison, the seroprotection rate among children in this study is consistent with a similar study in Iran which reported a pooled rate of 89% (95% CI 86–93%) among children under 5 years of age [81]. However, our result was higher than the rates reported by Le and colleagues in the US [82]. In their cross-sectional study of data for children and adolescents from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2016, among children aged 2–5 years who completed the HBV vaccine series, the seroprotection rate increased from 60.7% (95% CI 48.8–71.4%) to 65.2% (95% CI 57.4–72.3%) (protective immune response was defined as anti-HBs titer > 10 IU/L from 1999 to 2006 and ≥ 12 IU/L from 2007 to 2016). A small study in rural areas in Yemen also found a lower rate, with 72.2% of the under-five children having an anti-HBs level ≥ 10 IU/L [83]. The reasons for these differences are not apparent. The nature of the vaccines or vaccination may be responsible. Further investigation into the possibility of geographical variations in immune response to HBV vaccine is warranted.
Stratified by different groups, the results showed variations in the seroprotection rates. For instance, there was a significant variation in the assay method with the chemiluminescence assay having the highest immune response rate at 97.61% (95%CI 94.30–99.60%). Although the sensitivity and specificity of test assays vary by manufacturer, automated assays such as chemiluminescence tend to detect higher values of anti-HBs [84]. Compared with enzyme immunoassay which is more commonly used, chemiluminescence immunoassay has a lower turnaround time and requires less technical expertise [85]. There was also a significant subgroup difference by vaccine combination. Previous studies comparing the immunogenicity between hexavalent and pentavalent [86] and monovalent and pentavalent [87] vaccines found similar rates. Thus, more evidence is needed on this possible variation by vaccine combination. Of note, we did not find any significant difference between plasma-derived vaccine and recombinant vaccine. Safety concerns associated with plasma-derived HBV vaccine relegated its use [17]. Interestingly, the pooled seroprotection rate with four doses (i.e., a birth dose and three doses) was significantly higher than three doses, demonstrating the importance of HepB-BD. Despite the WHO recommendations of universal HepB-BD and its cost-effectiveness [88], many countries in Africa have not introduced routine HepB-BD into their national immunization programs due to reasons such as cost of implementation, the high proportion of non-institutional delivery, and limited evidence on the burden of HBV and perinatal transmission [19, 89,90,91].
From the few studies included in this meta-analysis, HIV was associated with lower odds of achieving seroprotection. Although there is no comparative study among children, our finding is consistent with results observed among adults in Africa [92]. The lack of protective immune response among people living with HIV has been linked with reduced CD4 cell count and B-cell dysfunction [35, 38] and factors such as viral load, sex, and age also influence immune response to HBV vaccine among people living with HIV [92, 93]. Our finding further supports routine PVST for children living with HIV after completion of the primary series. Although not included in our meta-analysis, other factors such as vitamin A supplementation [70] and EBV infection [58] were reported to be associated with immune response by single studies. These factors need to be further explored in future studies.
Several factors including perinatal host, nutritional, environmental, and immunization-related factors such as suboptimal dosing, site of administration, and reduced potency due to poor vaccine storage and handling conditions, could have accounted for the nonresponse among the under 5 children in the studies [31, 94]. While the cellular mechanisms involved in nonresponse to HBV vaccination remain unclear, impaired lymphocyte activation has been implicated [38, 95]. Interventions to address nonresponse among children will depend on largely the prevailing health status. A booster HBV vaccine dose can induce anamnestic response in most children without an immune response [96]. However, it is not recommended by the WHO for persons with normal immune status who have received a full primary course of HBV vaccine [18]. Some studies indicate long-term protection of the HBV vaccine regardless of the level of measurable anti-HBs titer [97,98,99]. On the other hand, for non-responding HIV-positive children, a second HBV vaccine series using larger or additional doses is recommended [34, 100, 101] or vaccination could be repeated after an increase in CD4 cell count or viral load suppression [18]. New vaccines with simplified schedules, and that can elicit higher anti-HBs response more rapidly are currently available and underway [17, 102]. However, these vaccines are still mostly for adults.
This systematic review has a few limitations. We only searched three databases and did not conduct hand-searches. The cutoff for seroprotection was not consistent across the study. Although most of the studies used ≥ 10 IU/L, four studies [58, 59, 66, 67] used > 10 IU/L. Our subgroup analysis showed no statistically significant difference between the two (data not shown). Secondly, the interval between the last dose and the immunoassay was not reported in some studies and varied across those that reported it. The optimal timing of PVST is within one to two months of the final dose. An immune response may decrease with longer intervals [103, 104]. In two studies, [62, 65] some of the participants received investigational vaccines that were yet to be licensed for use in the general public as at the time of the study. We were only able to examine a few factors associated with seroprotection because they were reported by a small number of the included studies for children under 5 years. Some of the pooled rates were also from a limited number of studies, thus the results should be interpreted cautiously.
Conclusions
Our study findings indicate that HBV vaccine induces a protective immune response in the majority of children under 5 years who complete three or four doses. However, the rate is lower among children living with HIV. This calls for interventions to timely identify and address nonresponse to HBV vaccine, particularly among children living with HIV. The high immunogenicity of HBV vaccine observed in this study further supports the need to scale up HBV vaccination coverage rates among under-five children in Africa. More studies are needed to better understand the factors associated with immune response to HBV vaccine among children under 5 years in Africa.
Availability of data and materials
The data set used in the meta-analysis is available from the corresponding author on request.
References
Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–9. https://doi.org/10.1016/j.vaccine.2011.12.116.
Sheena BS, Hiebert L, Han H, Ippolito H, Abbasi-Kangevari M, Abbasi-Kangevari Z, et al. Global, regional, and national burden of hepatitis B, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796–829. https://doi.org/10.1016/S2468-1253(22)00124-8.
World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies, 2016–2021: actions for impact. Geneva; 2021.
World Health Organization. Hepatitis B 2023. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b; 2023. Accessed 13 Nov 2023.
Spearman CW, Afihene M, Ally R, Apica B, Awuku Y, Cunha L, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol. 2017;2:900–9. https://doi.org/10.1016/S2468-1253(17)30295-9.
Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402–9. https://doi.org/10.1016/S1473-3099(07)70135-4.
Indolfi G, Easterbrook P, Dusheiko G, Siberry G, Chang MH, Thorne C, et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4:466–76. https://doi.org/10.1016/S2468-1253(19)30042-1.
Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992–1000. https://doi.org/10.1093/clinids/20.4.992.
World Health Organization. Global health sector strategy on viral hepatitis 2016–2021:towards ending viral hepatitis. Geneva; 2016.
Maupas P, Chiron JP, Barin F, Coursaget P, Goudeau A, Perrin J, et al. Efficacy of hepatitis B vaccine in prevention of early HBsAg carrier state in children. Controlled trial in an endemic area (Senegal). Lancet. 1981;1:289–92. https://doi.org/10.1016/S0140-6736(81)91908-5.
Francis DP, Hadler SC, Thompson SE, Maynard JE, Ostrow DG, Altman N, et al. The prevention of hepatitis B with vaccine. Report of the centers for disease control multi-center efficacy trial among homosexual men. Ann Intern Med. 1982;97:362–6. https://doi.org/10.7326/0003-4819-97-3-362.
Wong VC, Ip HM, Reesink HW, Lelie PN, Reerink-Brongers EE, Yeung CY, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet. 1984;1:921–6. https://doi.org/10.1016/S0140-6736(84)92388-2.
Szmuness W, Stevens CE, Harley EJ, Zang EA, Oleszko WR, William DC, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303:833–41. https://doi.org/10.1056/NEJM198010093031501.
Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395–403. https://doi.org/10.1016/S1473-3099(02)00315-8.
Chen DS. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50:805–16. https://doi.org/10.1016/J.JHEP.2009.01.002.
Meireles LC, Marinho RT, Van Damme P. Three decades of hepatitis B control with vaccination. World J Hepatol. 2015;7:2127–32. https://doi.org/10.4254/WJH.V7.I18.2127.
Zhao H, Zhou X, Zhou YH. Hepatitis B vaccine development and implementation. Hum Vaccin Immunother. 2020;16:1533–44. https://doi.org/10.1080/21645515.2020.1732166.
World Health Organization. Hepatitis B vaccines: WHO position paper, July 2017—recommendations. Vaccine. 2019;37:223–5. https://doi.org/10.1016/j.vaccine.2017.07.046.
Breakwell L, Tevi-Benissan C, Childs L, Mihigo R, Tohme R. The status of hepatitis B control in the African region. Pan Afr Med J. 2017;27:17. https://doi.org/10.11604/pamj.supp.2017.27.3.11981.
World Health Organization. Hepatitis B vaccination coverage. https://immunizationdata.who.int/pages/coverage/hepb.html. 2023. Accessed 14 Nov 2023.
World Health Organization. Introduction of HepB birth dose, https://immunizationdata.who.int/pages/vaccine-intro-by-antigen/hepb_bd.html?ISO_3_CODE=&YEAR=&CODE=AFRO. 2023. Accessed 15 Nov 2023.
Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–92. https://doi.org/10.1086/314578.
Zuckerman JN. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol. 2006;78:169–77. https://doi.org/10.1002/JMV.20524.
Van Den EC, Marano C, Van AA, Bunge EM, De ML. The immunogenicity and safety of GSK’s recombinant hepatitis B vaccine in adults: a systematic review of 30 years of experience. Expert Rev Vaccines. 2017;16:811–32. https://doi.org/10.1080/14760584.2017.1338568.
Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C, et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. 2016;6:27251. https://doi.org/10.1038/srep27251.
Fan W, Chen XF, Shen C, Guo ZR, Dong C. Hepatitis B vaccine response in obesity: a meta-analysis. Vaccine. 2016;34:4835–41. https://doi.org/10.1016/J.VACCINE.2016.08.027.
Fisman DN, Agrawal D, Leder K. Effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis. 2002;35:1368–75. https://doi.org/10.1086/344271.
Sangare L, Manhart L, Zehrung D, Wang CC. Intradermal hepatitis B vaccination: a systematic review and meta-analysis. Vaccine. 2009;27:1777–86. https://doi.org/10.1016/J.VACCINE.2009.01.043.
Wood RC, MacDonald KL, White KE, Hedberg CW, Hanson M, Osterholm MT. Risk factors for lack of detectable antibody following hepatitis B vaccination of minnesota health care workers. JAMA. 1993;270:2935–9. https://doi.org/10.1001/JAMA.1993.03510240047030.
Kubba AK, Taylor P, Graneek B, Strobel S. Non-responders to hepatitis B vaccination: a review. Commun Dis Public Health. 2003;6:106–12.
Hollinger BF. Factors influencing the immune response to hepatitis B vaccine, booster dose guidelines, and vaccine protocol recommendations. Am J Med. 1989;87:36S-40S. https://doi.org/10.1016/0002-9343(89)90530-5.
Schillie SF, Spradling PR, Murphy TV. Immune Response of hepatitis B vaccine among persons with diabetes. Diabetes Care. 2012;35:2690–7. https://doi.org/10.2337/DC12-0312.
Opri R, Veneri D, Mengoli C, Zanoni G. Immune response to Hepatitis B vaccine in patients with celiac disease: a systematic review and meta-analysis. Hum Vaccin Immunother. 2015;11:2800–5. https://doi.org/10.1080/21645515.2015.1069448.
Tian Y, Hua W, Wu Y, Zhang T, Wang W, Wu H, et al. Immune response to hepatitis B virus vaccine among people living with HIV: a meta-analysis. Front Immunol. 2021;12: 745541. https://doi.org/10.3389/FIMMU.2021.745541.
Saco TV, Strauss AT, Ledford DK. Hepatitis B vaccine nonresponders: possible mechanisms and solutions. Ann Allergy Asthma Immunol. 2018;121:320–7. https://doi.org/10.1016/J.ANAI.2018.03.017.
Walayat S, Ahmed Z, Martin D, Puli S, Cashman M, Dhillon S. Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J Hepatol. 2015;7:2503–9. https://doi.org/10.4254/WJH.V7.I24.2503.
David MC, Ha SH, Paynter S, Lau C. A systematic review and meta-analysis of management options for adults who respond poorly to hepatitis B vaccination. Vaccine. 2015;33:6564–9. https://doi.org/10.1016/J.VACCINE.2015.09.051.
Di Lello FA, Martínez AP, Flichman DM. Insights into induction of the immune response by the hepatitis B vaccine. World J Gastroenterol. 2022;28:4249–62. https://doi.org/10.3748/WJG.V28.I31.4249.
Lee JH, Hong S, Im JH, Lee JS, Baek JH, Kwon HY. Systematic review and meta-analysis of immune response of double dose of hepatitis B vaccination in HIV-infected patients. Vaccine. 2020;38:3995–4000. https://doi.org/10.1016/J.VACCINE.2020.04.022.
Rahmani A, Montecucco A, Kusznir Vitturi B, Debarbieri N, Dini G, Durando P. Long-term effectiveness of hepatitis B Vaccination in the protection of healthcare students in highly developed countries: a systematic review and meta-analysis. Vaccines. 2022;10:1841. https://doi.org/10.3390/VACCINES10111841.
Mahmood S, Shah KU, Khan TM. Immune persistence after infant hepatitis-B vaccination: a systematic review and meta-Analysis. Sci Rep. 2018;8:12550. https://doi.org/10.1038/s41598-018-30512-8.
Jiang H-Y, Wang S-Y, Deng M, Li Y-C, Ling Z-X, Shao L, et al. Immune response to hepatitis B vaccination among people with inflammatory bowel diseases: a systematic review and meta-analysis. Vaccine. 2017;35:2633–41. https://doi.org/10.1016/J.VACCINE.2017.03.080.
Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine. 2013;31:2506–16. https://doi.org/10.1016/J.VACCINE.2012.12.012.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6: e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid-based Nurs. 2004;1:176–84. https://doi.org/10.1111/J.1524-475X.2004.04006.X.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. https://doi.org/10.1186/2049-3258-72-39.
Miller JJ. The inverse of the freeman-tukey double arcsine transformation. Am Stat. 1978;32:138. https://doi.org/10.1080/00031305.1978.10479283.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. https://doi.org/10.1016/0197-2456(86)90046-2.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. https://doi.org/10.1002/sim.1186.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane; 2021.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–34. https://doi.org/10.1136/bmj.315.7109.629.
Abushady EAE, Gameel MMA, Klena JD, Ahmed SF, Abdel-Wahab KSE, Fahmy SM. HBV vaccine efficacy and detection and genotyping of vaccineé asymptomatic breakthrough HBV infection in Egypt. World J Hepatol. 2011;3:147–56. https://doi.org/10.4254/WJH.V3.I6.147.
Accrombessi M, Adetola CV, Bacharou S, Dossou Y, Avokpaho E, Yakoubou A, et al. Assessment of the anti-HBs antibody response in Beninese infants following 4 doses of HBV vaccine, including administration at birth, compared to the standard 3 doses regime; a cross-sectional survey. Vaccine. 2020;38:1787–93. https://doi.org/10.1016/J.VACCINE.2019.12.031.
Anutebeh EN, Tatah L, Feteh VF, Aroke D, Assob JCN, Choukem SP. Immune response to hepatitis B vaccine following complete immunization of children attending two regional hospitals in the Southwest region of Cameroon: a cross sectional study. BMC Infect Dis. 2021;21:1205. https://doi.org/10.1186/S12879-021-06913-Y.
Apiung T, Ndanu TA, Mingle JA, Sagoe KW. Hepatitis B virus surface antigen and antibody markers in children at a major paediatric hospital after the pentavalent DTP-HBV-Hib vaccination. Ghana Med J. 2017;51:13–9. https://doi.org/10.4314/GMJ.V51I1.3.
Aspinall S, Kocks D. Immunogenicity of a lowcost hepatitis Bvaccine in the South African Expanded Programme on Immunisation. South African Med J. 1998;88:36–9.
Aspinall S, Traynor D, Bedford P, Hartmann K. Lot-to-lot consistency study of the fully liquid pentavalent DTwP-HepB-Hib vaccine Quinvaxem (®) demonstrating clinical equivalence, suitability of the vaccine as a booster and concomitant administration with measles vaccine. Hum Vaccin Immunother. 2012;8:1109–18. https://doi.org/10.4161/HV.21095.
Baroncelli S, Galluzzo CM, Liotta G, Andreotti M, Orlando S, Ciccacci F, et al. HIV-exposed infants with EBV infection have a reduced persistence of the immune response to the HBV vaccine. AIDS Res Ther. 2021;18:48. https://doi.org/10.1186/S12981-021-00375-7.
Coursaget P, Bringer L, Sarr G, Bourdil C, Fritzell B, Blondeau C, et al. Comparative immunogenicity in children of mammalian cell-derived recombinant hepatitis B vaccine and plasma-derived hepatitis B vaccine. Vaccine. 1992;10:379–82. https://doi.org/10.1016/0264-410X(92)90067-T.
El-Asheer OM, Darwish MM, Abdou MA, Saad K. Immunogenicity of recombinant hepatitis B vaccine among routinely vaccinated healthy and chronically ill children in Assiut, Upper Egypt. Gastroenterol Res. 2015;8:222–7. https://doi.org/10.14740/GR636E.
Fortuin M, Chotard J, Jack AD, Maine NP, Mendy M, Hall AJ, et al. Efficacy of hepatitis B vaccine in the Gambian expanded programme on immunisation. Lancet. 1993;341:1129–32. https://doi.org/10.1016/0140-6736(93)93137-P.
Hodgson A, Forgor AA, Chandramohan D, Reed Z, Binka F, Bevilacqua C, et al. A phase II, randomized study on an investigational DTPw-HBV/Hib-MenAC conjugate vaccine administered to infants in Northern Ghana. PLoS ONE. 2008;3: e2159. https://doi.org/10.1371/JOURNAL.PONE.0002159.
Koen A, Madhi S, Lyabis O, Vidor E, Cowper B, Marais T, et al. Immunogenicity and safety of a hexavalent pediatric vaccine in HIV-exposed infected and uninfected infants in Republic of South Africa. Hum Vaccin Immunother. 2021;17:1770–8. https://doi.org/10.1080/21645515.2020.1839289.
Madhi SA, Cutland C, Jones S, Groome M, Ortiz E. Immunogenicity and safety of an acellular pertussis, diphtheria, tetanus, inactivated poliovirus, Hib-conjugate combined vaccine (Pentaxim) and monovalent hepatitis B vaccine at 6, 10 and 14 weeks of age in infants in South Africa. S Afr Med J. 2011;101:126–31. https://doi.org/10.7196/SAMJ.4401.
Madhi SA, Mitha I, Cutland C, Groome M, Santos-Lima E. Immunogenicity and safety of an investigational fully liquid hexavalent combination vaccine versus licensed combination vaccines at 6, 10, and 14 weeks of age in healthy South African infants. Pediatr Infect Dis J. 2011;30:e68-74. https://doi.org/10.1097/INF.0B013E31820B93D2.
Magoni M, Ekra KD, Aka LN, Sita KS, Kanga K. Effectiveness of hepatitis-B vaccination in Ivory Coast: the case of the Grand Bassam health district. Ann Trop Med Parasitol. 2009;103:519–27. https://doi.org/10.1179/136485909X451816.
Mancinelli S, Pirillo MF, Liotta G, Andreotti M, Mphwere R, Amici R, et al. Antibody response to hepatitis B vaccine in HIV-exposed infants in Malawi and correlation with HBV infection acquisition. J Med Virol. 2018;90:1172–6. https://doi.org/10.1002/JMV.25049.
Mbuthia JK, Kabera BM, Karuga R, Ivui G, Mainye S, Chanzu NM, et al. A cross-sectional study to compare hepatitis B immunity in HIV-infected and HIV-uninfected Kenyan children after primary hepatitis b immunization. Pediatr Infect Dis J. 2018;37:e214–5. https://doi.org/10.1097/INF.0000000000001902.
Metodi J, Aboud S, Mpembeni R, Munubhi E. Immunity to hepatitis B vaccine in Tanzanian under-5 children. Ann Trop Paediatr. 2010;30:129–36. https://doi.org/10.1179/146532810X12703902516167.
Newton S, Owusu-Agyei S, Ampofo W, Zandoh C, Adjuik M, Adjei G, et al. Vitamin A supplementation enhances infants’ immune responses to hepatitis B vaccine but does not affect responses to Haemophilus influenzae type b vaccine. J Nutr. 2007;137:1272–7. https://doi.org/10.1093/JN/137.5.1272.
Nlend AEN, Nguwoh PS, Ngounouh CT, Tchidjou HK, Pieme CA, Otélé JM, et al. HIV-Infected or -exposed children exhibit lower immunogenicity to hepatitis B vaccine in Yaoundé, Cameroon: An appeal for revised policies in tropical settings? PLoS ONE. 2016;11: e0161714. https://doi.org/10.1371/JOURNAL.PONE.0161714.
Ouedraogo HG, Kouanda S, Tiendrebeogo S, Liestman B, Tarnagda G, Bationo F, et al. Immune and Hepatitis B virus (HBV) infection status among children receiving hepatitis B immunization in Ouagadougou, Burkina Faso. J Pediatr Infect Dis. 2013;8:167–73. https://doi.org/10.3233/JPI-130399/BIB.
Rey-Cuille MA, Seck A, Njouom R, Chartier L, Sow HD, Mamadou BA, et al. Low immune response to hepatitis B vaccine among children in Dakar Senegal. PLoS ONE. 2012;7: e38153. https://doi.org/10.1371/JOURNAL.PONE.0038153.
Salama II, Sami SM, Said ZNA, El-Sayed MH, El Etreby LA, Rabah TM, et al. Effectiveness of hepatitis B virus vaccination program in Egypt: multicenter national project. World J Hepatol. 2015;7:2418–26. https://doi.org/10.4254/WJH.V7.I22.2418.
Schoub BD, Matai U, Singh B, Blackburn NK, Levin JB. Universal immunization of infants with low doses of a low-cost, plasma-derived hepatitis B vaccine in South Africa. Bull World Health Organ. 2002;80:277–81.
Shindano TA, Mbusa RK, Kabamba BM, Fiasse R, Horsmans Y. Immunisation after hepatitis B polyvalent vaccination among children in South Kivu Province, Democratic Republic of the Congo. S Afr Med J. 2019;109:319–22. https://doi.org/10.7196/SAMJ.2019.V109I5.12819.
Simani OE, Izu A, Violari A, Cotton MF, Van Niekerk N, Adrian PV, et al. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS. 2014;28:531–41. https://doi.org/10.1097/QAD.0000000000000127.
Tsebe KV, Burnett RJ, Hlungwani NP, Sibara MM, Venter PA, Mphahlele MJ. The first five years of universal hepatitis B vaccination in South Africa: evidence for elimination of HBsAg carriage in under 5-year-olds. Vaccine. 2001;19:3919–26. https://doi.org/10.1016/S0264-410X(01)00120-7.
Valéa I, Adjei S, Usuf E, Traore O, Ansong D, Tinto H, et al. Immune response to the hepatitis B antigen in the RTS, S/AS01 malaria vaccine, and co-administration with pneumococcal conjugate and rotavirus vaccines in African children: a randomized controlled trial. Hum Vaccin Immunother. 2018;14:1489–500. https://doi.org/10.1080/21645515.2018.1442996.
Whittle HC, Inskip H, Hall AJ, Mendy M, Downes R, Hoare S. accination against hepatitis B and protection against chronic viral carriage in The Gambia. Lancet. 1991;337:747–50. https://doi.org/10.1016/0140-6736(91)91367-4.
Najafi F, Sayehmiri K, Najafi R. Efficacy of hepatitis B vaccination in under five-year-old children in Iran: a systematic review and meta-analysis study. Hepat Mon. 2018;18: e65385. https://doi.org/10.5812/HEPATMON.65385.
Le MH, Yeo YH, So S, Gane E, Cheung RC, Nguyen MH. Prevalence of hepatitis B vaccination coverage and serologic evidence of immunity among US-born children and adolescents from 1999 to 2016. JAMA Netw Open. 2020;3: e2022388. https://doi.org/10.1001/JAMANETWORKOPEN.2020.22388.
Alssamei FAA, Al-Sonboli NA, Alkumaim FA, Alsayaad NS, Al-Ahdal MS, Higazi TB, et al. Assessment of immunization to hepatitis b vaccine among children under five years in rural areas of Taiz, Yemen. Hepat Res Treat. 2017;2017:2131627. https://doi.org/10.1155/2017/2131627.
Huzly D, Schenk T, Jilg W, Neumann-Haefelin D. Comparison of nine commercially available assays for quantification of antibody response to hepatitis B virus surface antigen. J Clin Microbiol. 2008;46:1298–306. https://doi.org/10.1128/JCM.02430-07.
Madiyal M, Sagar S, Vishwanath S, Banerjee B, Kalwajeeshwara V, Chawla K. Comparing assay performance of ELISA and chemiluminescence immunoassay in detecting antibodies to hepatitis B surface antigen. J Clin Diagn Res. 2016;10:DC22–5. https://doi.org/10.7860/JCDR/2016/24108.8921.
Wanlapakorn N, Pruetarat N, Sarawanangkoor N, Phanphanit K, Srimuan D, Thatsanathorn T, et al. Immunogenicity of the pentavalent DTwP-HB-Hib vaccine (Shan-5) used in the Thai Expanded Program on Immunization compared to the hexavalent DTaP-HB-Hib-IPV and DTwP-HB-Hib (Quinvaxem) vaccines administered to infants at 2, 4, 6 months of age. Vaccine. 2023;41:3855–61. https://doi.org/10.1016/J.VACCINE.2023.05.014.
Lee LY, Chan SM, Ong C, Aw M, Wong F, Saw S, et al. Comparing monovalent and combination hepatitis B vaccine outcomes in children delivered by mothers with chronic hepatitis B. J Paediatr Child Health. 2019;55:327–32. https://doi.org/10.1111/JPC.14194.
Anderson S, Harper LM, Dionne-Odom J, Halle-Ekane G, Tita ATN. A decision analytic model for prevention of hepatitis B virus infection in Sub-Saharan Africa using birth-dose vaccination. Int J Gynaecol Obstet. 2018;141:126–32. https://doi.org/10.1002/IJGO.12434.
Solomon-Rakiep T, Olivier J, Amponsah-Dacosta E. Weak adoption and performance of hepatitis B birth-dose vaccination programs in Africa: time to consider systems complexity?—A scoping review. Trop Med Infect Dis. 2023;8:474. https://doi.org/10.3390/TROPICALMED8100474.
World Health Organization. Preventing perinatal hepatitis B virus transmission: a guide for introducing and strengthening hepatitis B birth dose vaccination. Geneva; 2015.
Boisson A, Goel V, Yotebieng M, Parr JB, Fried B, Thompson P, et al. Implementation approaches for introducing and overcoming barriers to hepatitis B birth-dose vaccine in sub-Saharan Africa. Glob Health Sci Pract. 2022;10: e2100277. https://doi.org/10.9745/GHSP-D-21-00277.
Irungu E, Mugo N, Ngure K, Njuguna R, Celum C, Farquhar C, et al. Immune response to hepatitis B virus vaccination among HIV-1 infected and uninfected adults in Kenya. J Infect Dis. 2013;207:402–10. https://doi.org/10.1093/INFDIS/JIS695.
Nie L, Hua W, Liu X, Pang X, Guo C, Zhang W, et al. Associated factors and immune response to the hepatitis B vaccine with a standard schedule: a prospective study of people with HIV in China. Vaccines. 2023;11:921. https://doi.org/10.3390/VACCINES11050921.
Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32:e00084-e118. https://doi.org/10.1128/CMR.00084-18.
Goncalves L, Albarran B, Salmen S, Borges L, Fields H, Montes H, et al. The nonresponse to hepatitis B vaccination is associated with impaired lymphocyte activation. Virology. 2004;326:20–8. https://doi.org/10.1016/j.virol.2004.04.042.
Salama II, Sami SM, Said ZN, Salama SI, Rabah TM, Abdel-Latif GA, et al. Early and long term anamnestic response to HBV booster dose among fully vaccinated Egyptian children during infancy. Vaccine. 2018;36:2005–11. https://doi.org/10.1016/J.VACCINE.2018.02.103.
Simons BC, Spradling PR, Bruden DJT, Zanis C, Case S, Choromanski TL, et al. A longitudinal hepatitis B vaccine cohort demonstrates long-lasting hepatitis B virus (HBV) cellular immunity despite loss of antibody against HBV surface antigen. J Infect Dis. 2016;214:273–80. https://doi.org/10.1093/INFDIS/JIW142.
Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, et al. Antibody levels and protection after hepatitis b vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016;214:16–22. https://doi.org/10.1093/INFDIS/JIV748.
Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53:68–75. https://doi.org/10.1093/CID/CIR270.
Whitaker JA, Rouphael NG, Edupuganti S, Lai L, Mulligan MJ. Strategies to increase responsiveness to hepatitis B vaccination in adults with HIV-1. Lancet Infect Dis. 2012;12:966–76. https://doi.org/10.1016/S1473-3099(12)70243-8.
Vargas JI, Jensen D, Martínez F, Sarmiento V, Peirano F, Acuña P, et al. Comparative efficacy of a high-dose vs standard-dose hepatitis B revaccination schedule among patients with HIV: a randomized clinical trial. JAMA Netw Open. 2021;4: e2120929. https://doi.org/10.1001/JAMANETWORKOPEN.2021.20929.
Pattyn J, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B vaccines. J Infect Dis. 2021;224:S343–51. https://doi.org/10.1093/INFDIS/JIAA668.
Koc ÖM, van Oorschot E, Brandts L, Oude LA. Timing of primary three-dose hepatitis B vaccination and postvaccination serologic testing among a large cohort of healthy adults. J Med Virol. 2022;94:4433–9. https://doi.org/10.1002/JMV.27848.
Huang H, Zhang X, Luo Y, Chen J, Feng J, Dai Y, et al. The optimal interval for post-vaccination serological test in infants born to mothers with positive hepatitis B surface antigen. Hum Vaccin Immunother. 2021;17:5585–9. https://doi.org/10.1080/21645515.2021.1992213.
Acknowledgements
None.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
BOO, IMI, DAA conceived the study. BOO, OAO, TO, and DAA conducted the data screening and abstraction. BOO and IAI conducted the analysis. BOO and DAA wrote the first draft. IAI, OAO, TO, JOO, and EEE revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was a secondary analysis of already published articles.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search Strategy for PubMed.
Additional file 2: Fig. S1.
Forest plots of the seroprotection rates after HBV vaccination among children under 5 years in Africa by (A) study region, (B) vaccine dose, (C) assay method, (D) vaccine combination, and (E) vaccine type.
Additional file 3: Fig. S2.
Meta-regression of the seroprotection rates after HBV vaccination children under 5 years in Africa against (A) sample size and (B) publication year.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olakunde, B.O., Ifeorah, I.M., Adeyinka, D.A. et al. Immune response to hepatitis B vaccine among children under 5 years in Africa: a meta-analysis. Trop Med Health 52, 28 (2024). https://doi.org/10.1186/s41182-024-00594-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-024-00594-4