Abstract
Background
Zika virus (ZIKV) is a mosquito-borne flavivirus classified in Flaviviridae family such as dengue (DENV), yellow fever, and West Nile virus. An outbreak of ZIKV infection can pose a major public health risk because the contagion is unpredictable and induces severe pathology such as Guillan-Barre syndrome and neonatal microcephaly. However, an authorized ZIKV vaccine is not yet available, while several vaccine candidates are under development.
Methods
In this study, we constructed a recombinant ZIKV vaccine (Z_EDIII) that includes ZIKV envelope protein domain III using E. coli expression system. Then both humoral and cellular immunity were examined in C57BL/6 (female, 8-weeks-old) mice via Indirect ELISA assay, PRNT, ELISpot and cytokine detection for IFN-γ, TNF-α, and IL-12. In addition, the cross protection against DENV was evaluated in pups from Z_EDIII vaccinated and infected dam.
Results
Mice immunized by Z_EDIII produced a significant amount of ZIKV EDIII-specific and neutralizing antibodies. Together with antibodies, effector cytokines, such as IFN-γ, TNF-α, and IL-12 were induced. Moreover, vaccinated females delivered the adaptive immunity to neonates who are protective against ZIKV and DENV challenge.
Conclusions
This study observed Z-EDIII-induced humoral and cellular immunity that protected hosts from both ZIKV and DENV challenges. The result suggests that our ZIKV EDIII recombinant vaccine has potential to provide a new preventive strategy against ZIKV infection.
Similar content being viewed by others
Background
Zika virus (ZIKV) is a flavivirus mediated by mosquito bite. Since the first case of ZIKV infection reported in 1947, serious clinical symptoms including Guillan-Barre syndrome and neonatal microcephaly have been reported [1,2,3]. In the past decade, ZIKV infected more than 1 million people worldwide, with Brazil among the hardest-hit countries, but neither authorized vaccines nor medicines have been available [4, 5]. Therefore, it is critical to develop a strategy for a safe and effective ZIKV vaccine. ZIKV is genetically correlated with other flaviviruses such as Dengue virus (DENV), West Nile virus (WNV), and yellow fever virus (YFV) [6,7,8]. ZIKV envelope (E) protein consists of three ectodomain structures (EDI, EDII, and EDIII) [4, 9]. The E protein facilitates viral invasion by activating receptor binding, cellular attachment, viral entry, and fusion [10,11,12]. Neutralizing antibody production is an essential procedure for ZIKV protection, and its significance has been shown in other viruses, such as YFV and Tick-borne encephalitis virus (TBEV) [13,14,15]. The EDIII-inducing neutralizing antibody has a cellular receptor-binding motif that is critical for overall host antibody response and ZIKV-specific immunity [13, 16,17,18]. Therefore, a vaccine that borrows the EDIII structure can facilitate protective immunity.
A number of ZIKV vaccines are under development in various forms, including purified-inactivated, live-attenuated, DNA, mRNA, and recombinant vaccines [19]. The recombinant subunit vaccines are based on the ZIKV protein by the expression host system that utilizes bacteria [16, 20], yeast [21], mammalian cells [22], insect cells [23], and transgenic plants [24]. Among these, E. coli expression system is the most frequently used because E. coli grows fast and has a high protein yield [25]. In this study, we developed a ZIKV recombinant subunit vaccine (Z_EDIII) that carries E. coli-expressed EDIII protein and evaluated the immune responses by Z_EDIII. Z_EDIII-immunized mice produced a sizable amount of ZIKV-specific antibody and effector cytokines such as IFN-γ, IL-12, and TNF-α. Neonates from Z_EDIII-immunized females were protected against ZIKV and Dengue virus (DENV) challenge. Therefore, we suggest that Z_EDIII is a potential ZIKV vaccine candidate.
Methods
Cells and viruses
Vero cells (KCLB, Seoul, Korea) were grown in Minimum Essential Medium (MEM)-α supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), and Vero 76 cells (ATCC, Manassas, VA, USA) were grown in Dulbecco’s Minimal Essential Medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% FBS at 37 °C in 5% CO2 until monolayers were observed. We used two ZIKV strains, PRVABC59 and MR766 which were propagated in Vero and Vero 76 cells respectively at a multiplicity of infection of 0.01. ZIKV stocks were titrated by plaque assay using two cell lines and stored at − 80 °C.
Plasmids construction and protein expression
The recombinant BL21 star (DE3) was commercially constructed (GenScript, Piscataway, NJ, USA) using the E. coli expression system. Briefly, the ZIKV EDIII protein of strain ZBRX6 (amino acid 592–745, Genbank Acc.No. AQS26809.1) was synthesized with optimized E. coli codons (GenScript, Piscataway, NJ, USA). Recombinant BL21 star (DE3) was inoculated into TB medium containing related antibiotic and cultured at 37 ℃. When the OD600 reached about 1.2, cell culture was induced with IPTG at 37 ℃ for 4 h. Cells were harvested by centrifugation. Cell pellets were resuspended with lysis buffer followed by sonication. The precipitate after centrifugation was dissolved using a denaturing agent. Z_EDIII was obtained by two-step purification using an Ni column and a Superdex 75 column. Z_EDIII was sterilized using a 0.22 μm filter before being stored in aliquots. The protein purity and molecular weight were determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis. The primary antibody for Western blot was mouse-anti-His mAb (GenScript, Piscataway, NJ, USA). The protein concentration was determined using a Bradford protein assay with bovine serum albumin (BSA) standard curve.
Mice and immunization
C57BL/6 (female, 8-weeks-old) mice were purchased from Orient Bio Inc (Gyeonggi-do, Korea). The mice were housed in a specific pathogen-free facility (the Animal Laboratory Center of Kangwon National University) under a 12-h light–dark cycle and given free access to food and water. Two groups of 8-week-old female C57BL/6 mice (n = 5 in each group) were vaccinated with 10 μg recombinant ZIKV EDIII protein (Z_EDIII) or phosphate buffered saline (PBS). The vaccine was mixed with 500 μg of aluminum hydroxide gel (Invivogen, San Diego, CA, USA) and 20 μg of monophosphoryl Lipid A (MPLA; Sigma‐Aldrich, St. Louis, MO, USA). The vaccine–adjuvant mixture was administered via the intramuscular route at 0, 14, and 42 days. At 7, 17 and 45 days post-immunization (dpi), Some groups mice were euthanized with CO2 and collected splenocytes for examination of cellular immune response. Mouse sera were collected at 0, 14, and 49 dpi and immunized females were mated with homozygous males. One-day-old neonates were inoculated subcutaneously with each ZIKV strain (MR766, PRVABC59) or PBS as a negative control. Mice were inoculated with 106.8 tissue-culture-infected doses (TCID)50/mouse for the MR766 strain and 106.3 TCID50/mouse for the PRVABC59 ZIKV strain and were monitored daily until 21 dpi to assess survival rate and weight loss. Serum was collected at 0 and 2 dpi. Mice were euthanized with CO2 at the end of the experiment. For this study, no animals were euthanized for sickness or distress. This work was carried out in compliance with the ARRIVE guidelines and approved by the Institutional Animal Care and Use Committee of Kangwon National University (Nos. KW-190131-1, KW-190515-2). All methods were carried out in accordance with relevant guidelines and regulations.
Enzyme-linked immunosorbent assay (ELISA)
The ZIKV E protein-specific antibodies in sera were detected using an ELISA kit (Alpha Diagnostic, USA) according to the manufacturer’s instructions. Briefly, 100 μl aliquots of diluted serum (1:50,000) were added per well. Z_EDIII-specific antibodies (IgG, IgG1, and IgG2c) in sera were determined by indirect ELISA as described previously [26, 27]. Microplates (Nunc-Immuno Plates; Thermo Scientific, UK) were coated with Z_EDIII (1 μg/ml) at 4 °C overnight. Each well was washed with 0.05% Tween 20 in PBS (PBST) and then with 1% BSA in PBS and incubated at 37 °C for 2 h. The plates were washed and then diluted serum was incubated at 37 °C for 2 h. Plates were washed and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG heavy and light chain antibody (1:10,000; Bethyl Laboratories, TX, USA), goat anti-mouse IgG1 (1:10,000; Bethyl Laboratories), or goat anti-mouse IgG2c (1:8000; Southern Biotech, USA) at 37 °C for 1 h and washed with PBST. Color development was performed using tetramethylbenzidine substrate (Surmodics, USA) and stopped with 2 N H2SO4. The optical density of the plates was read at 450 nm in an ELISA plate reader (BioTek, Winooski, NT, USA).
Plaque reduction neutralization test
A plaque reduction neutralization test (PRNT) was performed as described previously [27]. Vero or Vero 76 cells (1 × 105 cells/well) were seeded in 24-well plates and cultured overnight in DMEM medium containing 10% FBS. Serum samples were heat inactivated at 56 °C for 30 min and serially diluted in DMEM. ZIKV and DENV were diluted to 2 × 102 plaque-forming units (PFU)/ml in DMEM, mixed with an equal volume of diluted serum and incubated at 37 °C for 30 min. Then, Vero or Vero 76 cells were incubated with the serum mixture at 37 °C for 2 h. Cells were then washed and cultured at 37 °C for 5 or 14 days in DMEM containing 1% sea plaque agar or 1.4% methyl cellulose. Cells were fixed in 4% paraformaldehyde and stained with 0.1% crystal violet dye. The percentage inhibition of virus infectivity was calculated by counting the number of plaques in immune sera.
Measurement of cytokine and T cell phenotype
The IFN-γ-secreting splenocytes were measured at 7, 17, and 45 dpi using a mouse IFN-γ enzyme-linked immune absorbent spot (ELISpot) assay (BD Life Sciences, USA). Briefly, 5 × 105 splenocytes/well were stimulated with Z_EDIII (1 μg/ml). Spots were counted under a dissecting microscope (Olympus, model no. SZH-ILLB). To measure secreted cytokines, splenocytes (5 × 105/well) were stimulated with Z_EDIII (1 μg/ml) for 48 h and the supernatant of splenocytes was collected. The levels of cytokines were determined using mouse tumor necrosis factor (TNF)-α ELISA MAX™ standard set, mouse IFN-γ ELISA MAX™ standard set, and mouse interleukin (IL)-12 ELISA MAX™ standard set (BioLegend, USA). Each assay was performed in triplicate. To analyze T lymphocyte subtypes, splenocytes were stained with the following antibodies at a dilution of 1:200 with 1% BSA in PBS at 4 °C for 1 h: anti-mouse CD3 (clone 145-2C11, BD Biosciences), and anti-mouse CD4 (clone RM4-5, BD Biosciences) or anti-mouse CD8 (clone 53–6.7, BD Biosciences) as described previously [27]. Cell phenotype was analyzed using a FACS Calibur flow cytometer (Becton Dickinson).
Tissue culture infective dose50 (TCID50)
The TCID50 assay was performed as described previously [27]. Vero or Vero 76 cells were seeded in 96-well plates (2 × 104 cells/well) and cultured overnight in DMEM medium containing 10% FBS. Serum samples were heat inactivated at 56 °C for 30 min and serially diluted (101–103) in PBS. The serum samples were used to infect Vero or Vero 76 cells at 37 °C for 1 h. Cells were then washed and cultured at 37 °C for 14 days in complete DMEM. TCID50 was calculated using the end-point method, and virus infectivity was determined using the Karber method.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (v. 5.0; GraphPad Software, La Jolla, CA, USA) using one-way analysis of variance with Tukey’s multiple comparisons test to compare groups. P values < 0.05 were considered significant.
Results
Expression of recombinant ZIKV envelope protein domain III
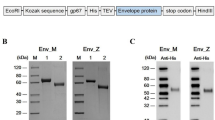
Previous studies have shown that EDIII is a major target of ZIKV-specific antibodies [13, 16] and we constructed a recombinant subunit vaccine that includes the EDIII protein. The coding sequence of the EDIII protein (amino acid 592–745, Genbank Acc.No. AQS26809.1) in a Brazilian ZIKV strain (ZBRX6) was inserted into an expression vector (pET28a) and cloned in E. coli cells (Fig. 1a). Viral protein was purified using an Ni column and a Superdex75 column and the expression of EDIII (18 kDa) protein was confirmed by SDS-PAGE and Western blotting (Fig. 1b and c). The recombinant vaccine is termed Z_EDIII.
Construction of Z_EDIII. a Schematic of constructs expressing Z_EDIII (TEV: TEV protease site). b, c Purified Z_EDIII proteins were confirmed by SDS-PAGE b and Western blot c. b Lane M: Protein Marker (GenScript, Cat. No. M00516). Lane 1: BSA (2 μg). Lane 2: Target protein (Z_EDIII 2 μg). c The primary antibodies used anti-His antibody (Mouse-anti-His mAb, GenScript, Cat. No. A00186)
Z_EDIII-induced humoral immune response
During ZIKV infection, IgG for envelope glycoprotein (E) is produced [6]. The major target of human monoclonal antibodies is EDIII, but other transmembrane proteins (EDI and EDII) are also covered by antibodies [28,29,30,31,32,33]. Moreover, previous studies suggest that EDIII provides epitopes that are targeted by both B cell repertoire and multiple fractions in polyclonal immune sera [8, 29, 31, 33, 34]. To examine whether our Z_EDIII protein induces ZIKV envelope protein domain III-specific IgG antibodies, we performed an ELISA assay. Compared with PBS-injected control, Z_EDIII immunization significantly increased Z_EDIII-specific IgG in immunocompetent mice 4 weeks after immunization (Fig. 2a and b). Z_EDIII immunization in immunocompetent mice 7-weeks after immunization boosted the pathogen-specific antibody production by 1.7 times. This indicates that our Z_EDIII immunization induces sufficient amount of Ag-specific antibodies.
ZIKV envelope protein domain III-specific antibody responses elicited by Z_EDIII. a Experimental strategies. C57BL/6 mice (n = 5 per group) were immunized with Z_EDIII or PBS at 0, 14, and 42 dpi. For Ag-specific IgG antibodies, blood samples were collected at 0, 28, and 49 dpi. b Serum samples were diluted (1:50,000) and OD 450 values were measured using ZIKV envelope protein domain III-coated ELISA kit. The ZIKV envelope protein domain III-specific IgG antibody activity units were calculated. c Serum samples were diluted (1:100) and OD 450 values were measured. d IgG2c-to-IgG1 ratio in Z_EDIII- or PBS-immunized mice is shown. Data show the mean ± SD of five mice per group. Each sample was assayed in duplicate. Statistical analysis *p < 0.05, **p < 0.01, ***p < 0.001
By Ag-specific detection and a plaque reduction neutralization test, we evaluated the neutralizing capacity by Z_EDIII immunization against two different ZIKV strains: MR766 and PRVABC59. The neutralizing antibody was detected from serum in 7-week-old mice after Z_EDIII immunization but not from PBS-injected control (Table 1). We further analyzed Ag-specific IgG1 and IgG2c from Z_EDIII immunized mice and evaluated the type of immune responses. Both IgG subtypes were promoted by Z_EDIII immunization; the level of IgG2 was lower than that of IgG1 (Fig. 2c and d). Accordingly, we speculate that Z_EDIII is a good immune stimulator, and the administration is more likely to induce Th2 than Th1 response.
Z_EDIII-induced cellular immune response
To evaluate vaccination-induced cellular immunity, we examined the effect of Z_EDIII doses on T cell phenotype (Fig. 3a). Total splenocytes were stained with antibodies to CD4 and CD8 molecules. Single and double doses of Z_EDIII did not change the frequencies of CD4+ and CD8+ T cells (Fig. 3b–g). However, triple immunizations increased the CD4+ T cell but slightly suppressed the CD8+ T cell population. Additionally, more CD4+ T cells were observed than CD8+ T cells in the spleen after triple immunizations (Fig. 3h–j). Next, we evaluated cellular immunity function by examining effector cytokines from immunized animals. IFN-γ and TNF-α are pivotal cytokines that are produced by CD4+ and CD8+ T cells after ZIKV antigen stimulation [35]. When we quantified IFN-γ-secreting cells by ELISpot assay, a single dose increased cytokine production by 12 times (Fig. 4a). Double and triple doses also promoted IFN-γ-producing cells but not by as many as a single dose (Fig. 4b, c).
CD4+ and CD8+ T cells population in immunized mice. a Experimental procedure for cellular immune response. C57BL/6 mice (n = 5) were immunized with Z_EDIII or PBS at 0, 14, and 42 dpi. Mice were euthanized at 7, 17, and 45 dpi (7 dpi; b–d, 17 dpi; e–g, 45 dpi; h–j). Total splenocytes were collected from five mice in each group and stained with antibodies to CD3, CD4, and CD8 molecule
Number of IFN-γ-secreting splenocytes in immunized mice. C57BL/6 mice (n = 5) were immunized with Z_EDIII or PBS at 0, 14, and 42 dpi. Mice were euthanized 7, 17, and 45 dpi (7 dpi; a, 17 dpi; b, 45 dpi; c). Splenocytes were stimulated with Z_EDIII (10 μg/ml) or PBS. Ag-specific IFN-γ response was identified by ELISPOT assay at 48 h after stimulation. Data show mean ± SD of five mice per group. Each sample was assayed in triplicate. Splenocytes were pooled from five mice in each group. Statistical analysis *p < 0.05, **p < 0.01, ***p < 0.001
Effector cytokine production by Z_EDIII was also investigated by ELISA assay. Levels of IFN-γ and TNF-α were identified and we additionally examined IL-12 level, which is originated antigen presenting cells and activates Th1 differentiation [36, 37]. Like the ELISpot assay, splenocytes from single-dosed mice secreted a high level of cytokines when cells were restimulated with ZIKV antigen (Fig. 5a–c). Double doses also promoted cytokine secretion, but the amount was lower compared with that induced by a single dose (Fig. 5d–f). On the other hand, triple doses completely blocked cytokine secretion (data not shown). The results suggest that Z_EDIII immunization is an effective inducer for effector cytokines, though multiple administrations make immune cells exhausted.
Cytokine production elicited by Z_EDIII immunization. C57BL/6 mice (n = 5) were immunized with Z_EDIII or PBS at 0 and 14 dpi. Mice were euthanized at 7 and 17 dpi (7 dpi; a–c, 17 dpi; d–f). Splenocytes were pooled from five mice in each group and stimulated with Z_EDIII (10 μg/ml) or PBS for 48 h. Concentrations of IFN-γ, TNF-α and IL-12 from the supernatant by ELISA. Data show the mean ± SD of five mice per group. Each sample was assayed in triplicates. Statistical analysis *p < 0.05, **p < 0.01, ***p < 0.001
Immunization with Z_EDIII protects against ZIKV or DENV challenge in immunocompetent neonatal mice
Controlling ZIKV spread during gestation is critical because the viral transmission across placenta induces high risk of intrauterine growth restriction (IUGR), spontaneous miscarriage, and microcephaly [38]. Accordingly, the impact of trans-generational immune priming by Z_EDIII was evaluated. Circulating Z_EDIII-specific IgG level was measured in neonates from triple-dosed parents. As a result, the pups showed a significantly increased level of Ag-specific IgG compared with the PBS-administered group (Fig. 6).
The level of Z-EDIII specific antibody after immunization. C57BL/6 mice (n = 5 per group) were immunized with Z_EDIII or PBS at 0, 14, and 42 dpi. Immunized mice were mated with homozygous breeding pairs. To measure Z_EDIII-specific IgG antibodies, blood samples were collected at day 2. Serum samples were serially diluted from 1:100 to 1:3,200 and the antibody level was determined by ELISA. Data show the mean ± SD of five mice per group. Each sample was assayed in duplicate. Statistical analysis *p < 0.05, **p < 0.01, ***p < 0.001
Next, we evaluated the protective effect of Z_EDIII against two ZIKV strains, MR766 and PRVABC59. Pups from immunized parents were challenged with ZIKV strains at a dose of 106.8, 106.3 TCID50/mouse and the viral antigenicity was observed in serum at 48 h post-infection. In addition, the survival rates were examined. Despite the level of MR766 in circulation being attenuated by immunization, infected animals failed to survive longer than 10 days. On the other hand, PRVACB59 was not detected when Z_EDIII was administrated and about 60% of infected pups survived (Table 2, Fig. 7a–f). This demonstrates that the Z_EDIII vaccination works as a potential blocker for viral spread.
Clinical results in neonates against ZIKV and DENV challenge. Body weight changes and survival rates were observed. C57BL/6 mice (n = 5 per group) were immunized with Z_EDIII or PBS at 0, 14, and 42 dpi. Immunized females were mated with homozygous breeding pairs. The survival rate and body weight changes in neonatal mice (n = 5 per group) were observed. Two-day-old neonatal mice were inoculated with PBS (a, b), MR766 (c, d), PRVABC59 (e, f), or DENV-2 (g, h) strains. Data show the mean ± SD of five mice per group. Statistical analysis *p < 0.05, **p < 0.01, ***p < 0.001
Moreover, we found that Z_EDIII provides cross-protection immunity against a different flavivirus, DENV-type 2. The level of neutralizing antibody against DENV induced by triple vaccination was 20, and the result was comparable against MR766 and PRVABC59 strains (Table 1). The viral detections in neonates from Z_EDIII-immunized and DENV-infected females were lower than in the non-immunized group. The immunization was also beneficial for clinical symptoms and survival. When females were vaccinated with Z_EDIII, their pups were resistant to DENV challenge (Fig. 7g and h).
Discussion
ZIKV infection can have serious medical consequences, such as Guillain–Barre syndrome, fetal microcephaly, and malformation. Thus, various ZIKV vaccine forms, including purified-inactivated, live-attenuated, and recombinant vaccines have been under development and some of them are under pre-clinical trials [19]. However, no authorized vaccine is available yet, and indeed the current vaccine forms do not fully assure safety and reliability. For example, a subunit vaccine composed of protein fragments can induce toxic immune responses and the pre-existing immunity to viral vector can suppress vaccine efficacy [39]. Moreover, potential tumorigenesis elicited by the insertion of a gene into a DNA or mRNA vaccine [40, 41], as well as unexpected immune response by viral-vectored vaccines [24, 42], can be risk factors. Lately, subunit vaccines utilizing ZIKV EDIII region to provide safety and efficacy has being developed and they protected hosts from ZIKV challenge [16, 21, 43]. An EDIII-encoding subunit vaccine using the E. coli expression system is considered as a potent inducer for host immune response with the least side effects [34, 44]. Thus, we evaluated immunogenicity by Z_EDIII immunization and immune responses to ZIKV and DENV infections in immunocompetent mice and their offspring.
The EDIII domain in ZIKV includes a cellular receptor-binding motif and is a potent stimulator of adaptive immunity that stimulates production of neutralizing antibodies and cytokines during infection [13, 16,17,18,19]. Z_EDIII immunization promoted humoral response: antigen-specific antibody secretion. We found that Z_EDIII induced protected immunity with a 20-fold increase in neutralizing antibody production when mice were challenged with ZIKV strains (MR766 and PRVABC). Considering that the authorized flavivirus vaccines are to have at least a tenfold boosting effect [39, 45], our vaccine provided partial protective immunity. The lower IgG2c-to-IgG1 ratio by Z_EDIII administration suggests that the cellular immunity is biased to Th2 response. Adjuvants in ZIKV subunit vaccines support neutralizing antibody production [26]. This study used two adjuvants, MPLA and Alum. MPLA is a toll-like receptor 4 (TLR4) inducer that induces a type I response. In contrast, Alum tends to stimulate Th2-oriented response; an established Th2 orientation is not switching to Th1 response [46]. This leads us to assume that repeated Z_EDIII immunizations with Alum adjuvant provides a Th2-dominant condition.
Both vaginal and subcutaneous ZIKV infections induce virus-specific T cell proliferation and the adoptive transfer of the T cells suppresses viral titers during infection. Structural proteins of ZIKV such as E, prM, and C are a major target of CD4+ and CD8+ T cells [19, 47, 48]. IFN-γ production in T cells is critical in protective immunity during ZIKV infection [39, 47, 49,50,51]. The CD4+ T cell population was the largest by triple immunization and we identified IFN-γ-producing cells. The numbers of such cells was the highest in single-dosed animals and the cell numbers declined as the vaccination was repeated. Production of effector cytokine such as IFN-γ, IL-12, and TNF-α was also stimulated the most by a single dose and the level went down with additional immunization. This is associated with the IgG2c-to-IgG1 ratio; multiple doses induce a Th2-dominant immune response.
Congenital ZIKV transmission is fatal to neonates, and thus a ZIKV vaccine needs to provide protection in fetal and neonatal stages [27, 52, 53]. We immunized females with the Z_EDIII vaccine and examined protection in their neonates. We immunized females with the Z_EDIII vaccine and examined protection against an African (MR766) and ab Asian (PRVABC56) ZIKV strains in neonates [54]. During the infection, antigen specific antibodies were detected in pups and they showed less viremia and increased survival rates. However, the protection was not complete in both African and Asian strain challenged groups. Among 3424 amino acids of ZIKV ORF, 75 to100 amino acid residues showed dissimilarity between the two strains [55]. We speculate that further study is needed to dissect how the genotypes of ZIKV strains regulates immunopathogenesis.
Also, the Z_EDIII induced cross-protection against DENV. The cross-reactivity can induce antibody-dependent enhancement (ADE). This is mediated by the binding of Fcγ receptors and IgG and the structural homology of E protein between ZIKV and other flaviviruses [51, 56, 57]. This similarity provided neutralizing activity for DENV and a protected infected host, but further studies should be conducted to determine whether Z_EDIII induces ADE and protection is acquired from ZIKV-specific neutralizing antibodies.
Conclusions
This study observed Z_EDIII-mediated humoral immunity that induced antigen-specific IgG and neutralizing antibodies and cellular immunity that promoted effector cytokine production such as IFN-γ, TNF-α, and IL-12. While there was partial protection against ZIKV Asian strain, vaccination induced neutralizing antibodies to another flavivirus, DENV, further manipulations and improvements on the Z_EDIII vaccine platform would be needed to develop a pan-flavivirus vaccine.
Availability of data and materials
All the data generated during the current study are included in the manuscript.
References
Dick GW, Kitchen SF, Haddow AJ. Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–20.
Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165(5):1081–91.
Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–8.
Lazear HM, Diamond MS. Zika virus: new clinical syndromes and its emergence in the Western Hemisphere. J Virol. 2016;90(10):4864–75.
Lessler J, Chaisson LH, Kucirka LM, Bi Q, Grantz K, Salje H, et al. Assessing the global threat from Zika virus. Science. 2016;353(6300):aaf8160.
Slon Campos JL, Mongkolsapaya J, Screaton GR. The immune response against flaviviruses. Nat Immunol. 2018;19(11):1189–98.
Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet. 2017;390(10107):2099–109.
Gallichotte EN, Young EF, Baric TJ, Yount BL, Metz SW, Begley MC, et al. Role of Zika virus envelope protein domain III as a target of human neutralizing antibodies. MBio. 2019;10(5):e01485-e1519.
Dai L, Song J, Lu X, Deng Y-Q, Musyoki AM, Cheng H, et al. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe. 2016;19(5):696–704.
Pierson TC, Diamond MS. Flaviviruses. Fields Virology: Sixth Edition: Wolters Kluwer Health Adis (ESP); 2013.
Fontes-Garfias CR, Shan C, Luo H, Muruato AE, Medeiros DBA, Mays E, et al. Functional analysis of glycosylation of Zika virus envelope protein. Cell Rep. 2017;21(5):1180–90.
Heinz FX, Stiasny K. Flaviviruses and their antigenic structure. J Clin Virol. 2012;55(4):289–95.
Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, et al. Structural basis of Zika virus-specific antibody protection. Cell. 2016;166(4):1016–27.
Belmusto-Worn VE, Sanchez JL, McCarthy K, Nichols R, Baustista CT, Magill AJ, et al. Randomized, double-blind, phase III, pivotal field trial of the comparative immunogenicity, safety, and tolerability of two yellow fever 17D vaccines (Arilvax and YF-VAX) in healthy infants and children in Peru. Am J Trop Med Hyg. 2005;72(2):189–97.
Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine. 2007;25(43):7559–67.
Yang M, Dent M, Lai H, Sun H, Chen Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vaccine. 2017;35(33):4287–94.
Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11(5):522–30.
Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, et al. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J Virol. 2010;84(18):9227–39.
Poland GA, Ovsyannikova IG, Kennedy RB. Zika vaccine development: current status. Mayo Clin Proc. 2019;94(12):2572–86.
Liang H, Yang R, Liu Z, Li M, Liu H, Jin X. Recombinant Zika virus envelope protein elicited protective immunity against Zika virus in immunocompetent mice. PLoS ONE. 2018;13(3): e0194860.
Zhang W, Qu P, Li D, Zhang C, Liu Q, Zou G, et al. Yeast-produced subunit protein vaccine elicits broadly neutralizing antibodies that protect mice against Zika virus lethal infection. Antiviral Res. 2019;170: 104578.
Roldán JS, Cassola A, Castillo DS. Optimization of recombinant Zika virus NS1 protein secretion from HEK293 cells. Biotechnol Rep. 2020;25: e00434.
Dai S, Zhang T, Zhang Y, Wang H, Deng F. Zika virus baculovirus-expressed virus-like particles induce neutralizing antibodies in mice. Virol Sin. 2018;33(3):213–26.
Yang M, Sun H, Lai H, Hurtado J, Chen Q. Plant-produced Zika virus envelope protein elicits neutralizing immune responses that correlate with protective immunity against Zika virus in mice. Plant Biotechnol J. 2018;16(2):572–80.
Tripathi NK, Shrivastava A. Recent developments in recombinant protein–based dengue vaccines. Front Immunol. 2018;9:1919.
Cibulski S, Varela APM, Teixeira TF, Cancela MP, Sesterheim P, Souza DO, et al. Zika virus envelope domain III recombinant protein delivered with saponin-based nanoadjuvant from Quillaja brasiliensis enhances anti-Zika immune responses, including neutralizing antibodies and splenocyte proliferation. Front Immunol. 2021;12: 632714.
Shin M, Kim K, Lee H-J, Lee R, Jung Y-J, Park J, et al. Zika virus baculovirus-expressed envelope protein elicited humoral and cellular immunity in immunocompetent mice. Sci Rep. 2022;12(1):660.
Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353(6301):823–6.
Robbiani DF, Bozzacco L, Keeffe JR, Khouri R, Olsen PC, Gazumyan A, et al. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell. 2017;169(4):597–609.
Rogers TF, Goodwin EC, Briney B, Sok D, Beutler N, Strubel A, et al. Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors. Sci Immunol. 2017;2(14):eaan6809.
Sapparapu G, Fernandez E, Kose N, Cao B, Fox JM, Bombardi RG, et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016;540(7633):443–7.
Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, et al. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci Transl Med. 2016;8(369):369ra179.
Yu L, Wang R, Gao F, Li M, Liu J, Wang J, et al. Delineating antibody recognition against Zika virus during natural infection. JCI insight. 2017;2(12): e93042.
Premkumar L, Collins M, Graham S, Liou GA, Lopez CA, Jadi R, et al. Development of envelope protein antigens to serologically differentiate Zika virus infection from dengue virus infection. J Clin Microbiol. 2018;56(3):e01504-e1517.
Pantoja P, Pérez-Guzmán EX, Rodríguez IV, White LJ, González O, Serrano C, et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun. 2017;8:15674.
Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76.
Dinarello CA. Proinflammatory cytokines. Chest. 2000;118(2):503–8.
Mysorekar IU. Zika virus takes a transplacental route to infect fetuses: insights from an animal model. Mo Med. 2017;114(3):168–70.
Pattnaik A, Sahoo BR, Pattnaik AK. Current status of Zika virus vaccines: successes and challenges. Vaccines. 2020;8(2):266.
Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543(7644):248–51.
Luo D, Miao Y, Ke X, Tan Z, Hu C, Li P, et al. Baculovirus surface display of Zika virus envelope protein protects against virus challenge in mouse model. Virol Sin. 2020;35(5):637–50.
Moyle PM, Toth I. Modern subunit vaccines: development, components, and research opportunities. ChemMedChem. 2013;8(3):360–76.
Tai W, He L, Wang Y, Sun S, Zhao G, Luo C, et al. Critical neutralizing fragment of Zika virus EDIII elicits cross-neutralization and protection against divergent Zika viruses. Emerg Microbes Infect. 2018;7(1):7.
Araujo SC, Pereira LR, Alves RPS, Andreata-Santos R, Kanno AI, Ferreira LCS, et al. Anti-flavivirus vaccines: review of the present situation and perspectives of subunit vaccines produced in Escherichia coli. Vaccines. 2020;8(3):492.
Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65.
Cao H, Yang S, Wang Y, Luan N, Yin X, Lin K, et al. An established Th2-oriented response to an alum-adjuvanted SARS-CoV-2 subunit vaccine is not reversible by sequential immunization with nucleic acid-adjuvanted Th1-oriented subunit vaccines. Vaccines. 2021;9(11):1261.
Scott JM, Lebratti TJ, Richner JM, Jiang X, Fernandez E, Zhao H, et al. Cellular and humoral immunity protect against vaginal Zika virus infection in mice. J Virol. 2018;92(7):e00038-e118.
Grifoni A, Pham J, Sidney J, O’rourke PH, Paul S, Peters B, et al. Prior Dengue virus exposure shapes T cell immunity to Zika virus in humans. J Virol. 2017;91(24):e01469-e1517.
Pardy RD, Rajah MM, Condotta SA, Taylor NG, Sagan SM, Richer MJ. Analysis of the T cell response to Zika virus and identification of a novel CD8+ T cell epitope in immunocompetent mice. PLoS Pathog. 2017;13(2): e1006184.
Lucas CGO, Kitoko JZ, Ferreira FM, Suzart VG, Papa MP, Coelho SVA, et al. Critical role of CD4(+) T cells and IFNγ signaling in antibody-mediated resistance to Zika virus infection. Nat Commun. 2018;9(1):3136.
Grubor-Bauk B, Wijesundara DK, Masavuli M, Abbink P, Peterson RL, Prow NA, et al. NS1 DNA vaccination protects against Zika infection through T cell–mediated immunity in immunocompetent mice. Sci Adv. 2019;5(12):eaax2388.
Rathore APS, Saron WAA, Lim T, Jahan N, St John AL. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci Adv. 2019;5(2):eaav3208.
Kurup D, Wirblich C, Schnell MJ. Measles-based Zika vaccine induces long-term immunity and requires NS1 antibodies to protect the female reproductive tract in the hCD46 IFNα/β receptor knockout mice. bioRxiv. 2020;29:290.
Shin M, Kim J, Park J, Hahn TW. Clinical profile of Asian and African strains of Zika virus in immunocompetent mice. Korean J Vet Res. 2021;61(2):1–9.
Smith DR, Sprague TR, Hollidge BS, Valdez SM, Padilla SL, Bellanca SA, et al. African and Asian Zika virus isolates display phenotypic differences both in vitro and in vivo. Am J Trop Med Hyg. 2018;98(2):432–44.
Paul LM, Carlin ER, Jenkins MM, Tan AL, Barcellona CM, Nicholson CO, et al. Dengue virus antibodies enhance Zika virus infection. Clin Transl Immunol. 2016;5(12): e117.
Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, et al. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016;352(6284):467–70.
Acknowledgements
This research was supported by funding from the Korea Centers for Disease Control and Prevention (2016-ER4203-02). We also would like to thank Eunjin Hong for providing technical assistance.
Funding
This research was supported by funding from the Korea Centers for Disease Control and Prevention (2016-ER4203-02).
Author information
Authors and Affiliations
Contributions
MS: Conceptualization, data curation, formal analysis, methodology, writing—original draft. KK, HL, YJ: Data curation, formal analysis, methodology. JP: Formal analysis, writing—reviewing and editing. TH: Conceptualization, methodology, project administration, writing—reviewing and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Animal Care and Use Committee of Kangwon National University (Nos. KW-190131-1, KW-190515-2).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shin, M., Kim, K., Lee, HJ. et al. Vaccination with a Zika virus envelope domain III protein induces neutralizing antibodies and partial protection against Asian genotype in immunocompetent mice. Trop Med Health 50, 91 (2022). https://doi.org/10.1186/s41182-022-00485-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-022-00485-6