Abstract
Background
Pericardial effusion is a late manifestation of HIV more commonly observed in individuals with depressed CD4 counts. Although Mycobacterium tuberculosis remains to be one of the most frequently identified pathogens in the pericardial fluid among people living with HIV, less commonly described etiologies include SARS-CoV-2 that causes coronavirus disease and human herpesvirus-8 which is associated with Kaposi sarcoma. Isolation of more than one pathogen in normally sterile sites remains challenging and rare. We report the first documentation of both SARS-CoV-2 and HHV-8 in the pericardial fluid.
Case presentation
We present the case of a young man in his 20s with a recent history of clinically diagnosed pulmonary tuberculosis who was admitted for progressive dyspnea and cough. He had multiple violaceous cutaneous lesions on the face, neck, and trunk and diffused lymphadenopathies. He tested positive for SARS-CoV-2 on admission. The patient was clinically diagnosed with pneumonia, Kaposi sarcoma, and HIV/AIDS. Empiric broad spectrum antimicrobial regimen was subsequently initiated. HIV with low CD4 count was confirmed during hospitalization. Echocardiography revealed a large pericardial effusion, in impending cardiac tamponade. Frond-like fibrin strands, extending to the parietal pericardium, were also observed. Pericardiostomy yielded hemorrhagic, exudative effusion with lymphocytic predominance. SARS-CoV-2 and HHV-8 were detected in the pericardial fluid, and bacterial, fungal, and tuberculous studies were negative. The patient had clinical improvement after pericardial drainage. However, despite our best clinical care, he developed a nosocomial infection leading to clinical deterioration and death.
Conclusion
Detection of SARS-CoV-2 and HHV-8 in the pericardial fluid is rare, and interpretation of their significance in clinical care is challenging. However, coronavirus disease and Kaposi sarcoma must be considered and adequately addressed in immunocompromised adults presenting with large pericardial effusion.
Similar content being viewed by others
Background
Pericardial effusion was a common manifestation among patients with HIV in the pre-antiretroviral therapy (ART) era [1]. In countries with widespread access to ART, the incidence of symptomatic pericardial disease has decreased dramatically [2]. However, it remains a significant problem in resource-limited settings and among patients with depressed CD4 and/or Acquired Immune Deficiency Syndrome (AIDS)-defining conditions.
Although M. tuberculosis is a highly prevalent cause of pericardial effusion in HIV and tuberculosis (TB)-endemic settings [3, 4], diverse etiologies have been reported including bacteria, fungi, and viruses [5,6,7,8]. HIV-associated lymphomas like Burkitt lymphoma and primary effusion lymphoma also result in pericardial effusion and tumors [9]. Majority of these malignancies are associated with Epstein–Barr virus (EBV) infection [10]. AIDS-related Kaposi sarcoma (KS), caused by Human Herpesvirus-8 (HHV-8), has been shown to involve the pericardium during autopsies. Recent reports suggest that SARS-CoV-2 also causes pericardial effusion presenting with myopericarditis, Takotsubo cardiomyopathy, and acute respiratory distress syndrome. However, COVID-19-associated pericardial effusion in an HIV-positive patient has not been reported to the best of our knowledge [11]. In this report, we present the first documentation of SARS-CoV-2 and HHV-8 detection in the pericardial fluid of a young HIV-positive patient.
Case presentation
A male patient in his 20s was admitted to our hospital with a 2-week history of progressive dyspnea and cough. Five months prior to admission, he experienced non-productive cough accompanied by weight loss, night sweats, fever, and enlarged neck lymph nodes. He was clinically diagnosed with pulmonary TB. Intensive-phase therapy, comprising isoniazid–rifampicin–pyrazinamide–ethambutol combination, was initiated. He was adherent to treatment, and his symptoms improved after 2 months. Two-drug maintenance phase therapy was subsequently initiated. Two months prior to admission, the patient noticed multiple violaceous patches over the face, neck, and anterior chest. Eleven days prior to admission, he visited the outpatient department due to occurrence of cough, dyspnea, and fever. The patient was advised to undergo COVID-19 testing, but he refused due to fear of isolation. Worsening symptoms over the next week prompted emergency room (ER) visit. Further medical interview revealed history of unprotected sexual intercourse with multiple male partners beginning age 13. He was unvaccinated against COVID-19. The rest of the medical, social, and family history was unremarkable.
At the ER, the patient was normotensive (110/70 mmHg), tachycardic (108 beats/min), tachypneic (28 cycles/minute), afebrile, and with oxygen saturation of 99% on room air. He was underweight, with a BMI of 18 kg/m2. Physical examination revealed well-defined, variably sized, violaceous, non-tender patches, plaques, and nodules on the face, neck, and trunk, measuring 3 cm at most. There was a well-defined, purplish mass with overlying ulceration on the right lower lip extending to the gingiva, with discrete purplish nodules on the lower alveolar ridge (Fig. 1). Coating patches of white slough were present on the mouth and oropharynx. Multiple matted, non-tender bilateral lymphadenopathies were palpable on the neck. There was no neck vein distention. Auscultation revealed crackles in the bilateral lung fields, adynamic precordium, and distinct heart sounds. Multiple non-tender inguinal lymphadenopathies with maximum size of 1 cm were palpable. The rest of the examination was within normal limits.
Due to the history of cough and dyspnea, the high-risk sexual practices, the presence of violaceous patches and nodules, the white slough in the oropharynx, and multiple lymphadenopathies, the primary impression was pneumonia in the immunocompromised host (COVID-19, Pneumocystis jirovecii, bacterial, tuberculosis), to consider HIV/AIDS with Kaposi sarcoma and candidiasis. The following medications were given empirically on admission: remdesivir, cotrimoxazole, ceftazidime, azithromycin, rifampicin, isoniazid, fluconazole, and prednisolone.
Investigations and differential diagnoses
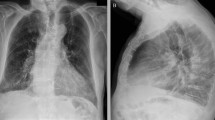
SARS-CoV-2 real-time reverse transcription polymerase chain reaction (RT-PCR) was positive on admission. The chest radiograph showed bilateral fibroreticular opacities, more pronounced on the right upper and middle lung fields. This finding was consistent with pulmonary TB (Fig. 2). Cardiac silhouette was visibly wider on admission radiograph compared to outpatient radiograph taken 5 months prior. Blood tests revealed slightly elevated C-reactive protein (18.71 µg/mL, normal value: < 10 µg/mL), normal procalcitonin (0.05 ng/mL, normal value: < 0.5 ng/mL), and normal lactate dehydrogenase (LDH, 391 U/L, normal range: 313–618 U/L). Kidney and liver function and cardiac troponins were within normal limits. Sputum acid-fast bacilli smear and GeneXpert® MTB/RIF assay were negative. Sputum bacterial culture later showed normal oral flora. Consequently, the empiric antibiotic therapy for bacterial pneumonia was discontinued.
HIV antibody/antigen test was positive, and confirmatory Western blot test was also positive. CD4 count was low (70 cells/µL), and HIV viral load was 784,000 copies/mL. Hepatitis B surface antigen was positive, and the remaining screening tests for sexually transmitted infections were negative.
Contrast chest and abdominal computed tomography (CT) scan revealed multiple nodules, air bronchograms, and surrounding ground-glass opacities in both lungs; prominent axillary lymph nodes; moderate pericardial effusion; and prominent mesenteric and peripancreatic lymph nodes, measuring 8 mm maximum.
Echocardiography showed large pericardial effusion, mild septal shift, and concentric left ventricular hypertrophy with preserved systolic function (Fig. 3). Frond-like fibrin strands were seen extending to the parietal pericardium.
Due to limited local available of more current anti-retroviral therapy regimen, lamivudine, tenofovir, and efavirenz single pill combination regimen was initiated on day 6. Moreover, dolutegravir-based regimen was not initiated due to potential enzyme-inducing effects of rifampicin, leading to reduced dolutegravir exposure [12]. In such cases, dosing may be increased, but single pill dolutegravir was not available locally.
Diagnostic and therapeutic pericardial drainage and tissue biopsy were contemplated, and transfer to a hospital with adequate facilities for post-cardiovascular surgical care was planned. However, logistic challenges associated with the patient’s COVID-19-positive status prevented the medical team from performing these interventions.
On days 9 to 18, the patient complained of worsening chest heaviness and dyspnea. There was no hypotension nor desaturation, and heart sounds were distinct on auscultation. Serial chest radiograph showed further increase in the size of the cardiac silhouette. He underwent emergency diagnostic and therapeutic pericardiostomy, and a drainage tube was placed, initially yielding 800 mL sanguineous, turbid fluid, which was sent for analysis.
From days 19 to 22, the patient was weaned off from oxygen support and had no episode of hemodynamic instability. Around 150–200 mL/day of serosanguinous pericardial fluid was drained. The effluent gradually decreased to < 10 mL/day.
Pericardial fluid analysis revealed hemorrhagic, exudative effusion with lymphocytic predominance (Table 1). Acid-fast bacilli were not isolated on Ziehl–Neelsen stain, and M. tuberculosis was not detected on GeneXpert® MTB/RIF assay. No fungal element was identified on the potassium hydroxide exam, and bacterial culture studies were negative. Cytology showed a chronic inflammatory pattern, and malignant cells were not identified. HHV-8 was detected on quantitative PCR (6,000 copies/mL). SARS-CoV-2 RdRP and E genes were detected on RT-PCR. Parallel nasopharyngeal/oropharyngeal (NP/OP) swab was also positive for SARS-CoV-2, albeit with lower cycle threshold (Ct) values. Multiplex PCR for common respiratory viruses including influenza A and B, adenovirus, bocavirus, parainfluenza, human metapneumovirus, respiratory syncytial virus, rhinovirus and seasonal coronavirus were all negative. Due to resource limitations, molecular tests for cardiotropic viruses, indirect TB detection methods, and tissue biopsy (pericardial, skin, and lymph node) were not performed.
Based on the heavy TB burden in the Philippines [13] and the patient’s history of pulmonary TB, we highly considered tuberculous pericarditis in our case. Echocardiography showed typical, but not specific findings of frond-like fibrin projections from the visceral pericardium [3]. However, the patient’s adequate response to initial anti-TB treatment and the negative results of nucleic acid tests for M. tuberculosis on both sputum and pericardial fluid made tuberculous effusion less likely.
We could not completely rule out HIV-associated lymphomas due to lack of tissue biopsy. However, the absence of mediastinal tumors, hepatosplenomegaly, and other constitutional symptoms and the presence of normal LDH levels made lymphomas less likely.
Disseminated KS could present in any organ including the pericardium. Typical cutaneous lesions and lymphadenopathies and low CD4 count supported the diagnosis of KS. Although not diagnostically conclusive, the detection of HHV-8 suggested a precursor infection to the development of the disease.
We did not initially consider COVID-19 as the cause of the pericardial effusion due to the presence of other more likely etiologies. However, detection of SARS-CoV-2 in both the pericardial fluid and NP/OP swab made COVID-19-associated effusion probable.
Outcome
Over the following week, the patient complained of gradually worsening dyspnea despite increasing oxygen support. He had coarse crackles on both lung fields and distinct heart sounds. Chest radiograph showed new bilateral reticular hazy opacities (Fig. 4). Chest CT scan revealed an interval progression of multifocal confluent and ground-glass opacities with air bronchograms in both lungs and minimal pericardial effusion. The patient was diagnosed with hospital acquired pneumonia, and empiric treatment with meropenem was added. On day 32, the patient developed acute respiratory failure and septic shock, prompting intubation, vasopressor initiation, and empiric vancomycin use. Despite maximal medical management, he died on day 35. The patient’s family did not consent for autopsy. Endotracheal aspirate culture showed extended spectrum beta-lactamase-producing Enterobacter cloacae. Throughout the hospitalization, the patient underwent SARS-CoV-2 tests every 7–10 days. N and ORF1ab genes were detected on four occasions (Fig. 5). Because of the declining Ct values coinciding with the patient’s deterioration, we explored the possibility of reinfection with a new variant. However, sequencing performed on the first and last specimens showed Delta variant (B1.617.2) congruently.
Discussion and conclusions
Cardiac manifestations in SARS-CoV-2 infection are increasingly characterized [14, 15]. Detection of SARS-CoV-2 in the pericardial fluid has only been reported three times [16,17,18]. The reported cases involved immunocompetent elderly with prior history of cardiovascular diseases (i.e., two with non-ST segment elevation myocardial infarction and one with pulmonary embolism). However, our patient was an immunocompromised young adult without known cardiac comorbidity. The presence of large effusions with low estimated viral load was common among these four cases.
Current theories of pericardial injury in COVID-19 include direct invasion of cardiomyocytes and pericardium by SARS-CoV-2 and indirect injury through exaggerated inflammatory response [11]. Detection of SARS-CoV-2 in effusions support direct pericardial invasion, but the exact mechanism remains unclear. Furthermore, the limited number of publications reporting the detection of SARS-CoV-2 in pericardial fluid may be due to the small proportion of individuals who undergo pericardial drainage among COVID-19 patients with pericardial effusion [19].
Large pericardial effusions are considered to be a late sequela of COVID-19, presenting within 2–3 weeks of pulmonary symptoms and, in some instances, with undetectable virus on NP/OP samples [16, 20,21,22,23]. Various treatment modalities have been used, including pericardial drainage with or without colchicine [16,17,18, 21, 22] and uniportal video-assisted thoracoscopic surgery [24]. Similarly, our patient’s pericardial effusion was diagnosed after a 2-week history of new-onset cough and dyspnea. SARS-CoV-2 was detected in both NP/OP swab and pericardial fluid despite being sampled 5 weeks after initial symptom onset. Our findings support the evidence base for prolonged SARS-CoV-2 shedding among HIV-positive and other immunocompromised patients [25,26,27].
Detection of HHV-8 in the pericardial fluid is uncommon. The first reported case involved an HIV-seronegative patient with relapsing plasmacytic multicentric Castleman’s disease and Kaposi lesions on the toes [28]. Both pericardial and pleural fluid specimen were positive using single target PCR. HHV-8 is more commonly detected through immunohistochemistry staining in malignant cells including those isolated from pericardial fluid cytology [29]. Most cases are immunocompromised and/or HIV positive, like our patient [30].
Despite the negative sputum and pericardial fluid Xpert MTB/RIF assay and acid-fast bacilli smear, we could not completely rule out the occurrence of tuberculosis in our patient due to local disease epidemiology and clinical presentation. Nevertheless, detection of two or more pathogens in the pericardial fluid as in our case is extremely rare. Two reported cases involved M. tuberculosis–Staphylococcus aureus and M. tuberculosis–Streptococcus pneumoniae coinfections in HIV-positive patients who presented with acute purulent pericarditis and cardiac tamponade [31, 32].
Due to the significant number of likely etiologies for the patient’s clinical presentation, we attempted to be exhaustive in our management. Tissue biopsy was central in establishing the definitive diagnosis, but policies covering the COVID-19 status of patients, regardless of the duration since the first positive PCR test, severely prevented us from referring the patient to appropriate specialists. Because the patient was admitted in an infectious disease hospital, we deemed transfer to a more suitable facility for post-cardiovascular surgical care to be important. However, identified centers could not readily accommodate our referral due to lack of source isolation unit vacancy to address the patient’s multiple coinfections. Delayed diagnosis of HIV status and initiation of treatment likely contributed to the unfortunate outcome of the patient. The impact of social stigma associated with HIV in the Philippines remained apparent. Systemic delays and resource limitations likely contributed to the patient’s prolonged hospitalization and development of hospital acquired infection. Despite these challenges, we maximized the pericardial fluid analysis to guide our clinical management and to better characterize this rare case.
Detection of viruses like SARS-CoV-2 and HHV-8 in the pericardial fluid is rare, and interpretation of their significance in clinical care is challenging. Kaposi sarcoma should be considered in people living with HIV with characteristic cutaneous lesions, lymphadenopathies, and pericardial effusion. COVID-19 status-based hospital policies may prevent timely diagnostic and therapeutic interventions and disproportionately impact prolonged shedders including HIV patients and immunocompromised hosts. Finally, HIV status should be screened for young patients with TB in endemic countries.
Availability of data and materials
All data used in this article are available and may be requested from the corresponding author.
Abbreviations
- AIDS:
-
Acquired immunodeficiency syndrome
- ART:
-
Antiretroviral therapy
- COVID-19:
-
Coronavirus disease-2019
- Ct:
-
Cycle threshold
- CT:
-
Computed tomography
- EBV:
-
Epstein–Barr virus
- ER:
-
Emergency room
- HHV-8:
-
Human herpesvirus-8
- HIV:
-
Human immunodeficiency virus
- KS:
-
Kaposi sarcoma
- LDH:
-
Lactate dehydrogenase
- NP/OP:
-
Nasopharyngeal/oropharyngeal
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- TB:
-
Tuberculosis
References
Heidenreich PA, Eisenberg MJ, Kee LL, Somelofski CA, Hollander H, Schiller NB, et al. Pericardial effusion in AIDS. Circulation. 1995;92(11):3229–34.
Lind A, Reinsch N, Neuhaus K, Esser S, Brockmeyer NH, Potthoff A, et al. Pericardial effusion of HIV-infected patients? Results of a prospective multicenter cohort study in the era of antiretroviral therapy. Eur J Med Res. 2011;16(11):480–3.
Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation. 2005;112(23):3608–16.
Syed FF, Sani MU. Recent advances in HIV-associated cardiovascular diseases in Africa. Heart. 2013;99(16):1146–53.
Chen Y, Brennessel D, Walters J, Johnson M, Rosner F, Raza M. Human immunodeficiency virus-associated pericardial effusion: report of 40 cases and review of the literature. Am Heart J. 1999;137(3):516–21.
Zakowski MF, Lanuale-Shanerman A. Cytology of pericardial effusions in aids patients. Diagn Cytopathol. 1993;9(3):266–9.
Puius Y, Scully B. Treatment of Candida albicans pericarditis in a heart transplant patient. Transpl Infect Dis. 2007;9(3):229–32.
Sung J, Perez IE, Feinstein A, Stein DK. A case report of purulent pericarditis caused by Candida albicans. Medicine (Baltimore). 2018;97(28): e11286.
Eisenberg MJ, Gordon AS, Schiller NB. HIV-associated pericardial effusions. Chest. 1992;102(3):956–8.
Foster WR, Bischin A, Dorer R, Aboulafia DM. Human herpesvirus type 8-associated large b-cell lymphoma: a nonserous extracavitary variant of primary effusion lymphoma in an HIV-infected man: a case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2016;16(6):311–21.
Kermani-Alghoraishi M, Pouramini A, Kafi F, Khosravi A. Coronavirus Disease 2019 (COVID-19) and severe pericardial effusion: from pathogenesis to management: a case report based systematic review. Curr Probl Cardiol. 2022;47(2): 100933.
Dooley KE, Kaplan R, Mwelase N, Grinsztejn B, Ticona E, Lacerda M, et al. Dolutegravir-based antiretroviral therapy for patients coinfected with tuberculosis and human immunodeficiency virus: a multicenter, noncomparative, open-label, randomized trial. Clin Infect Dis. 2020;70(4):549–56.
Lansang MAD, Alejandria MM, Law I, Juban NR, Amarillo MLE, Sison OT, et al. High TB burden and low notification rates in the Philippines: the 2016 national TB prevalence survey. PLoS ONE. 2021;16(6): e0252240.
Peiris S, Ordunez P, DiPette D, Padwal R, Ambrosi P, Toledo J, et al. Cardiac manifestations in patients with COVID-19: a scoping review. Glob Heart. 2022;17(1):2.
Guglin ME, Ballut K, Jones M, Ilonze O, Rao R. clinical variants of myocardial involvement in COVID-19 positive patients. J Am Coll Cardiol. 2021;77(18):582.
Farina A, Uccello G, Spreafico M, Bassanelli G, Savonitto S. SARS-CoV-2 detection in the pericardial fluid of a patient with cardiac tamponade. Eur J Intern Med. 2020;1(76):100–1.
Sauer F, Dagrenat C, Couppie P, Jochum G, Leddet P. Pericardial effusion in patients with COVID-19: case series. Eur Heart J Case Rep. 2020;4(FI1):1–7.
Falchetti E. Detection of SARS CoV-2 RNA in pericardial effusion in a patient with cardiac tamponade— a rare case report. Eur J Med Case Rep. 2020;4(12):432–5.
Ghantous E, Szekely Y, Lichter Y, Levi E, Taieb P, Banai A, et al. Pericardial involvement in patients hospitalized with COVID-19: prevalence, associates, and clinical implications. J Am Heart Assoc. 2022;11(7): e024363.
Sollie ZW, Vallepu SR, Tharumia Jagadeesan C, White LC, Nagalapuram V. Challenges in managing pericardial disease related to post viral syndrome after COVID-19 infection. Cureus. 2021;13(2): e13461.
Foster B, Liaqat A, Chib A, Bolton SS, Kendig AC. An unusual presentation of COVID-19: hemorrhagic pericardial effusion with tamponade physiology. Cureus. 2021;13(2): e13438.
García-Cruz E, Manzur-Sandoval D, Lazcano-Díaz EA, Soria-Castro E, Jiménez-Becerra S. Cardiac tamponade in a patient with myocardial infarction and COVID-19: electron microscopy. JACC Case Rep. 2020;2(12):2021–3.
Parsova KE, Pay L, Oflu Y, Hacıyev R, Çinier G. A rare presentation of a patient with COVID-19: Cardiac tamponade. Turk Kardiyol Dern Ars. 2020;48(7):703–6.
Essa RA, Ahmed SK. Life-threatening cardiac tamponade secondary to COVID-19 treated with uniportal video-assisted thoracoscopic surgery: a case report. Am J Case Rep. 2022;23:e935839–1-e935839–5.
Meiring S, Tempia S, Bhiman J, Kleynhans J, Buys A, Makhasi M, et al. Prolonged shedding of SARS-CoV-2 at high viral load amongst hospitalised immunocompromised persons living with HIV in South Africa. Int J Infect Dis. 2022;1(116):S25.
Yousaf M, Hameed M, Alsoub H, Khatib M, Jamal W, Ahmad M. COVID-19: prolonged viral shedding in an HIV patient with literature review of risk factors for prolonged viral shedding and its implications for isolation strategies. Clin Case Rep. 2021;9(3):1397–401.
Nakajima Y, Ogai A, Furukawa K, Arai R, Anan R, Nakano Y, et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother. 2021;27(2):387–9.
Pliakas G, Konstantinou E, Ignatiades T, Legakis N, Matsiota-Bernard P. Detection of Kaposi’s sarcoma herpesvirus DNA sequences in pericardial fluid in a patient with multicentric Castleman’s disease. Clin Microbiol Infect. 2002;8(6):381–2.
Marak CP, Ponea AM, Shim C, Shaheen S, Guddati AK. Extracavitary manifestation of primary effusion lymphoma as a right atrial mass. CRO. 2013;6(1):114–8.
Vega F, Miranda RN, Medeiros LJ. KSHV/HHV8-positive large B-cell lymphomas and associated diseases: a heterogeneous group of lymphoproliferative processes with significant clinicopathological overlap. Mod Pathol. 2020;33(1):18–28.
Louw A, Tikly M. Purulent pericarditis due to co-infection with Streptococcus pneumoniae and Mycobacterium tuberculosis in a patient with features of advanced HIV infection. BMC Infect Dis. 2007;7(1):12.
Lamas ES, Bononi RJR, Bernardes MVAA, Pasin JL, Soriano HAD, Martucci HT, et al. Acute purulent pericarditis due co-infection with Staphylococcus aureus and Mycobacterium tuberculosis as first manifestation of HIV infection. Oxf Med Case Rep. 2019;2019(2):omy127.
Acknowledgements
We acknowledge Dr. Samantha Punzalan, Dr. Duane Ymbong, and Dr. Ronnie Oriola for actively providing clinical care and supervision. We also acknowledge the services provided by the San Lazaro Hospital Cardiology Unit headed by Dr. Raymond Dela Cruz, the Radiology Department, and the Laboratory Department including the national and subnational reference laboratories.
Funding
This work was partly funded by Nagasaki University (salary support for GMBM, MJS, SS, CS, KA, and KT) and Japan Agency for Medical Research and Development (Grant No. JP21fk0108104). The funding source had no role in the design and implementation of the case report and in the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
RY and LKEG provided direct clinical care and participated in the acquisition of informed consent, literature review, data collection, and writing and review of the original and final manuscript. GMBM conceptualized the case report and overall study design and participated in the literature review, data interpretation, investigation, and writing and review of the original and final manuscript. He also provided data visualization. SAMR, RMS, and EME provided direct clinical supervision and medical care and participated in the review of the final manuscript. MJS participated in the data collection and synthesis and in the review of the final manuscript. SS coordinated diagnostics management. He also participated in the review of the final manuscript. CS and KA provided supervision, acquired funding, and reviewed the final manuscript. KT participated in the conceptualization, study design, funding acquisition, and review of the final manuscript. He also facilitated further laboratory analyses of the pericardial fluid specimen. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No institutional ethical approval was required in writing this case report. The proper informed consent process was observed in soliciting the patient’s consent to be included in the case report.
Consent for publication
Written informed consent was secured from the patient for the preparation and publication of this case report including the images taken as part of the report figures.
Competing interests
We declare no existing nor potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yanes, R.R., Malijan, G.M.B., Escora-Garcia, L.K. et al. Detection of SARS-CoV-2 and HHV-8 from a large pericardial effusion in an HIV-positive patient with COVID-19 and clinically diagnosed Kaposi sarcoma: a case report. Trop Med Health 50, 72 (2022). https://doi.org/10.1186/s41182-022-00464-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-022-00464-x