Abstract
Background
Antimicrobial resistance (AMR) is a global public health threat and is increasingly prevalent among enteric pathogens in low- and middle-income countries (LMICs). However, the burden of multidrug-resistant organisms (MDROs) in older children, adults, and elderly patients with acute diarrhea in LMICs is poorly understood. This study’s aim was to characterize the prevalence of MDR enteric pathogens isolated from patients with acute diarrhea in Dhaka, Bangladesh, and assess a wide range of risk factors associated with MDR.
Methods
This study was a secondary analysis of data collected from children over 5 years, adults, and elderly patients with acute diarrhea at the International Centre for Diarrhoeal Disease Research, Bangladesh Dhaka Hospital between March 2019 and March 2020. Clinical, historical, socio-environmental information, and a stool sample for culture and antimicrobial susceptibility testing were collected from each patient. Univariate statistics and multiple logistic regression were used to assess the prevalence of MDR among enteric pathogens and the association between independent variables and presence of MRDOs among culture-positive patients.
Results
A total of 1198 patients had pathogens isolated by stool culture with antimicrobial susceptibility results. Among culture-positive patients, the prevalence of MDR was 54.3%. The prevalence of MDR was highest in Aeromonas spp. (81.5%), followed by Campylobacter spp. (72.1%), Vibrio cholerae (28.1%), Shigella spp. (26.2%), and Salmonella spp. (5.2%). Factors associated with having MDRO in multiple logistic regression included longer transport time to hospital (>90 min), greater stool frequency, prior antibiotic use prior to hospital presentation, and non-flush toilet use. However, pseudo-R2 was low 0.086, indicating that other unmeasured variables need to be considered to build a more robust predictive model of MDR.
Conclusions
MDR enteric pathogens were common in this study population with clinical, historical, and socio-environmental risk factors associated with MDROs. These findings may help guide clinical decision-making regarding antibiotic use and selection in patients at greatest risk of complications due to MDROs. Further prospective research is urgently needed to determine what additional factors place patients at greatest risk of MDRO, and the best strategies to mitigate the spread of MDR in enteric pathogens.
Similar content being viewed by others
Background
Diarrheal diseases are a leading cause of morbidity and mortality worldwide, causing over 6.3 billion episodes and 1.3 million deaths annually, with the vast majority of cases occurring in low- and middle-income countries (LMICs) [1, 2]. While the majority of diarrhea cases are self-limiting, and the mainstay of treatment is rehydration, antibiotics are recommended by the World Health Organization (WHO) for treatment of certain pathogenic causes of diarrhea [3]. For example, treatment of patients with Vibrio cholerae (V. cholerae) with severe dehydration and Shigellosis is recommended to reduce the duration of symptoms and patient-to-patient transmission [3]. However, for other etiologies of diarrhea, antibiotics are generally not indicated [3]. Ideally, decisions regarding antimicrobial use should be guided by microbiological testing such as stool culture with susceptibilities or molecular diagnostics; however, these tests are unavailable in the vast majority of LMIC clinical settings [4, 5]. This lack of diagnostic testing availability together with shortages of healthcare providers to guide antibiotic use has often led to long-standing practices of antibiotic overuse, contributing to high antimicrobial resistance (AMR) rates among enteric pathogens in LMICs. Additionally, clinicians often make decisions regarding antibiotic use and selection based on syndromic guidelines and limited local susceptibility patterns with minimal consideration of individual risk factors for AMR [3, 5].
AMR has been identified by the World Health Organization (WHO) as a serious global public health concern that must be addressed with urgency [6]. In LMICs, AMR rates of various enteric pathogens have been increasing due to a multitude of reasons including widespread availability and unregulated sale of antibiotics, poor drug quality assurance, long-standing patient expectations for antibiotics, and limited public health knowledge of AMR [6,7,8]. Alarmingly, an AMR surveillance study in Nepal showed an increase in MDR to 100% among V. cholerae samples during the 2006-2016 period [9]. Furthermore, emerging multi-drug resistance (MDR) in LMICs threatens to limit the efficacy of commonly used and low-cost antimicrobials. Individuals with MDR infections are more likely to have longer hospital admissions, higher healthcare costs, prolonged time to recovery, and higher case fatality [6]. MDR infections not only negatively impact individuals but also create substantial challenges for clinicians and healthcare systems [6]. Despite the extent of the problem, AMR and especially the burden of multidrug-resistant organisms (MDRO) are very poorly understood in LMICs, with patterns of resistance fluctuating greatly between regions and over time, even within the same country [7, 10].
While several large recent studies, such as the Global Enteric Multicenter Study, have investigated the etiologies of diarrhea in children under 5 years of age in LMICs, there is a stark lack of data for older children, adolescents, adults, and the elderly [11]. Diarrheal etiologies may vary greatly among these age groups in whom a substantial burden of disease exists, with patients over 70 years constituting more than 40% of all global deaths due to diarrhea in 2016 [12]. Understanding the epidemiology and risk factors associated with MDR in common enteric pathogens among older children, adults, and elderly individuals with diarrhea can lead to more evidence-based decision-making regarding testing and treatment of patients at highest risk for complicated disease. The aim of this study was to characterize the epidemiology of MDR enteric pathogens isolated from patients over 5 years old with acute diarrhea in Dhaka, Bangladesh, and determine the clinical, historical, and socio-environmental risk factors associated with MDR. This information may be beneficial in guiding clinicians and public health practitioners in managing patients with acute diarrhea as well as reducing the impact of MDR on communities.
Methods
Study design
This was a secondary analysis of data collected from the “Novel, Innovative Research for Understanding Dehydration in Adults and Kids” (NIRUDAK) study, a prospective cohort study of patients over 5 years presenting with acute diarrhea to the rehydration unit at Dhaka Hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) [13]. Ethical approval for the NIRUDAK Study was obtained from the icddr,b Ethical Review Committee and Rhode Island Hospital Institutional Review Board. Data from the NIRUDAK study was obtained through the NIRUDAK Principal Investigator (ACL; last author on this study). Data for the present study consisted only of de-identified data with all personal identifiers removed, and therefore ethical approval for this secondary analysis was not necessary.
Study setting and population
The NIRUDAK study was conducted between March 2019 and March 2020 at icddr,b Dhaka Hospital, an urban referral hospital that provides free clinical services for over 100,000 patients annually. The inclusion and exclusion criteria used for the present study were the same as the parent (NIRUDAK) study. All patients over 5 years of age with acute diarrhea (using the World Health Organization definition of diarrhea as three or more loose stools in the past 24 h lasting less than 7 days) were eligible for enrollment [3, 14]. Exclusion criteria included the following: having less than three loose stools in the past 24 h, diarrhea lasting more than 7 days, a clear alternative diagnosis to gastroenteritis, and previous enrollment in the study. Research staff provided patients and/or their guardian with information about the risks, and benefits of the study and obtained verbal/written consent in the local language, Bangla. In cases where the patient or legal guardian could not read or write, research staff obtained verbal consent asked the parent or guardian to mark the consent form with a thumbprint. For children over the age of 8 years and under the age of 18, verbal or written assent was also obtained.
Study procedures
Enrolled subjects were clinically assessed by a study nurse for clinical signs and symptoms, historical, demographic, and socio-environmental data. Socio-environmental data included the following: monthly household income, highest education level obtained by participant or parent, water source, use of treated water, toilet facility type, number of people sharing waste facilities, number of people living in household and transport time to hospital. All baseline clinical data were obtained from either the patient and/or parent/guardian and recorded on a case report form. Study procedures were not allowed to delay emergent care, such as placing an intravenous line or delivering fluids. After initial assessment, patients were treated according to standard icddr,b protocols for management of acute diarrhea and per physician discretion including oral or intravenous rehydration and antibiotics. Percent weight change with rehydration was used as the criterion standard for percent dehydration [15, 16]. Patients were categorized as having severe (>9%) dehydration, some (3–9%) dehydration, or no (<3%) dehydration [14]. Prior antibiotic use was determined by patient/parent report of any prior medications used (including antibiotics) before hospital evaluation; those who answered “don’t know” in response to the question on prior medication use were coded as “non-antibiotic” use for the purpose of this analysis.
Laboratory data and microbiological evaluation
Two stool specimens (at least 2 ml/vial) were collected from each subject—one for analysis to the clinical microbiology laboratory and one for storage in 70% ethanol. Each specimen was screened for common enteric pathogens using stool culture. Isolation, identification, serogrouping, and biotyping of stool samples were performed using standard procedures [17]. Briefly, V. cholerae was isolated by growth on tellurite taurocholate gelatin agar (TTGA) media with enrichment in bile peptone broth. Salmonella spp. and Shigella spp. were isolated by growth on MacConkey agar and Salmonella-Shigella agar with enrichment in selenite broth followed by antisera panel testing (Denka Seiken, Tokyo, Japan), Campylobacter spp. were isolated by growth on Brucella agar, and Aeromonas spp. were isolated by growth on TTGA and gelatin agar followed by phenotypic characterization of long-sugar metabolism. Antimicrobial susceptibility testing (AST) was determined by the Kirby-Bauer standard disk diffusion method on Muller–Hinton agar. The results were reported as sensitive, intermediate, and resistant by a method based on the cutoff of the zone size for different antibiotics according to the latest available Clinical and Laboratory Standards Institute guidelines [18].
Pathogens resistant to at least one agent in ≥3 antimicrobial categories were defined as MDR based on consensus definitions from the European Centre for Disease Prevention and Control and the Centers for Disease Control and Prevention [19]. Isolates with a result of “intermediate” were grouped with those with a result of “resistant” for the purpose of this analysis. Aminoglycosides included amikacin and gentamycin; first/second-generation cephalosporins included cefoxitin, cefuroxime; third-generation cephalosporins included cefotaxime, cefixime, ceftazidime, and ceftriaxone; quinolones included ciprofloxacin and nalidixic acid; pencillins included amoxicillin, ampicillin, ticarcillin-clavulanic acid, mecillinam, piperacillin-tazobactam; tetracyclines included doxycycline and tetracycline; sulfonamides included trimethoprim-sulfamethoxazole; colistin and chloramphenicol were also included in AST.
Statistical analysis
Categorical variables were described using frequencies with percentages. Continuous variables with normal distribution were presented as means with standard deviations (SD). Variables were also stratified by age group (child, adult, elderly) in supplementary analysis (Additional file 1). Comparisons across age groups were conducted with one-way analysis of variance, Pearson’s chi-squared test, or Fisher’s exact test as appropriate. Bivariate analyses evaluated differences between those with and without presence of MDR enteric pathogens, with magnitudes of effect given as odds ratios (OR) and their respective 95% confidence intervals (CI). Multiple logistic regression analysis was performed to identify clinical, historical, and socio-environmental variables independently associated with MDR enteric pathogens with results expressed as adjusted odds ratios (aORs) and their respective 95% CIs. All candidate variables based on expert judgment were retained in the multiple regression analysis as this was an exploratory study with the aim of evaluating potential associations with MDR rather than the creation of a new prediction model. Additionally, due to the large number of observations, the study met a general rule of thumb from the literature of having at least 10 events per candidate variable [20]. Continuous variables were recoded as binary variables by using the median value of the distribution or clinically relevant cutoff points. Nagelkerke’s pseudo-R2, a measure analogous to the R2 used in logistic regression, was calculated to provide a global measure of the estimated explained variance of the final model on a new data set; the pseudo-R2 ranges between 0 and 1 where 1 is a fully explained model [21].
Two sensitivity analyses were conducted. As there remains significant uncertainty regarding the etiologic role of Aeromonas spp. as an enteric pathogen in diarrheal disease, an analysis was performed in which samples with isolation of Aeromonas spp. only were excluded [22, 23]. In the second sensitivity analysis, patients who had reported “don’t know” regarding prior medications taken were excluded from analysis. For all analyses, a two-tailed p value of 0.05 was considered statistically significant. STATA Version 14 (Stata Corp; College Station, USA) and R (R Foundation for Statistical Computing, Vienna, Austria) were used for all analyses.
Results
Enrollment and baseline characteristics
During the study period, 2172 patients ≥ 5 years of age with acute diarrhea presenting to icddr,b Dhaka Hospital were enrolled in the NIRUDAK study. Stool culture was completed for 2135 patients, with 1198 samples (56.1%) having pathogens isolated on stool culture (“positive culture”). For the present analysis, only records with growth on stool culture were included for further analysis as shown in Fig. 1. The median age of the study population was 30 years (IQR, 17-60 years; range, 5-100 years), and 577 (48.2%) patients were female.
Of the samples with growth on stool culture, the majority (1025 or 85.6%) had a single bacterial pathogen isolated. The prevalence of enteric pathogens isolated from stool samples of the study population are shown in Table 1. “Other” organisms were isolated in 13 samples (1.1%) and included Vibrio fluvialis, Vibrio parahaemolyticus, and Plesiomonas shigelloides. In 173 samples (14.4%), there was more than one bacterial pathogen isolated.
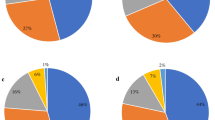
The antibiotic resistance pattern of isolated pathogens by antibiotic category and the prevalence of MDR by pathogen type are shown in Table 2. A total of 650 (30.4%) study patients had MDRO isolated from stool samples, representing 54.3% of patients with positive cultures. The prevalence of MDR was highest in Aeromonas spp. (81.5%), followed by Campylobacter spp. (72.1%), V. cholerae (28.2%), Shigella spp. (26.2%), and Salmonella spp. (5.2%).
In bivariate analysis, variables associated with the presence of MDRO included the following: having any sick contacts at home (OR 1.36; 95% CI 1.03-1.79), transport time to hospital > 90 min (OR 1.41; 95% CI 1.06-1.87), greater diarrhea frequency (>10 episodes OR 1.50; 95% CI 1.17-1.93; >20 episodes OR 2.13; 95% CI 1.47-3.09), antibiotic use prior to hospital presentation (OR 1.75, 95% CI 1.38-2.23), female sex (OR 1.26; 95% CI 1.00-1.58), and non-flush toilet use (OR 1.49; 95% CI 1.18-1.87). There was a small but statistically significant difference in temperature between the two groups with patients with MDRO having mean (SD) temperature 0.21 (0.07) degrees Fahrenheit lower than those without MDRO (OR 0.86, 95% CI 0.79-0.95). Results of the bivariate analyses are shown in Tables 3 and 4. Age was not found to be significantly associated with MDRO (OR 1.00, 95% CI 0.99-1.00); when age was categorized as a binary variable (child versus adult), age remained unassociated with MDROs (Table 3). Clinical, historical, and socio-environmental variables and presence of MDROs stratified by age group (child, adult, elderly) are shown in Additional file 1.
All variables except sick contacts at home, gender and temperature remained significant in the multiple logistic regression analysis (Table 5). Generally, the odds ratios were smaller in the multiple regression model with greater diarrhea frequency (>10 episodes OR 1.32, 95% CI 1.01-1.72; >20 episodes OR 1.88; 95% CI 1.26-2.80), transport time to hospital > 90 min (OR 1.43; 95% CI 1.06-1.95), prior antibiotic use (OR 1.70; 95% CI 1.32-2.20), and non-flush toilet use (OR 1.38; 95% CI 1.08-1.78) associated with MDRO. Nagelkerke’s pseudo-R2 was only 0.086 for the multiple logistic regression model, indicating that the model explained only a small part of the outcome variation.
In sensitivity analysis excluding 363 patients with Aeromonas spp. only isolated from culture, multiple logistic regression analysis results were similar with prior antibiotic use (OR 1.91; 95% CI 1.36-2.55) and greater transport time to hospital (OR 1.46; 95% CI 1.01-2.12) remaining significantly associated with MDRO (Additional file 1). However, diarrhea frequency (>10 episodes OR 1.13; 95% CI 0.81-1.58; >20 episodes OR 1.43; 95% CI 0.89-2.32) was no longer found to be associated with MDRO. Additionally, non-flush toilet use was marginally statistically insignificant (OR 1.33; 95% CI 0.98-1.80), while temperature (OR 0.74, 95% CI 0.63-0.87) and mid-upper arm circumference (MUAC) (OR 0.99, 95% CI 0.99-1.00) were negatively associated with MDRO. In the sensitivity analysis (Additional file 2) excluding 199 patients who had reported “don’t know” regarding the type of prior medication used, multiple logistic regression analysis results found the same significant variables as in the main analysis: greater diarrhea frequency (>10 episodes OR 1.47, 95% CI 1.10-1.97; >20 episodes OR 2.08; 95% CI 1.34-3.23), greater transport time to hospital (OR 1.41; 95% CI 1.10-1.98) prior antibiotic use (OR 1.74; 95% CI 1.32-2.29), and non-flush toilet use (OR 1.52, 95% CI 1.15-2.00).
Discussion
MDR enteric pathogens are an urgent public health threat in LMICs where the largest burden of diarrheal disease persists [24,25,26]. Such trends are not limited to LMICs, with high-income countries (HICs) also having substantial burdens of MDR, although antimicrobial stewardship efforts, infection control measures, and regulations on antibiotic use among humans and animals have generally been more widely instituted as HICs have more financial and human resources to implement these measures [27, 28]. In this study, over half of all culture-positive samples from this population of patients over 5 years with diarrhea in urban Bangladesh demonstrated MDR. This finding is consistent with a number of other recent studies showing that AMR in enteric pathogens has become commonplace in LMICs [9, 29,30,31].
Prior antibiotic use was found to be strongly associated with presence of MDROs in this study, similar to findings from prior studies among patients with acute infections from both HICs and LMICs [10]. These results emphasize the important role of assessing an individual’s antibiotic exposure history in determining risk for MDR infections. Antibiotic use was common with 36.3% of patients reporting use of antibiotics for their current illness. Actual antibiotic use is suspected to be even higher as 16% of culture-positive patients reported “I don’t know” and were coded as non-antibiotic in this analysis. These results are consistent with prior studies including a 2019 scoping review of non-prescription antibiotic use in LMICs showing rates from 8% to greater than 90% depending on patients’ level of education, monthly income, and gender [32].
Non-flush toilet use (i.e., pit latrine, open defecation) was associated with MDROs suggesting the important role that improved sanitation systems may play in disrupting cycles of oral-fecal transmission of resistant enteric pathogens, a finding which has been described previously in patients with ciprofloxacin-resistant Shigella in Bangladesh [33]. However, handwashing was not found to be associated with MDROs which may be due to limitations in the binary nature of how this question was asked rather than a lack association between personal hygiene practices and risk of MDROs. Prior studies that have evaluated individuals’ self-reported frequency of handwashing (categorized as “never,” “sometimes,” “usually,” or “always”) have shown that lower handwashing frequencies are associated with MDROs [31].
Longer transport time to hospital and greater stool frequency were also significantly associated with MDROs. Patients with longer transport times to hospital, such as those living in semi-urban or rural areas, may have more limited access to healthcare facilities and qualified healthcare providers to guide appropriate antibiotic use. Consequently, they may be more likely to purchase antibiotics directly from local pharmacies or unlicensed/unqualified vendors, potentially leading to higher levels of inappropriate antibiotic use [34]. This explanation is supported by a 2020 study in Bangladesh which found that rural healthcare providers (including qualified practitioners, semi-qualified, and unqualified vendors) had lower awareness of antibiotic resistance and correct antibiotic course durations compared to those in urban areas [35]. Differences in MDR patterns of Shigella and V. cholerae O1 between rural and urban locations in Bangladesh have also been previously documented and may explain the association between transport time and MDROs [10].
Greater diarrhea frequency, a marker of more severe illness, was found to be significantly associated with MDRO in this study. This may be due to difficult-to-treat MDROs causing more severe illness and symptoms and is consistent with a prior study in Bangladesh showing MDR shigellosis and V. cholerae 01 infections exhibited features of more severe illness (including greater stool frequency) compared to antibiotic-susceptible infections [10]. Furthermore, more severe illness may prompt individuals to travel longer distances to the hospital versus seeking care at other closer primary care facilities. The association between transport time to hospital (or distance to hospital, a close correlate) and illness severity has been previously reported in numerous LMIC and HIC settings. For example, a 2011 study of children requiring hospitalization at Kilifi District Hospital in Kenya found that distance to hospital was correlated with disease severity; the authors suspected this was due to families’ being more willing to travel to the hospital for more severe conditions or from delays in care-seeking [36]. Similarly, studies from Burkina Faso and Ethiopia have also shown that child mortality increases with greater transport times to hospital [37, 38]. In the USA, severity of illness has consistently been identified as a key factor for longer travel times to hospital, notably among elderly individuals living in rural areas [39].
Review of the literature shows a lack of consistency in the risk factors assessed in other studies evaluating MDR in enteric pathogens, limiting the ability to compare study findings between different populations. However, prior studies have illustrated that age, gender, and environmental conditions, such as crowding, poor sanitation, and contaminated foods, are highly associated with resistant enteric pathogens [31, 33]. In a study of MDR enteric pathogens among children under 15 years in Kenya, younger children (particularly those < 24 months), HIV exposure, acute malnutrition, and poor sanitation contributed to an increased risk of MDR enteric pathogens [31]. As HIV is much less common in Bangladesh compared to sub-Saharan Africa, HIV status was not measured or assessed in the present study, although HIV may be a significant risk factor for MDR in other populations [31]. Age was not found to be associated with MDR in this population of patients over 5 years old. Reasons for these findings may be due to a consistently high overall use of antibiotics among older children and adults, whereas greater discrepancies in antibiotic use may exist in younger children. A 2020 study from eight LMICs showed that an average of 24 antibiotics prescriptions were given to children by the time they were 5 years old [40]. While malnutrition has been associated with MDR, no association was seen in this study’s primary analysis [31]. This may be due to difficulties in defining malnutrition status among older populations compared to young children, or the role that other chronic comorbidities that affect immune function may play in older individuals relative to the role of malnutrition. However, lower MUAC was associated with MDR in sensitivity analysis excluding Aeromonas spp.; this finding may reflect an association between malnutrition states with MDR in other bacterial etiologies such as V. cholerae and Shigella versus Aeromonas spp.
Additionally, while this study found no associations between MDROs and income or education level, this may be due to relative socioeconomic homogeneity in the patient population. Dhaka Hospital, run by a non-profit organization icddr,b, does not charge fees for care, and thus serves a fairly uniformly impoverished population. Lastly, while statistically insignificant in adjusted analysis, an association between female gender and MDR was seen in univariate analysis. Prior studies have cited the influence that gender may have on AMR though differential health-seeking behaviors, educational levels, and antibiotic use between males and females [41].
Limitations and future directions
This study consisted of data from a single study site in urban Bangladesh and may not be generalizable to other populations given the high variability in resistance patterns between regions [10]. While the objective of this study was not to develop a new predictive model, but rather identify potential risk factors highly associated with MDR to advise further studies, the low pseudo-R2 statistic suggests a relatively low predictive accuracy. This indicates that other variables, which could not be collected given the nature of this study, should be considered to build a stronger predictive model of MDR. For example, frequency of antibiotic use, prior hospitalizations, and chronic co-morbidities have been shown to be highly associated with MDR in other studies [42].
In countries with large diarrheal burden such as Bangladesh, there may be high rates of asymptomatic bacterial pathogen carriage. This study used stool culture to determine etiologic causes of diarrhea; however, some culture-positive cases may indeed represent asymptomatic colonization. Conversely, culture has low detection rates for certain pathogens, particularly for Shigella [43]. Quantitative PCR approaches which consider the baseline levels of asymptomatic carriage may help overcome this limitation [44]. Lastly, while all pathogens were combined in the regression analysis, this may have created heterogeneous categories since different bacterial pathogens may have different mechanisms of non-susceptibility acquisition. Pathogen-specific models should be considered due to the potential difference in associated risk factors for MDR. This dataset was dominated by V. cholerae and Aeromonas spp.; while sensitivity analysis excluding Aeromonas spp. only samples found slight differences in associated risk factors, prior antibiotic use and transport time to hospital remained significantly associated, indicating the broad importance of these factors regardless of pathogen type. Further research defining the role of Aeromonas spp. as a significant enteric pathogen in LMICs is greatly needed.
Conclusion
In order to promote continued cost-effective and judicious use of antimicrobials for diarrhea, it is important to determine characteristics of patients at increased risk of MDROs. This information is beneficial in determining which patients warrant further testing, as well as to guide appropriate antibiotic selection and local antibiotic prescribing guidelines. MDROs were isolated in over half of culture-positive patients in this population over 5 years old in urban Bangladesh, underscoring the extent and need for strategies to reduce AMR in LMICs. Longer transport time to hospital, greater diarrhea frequency, non-flush toilet use, and prior use of antibiotics were all associated with MDROs. These findings highlight the importance of considering patient-specific factors such as access to healthcare facilities, sanitation resources, and practices of antibiotic use in determining individual and population risks of MDR enteric pathogens. Lastly, the pseudo-R2 of this multiple logistic regression model was low indicating that further prospective research is urgently needed to explore additional factors that may place patients at greatest risk of MDRO.
Availability of data and materials
The de-identified datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMR:
-
Antimicrobial resistance
- AST:
-
Antimicrobial susceptibility testing
- HIC:
-
High-income country
- LMIC:
-
Low- and middle-income country
- MDR:
-
Multidrug resistance
- MDRO:
-
Multidrug-resistant organism
- NIRUDAK:
-
Novel, Innovative Research for Understanding Dehydration in Adults and Kids
- TTGA:
-
Taurocholate gelatin agar
- WHO:
-
World Health Organization
References
Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SR, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–28. https://doi.org/10.1016/S1473-3099(18)30362-1.
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. https://doi.org/10.1016/S0140-6736(18)32279-7 Available from: http://www.sciencedirect.com/science/article/pii/S0140673618322797.
World Health Organization. The treatment of diarrhoea: a manual for physicians and other senior health workers. 2005.
Hatchette TF, Farina D. Infectious diarrhea: when to test and when to treat. CMAJ. 2011;183(3):339–44. https://doi.org/10.1503/cmaj.091495.
Pavlinac PB, Denno DM, John-Stewart GC, Onchiri FM, Naulikha JM, Odundo EA, et al. Failure of syndrome-based diarrhea management guidelines to detect shigella infections in Kenyan children. J Pediatric Infect Dis Soc. 2016;5(4):366–74. https://doi.org/10.1093/jpids/piv037.
World Health Organization. Global action plan on antimicrobial resistance. WHO. 2017; Available from: https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ [cited 2019 Aug 20]
Vila J, Pal T. Update on antibacterial resistance in low-income countries: factors favoring the emergence of resistance. Open Infect Dis J. 2010;4:38-54.
Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5(8):481–93. https://doi.org/10.1016/S1473-3099(05)70189-4.
Rijal N, Acharya J, Adhikari S, Upadhaya BP, Shakya G, Kansakar P, et al. Changing epidemiology and antimicrobial resistance in Vibrio cholerae: AMR surveillance findings (2006-2016) from Nepal. BMC Infect Dis. 2019;19(1):1–8.
Das SK, Klontz EH, Azmi IJ, Ud-Din AIMS, Chisti MJ, Afrad MH, et al. Characteristics of multidrug resistant Shigella and Vibrio cholerae O1 infections in patients treated at an urban and a rural hospital in Bangladesh. ISRN Microbiol. 2013;2013:213915.
Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. Available from: https://www.sciencedirect.com/science/article/pii/S0140673613608442 [cited 2019 Aug 24]
Collaborators G 2016 DD. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–28.
Levine AC, Barry MA, Gainey M, Nasrin S, Qu K, Schmid CH, et al. Derivation of the first clinical diagnostic models for dehydration severity in patients over five years with acute diarrhea. PLoS Negl Trop Dis. 2021;15(3):e0009266.
Levine A, Glavis-Bloom J, Modi P, Al E. Empirically derived dehydration scoring and decision tree models for children with diarrhea: assessment and internal validation in a prospective cohort study in Dhaka, Bangladesh. Glob Heal Sci Pr. 2015;3(405):18.
Hooper L, Abdelhamid A, Attreed NJ, Campbell WW, Channell AM, Chassagne P, et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst Rev. 2015;(4):CD009647. https://doi.org/10.1002/14651858.CD009647.pub2.
Shirreffs S. Markers of hydration status. Eur J Clin Nutr. 2003;57(2):9.
Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, Morgan DR. Manual of clinical microbiology (6th edn). Trends Microbiol. 1995;3(11):449.
Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard-CLSI Document M02-A11. 13th ed. Wayne: Clinical and Laboratory Standards Institute; 2018.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9. https://doi.org/10.1016/S0895-4356(96)00236-3.
Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–2. https://doi.org/10.1093/biomet/78.3.691.
Klontz EH, Faruque ASG, Das SK, Malek MA, Islam Z, Luby SP, et al. Clinical and epidemiologic features of diarrheal disease due to Aeromonas hydrophila and Plesiomonas shigelloides infections compared with those due to Vibrio cholerae Non-O1 and Vibrio parahaemolyticus in Bangladesh. ISRN Microbiol. 2012;2012:1–6. https://doi.org/10.5402/2012/654819.
Stang AS, Trudeau M, Vanderkooi OG, Lee BE, Chui L, Pang XL, et al. Diagnostic interpretation guidance for pediatric enteric pathogens: a modified delphi consensus process. Can J Infect Dis Med Microbiol. 2018;2018:1–11. https://doi.org/10.1155/2018/2589826.
Moharana SS, Panda RK, Dash M, Chayani N, Bokade P, Pati S, et al. Etiology of childhood diarrhoea among under five children and molecular analysis of antibiotic resistance in isolated enteric bacterial pathogens from a tertiary care hospital, Eastern Odisha, India. BMC Infect Dis. 2019;19(1):1–9.
O’Ryan G, Ashkenazi-Hoffnung L, O’Ryan-Soriano M, Ashkenazi S. Management of acute infectious diarrhea for children living in resource-limited settings. Expert Rev Anti-Infect Ther. 2014;12(5):621–32. https://doi.org/10.1586/14787210.2014.901168.
Parvin I, Shahunja KM, Khan SH, Alam T, Shahrin L, Ackhter MM, et al. Changing susceptibility pattern of Vibrio cholerae O1 isolates to commonly used antibiotics in the largest diarrheal disease hospital in Bangladesh during 2000–2018. Am J Trop Med Hyg. 2020;103(2):652-8.
García-Fernández A, Dionisi AM, Arena S, Iglesias-Torrens Y, Carattoli A, Luzzi I. Human campylobacteriosis in Italy: emergence of multi-drug resistance to ciprofloxacin, tetracycline, and erythromycin. Front Microbiol. 2018;9:1–8.
Brown JD, Willcox SJ, Franklin N, Hazelton B, Howard P, Reinten T, et al. Shigella species epidemiology and antimicrobial susceptibility: the implications of emerging azithromycin resistance for guiding treatment, guidelines and breakpoints. J Antimicrob Chemother. 2017;72(11):3181–6. https://doi.org/10.1093/jac/dkx268.
Okeke IN, Aboderin OA, Byarugaba DK, Ojo KK, Opintan JA. Growing problem of multidrug-resistant enteric pathogens in Africa. Emerg Infect Dis. 2007;13:1640–6 Centers for Disease Control and Prevention (CDC).
Mahbubur R, Shoma S, Rashid H, El Arifeen S, Baqui AH, Siddique AK, et al. Increasing spectrum in antimicrobial resistance of Shigella isolates in Bangladesh: resistance to azithromycin and ceftriaxone and decreased susceptibility to ciprofloxacin. J Health Popul Nutr. 2007;25(2):158.
Brander RL, Walson JL, John-Stewart GC, Naulikha JM, Ndonye J, Kipkemoi N, et al. Correlates of multi-drug non-susceptibility in enteric bacteria isolated from Kenyan children with acute diarrhea. PLoS Negl Trop Dis. 2017;11(10):1–18.
Torres NF, Chibi B, Middleton LE, Solomon VP, Mashamba-Thompson TP. Evidence of factors influencing self-medication with antibiotics in low and middle-income countries: a systematic scoping review. Public Health. 2019;168:92–101 Elsevier B.V.
Gruninger RJ, Johnson RA, Das SK, Nelson EJ, Spivak ES, Contreras JR, et al. Socioeconomic determinants of ciprofloxacin-resistant Shigella infections in Bangladeshi children. Pathog Immun. 2017;2(1):89 Available from: /pmc/articles/PMC5461975/?report=abstract [cited 2021 Jan 1].
Matin MA, Khan WA, Karim MM, Ahmed S, John-Langba J, Sankoh OA, Gyapong M, Kinsman J, Wertheim H What influences antibiotic sales in rural Bangladesh? A drug dispensers’ perspective. 13;1:20. doi: https://doi.org/10.1186/s40545-020-00212-8 [cited 2021 Mar 15]
Nahar P, Unicomb L, Lucas PJ, Uddin MR, Islam MA, Nizame FA, et al. What contributes to inappropriate antibiotic dispensing among qualified and unqualified healthcare providers in Bangladesh? A qualitative study. BMC Health Serv Res. 2020;20(1):656 Available from: https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-020-05512-y [cited 2021 Apr 23].
Moïsi J, Nokes D, Gatakaa H, Williams T, Bauni E, Levine O, et al. Sensitivity of hospital-based surveillance for severe disease: a geographic information system analysis of access to care in Kilifi district, Kenya. Bull World Health Organ. 2011;89(2):102–11. https://doi.org/10.2471/BLT.10.080796.
Schoeps A, Gabrysch S, Niamba L, Sié A, Becher H. The effect of distance to health-care facilities on childhood mortality in rural Burkina Faso. Am J Epidemiol. 2011;173(5):492–8 Available from: https://academic.oup.com/aje/article-lookup/doi/10.1093/aje/kwq386 [cited 2021 Apr 22].
Okwaraji YB, Cousens S, Berhane Y, Mulholland K, Edmond K. Effect of geographical access to health facilities on child mortality in rural Ethiopia: a community based cross sectional study. PLoS One. 2012;7(3):e33564 Available from: https://dx.plos.org/10.1371/journal.pone.0033564 [cited 2021 Apr 22], Noor AM, editor.
Basu J, Mobley LR. Illness severity and propensity to travel along the urban-rural continuum. Health Place. 2007;13:381–99 Available from: http://www.ers. [cited 2021 Apr 22].
Fink G, D’Acremont V, Leslie HH, Cohen J. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: a cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect Dis. 2020;20(2):179–87. https://doi.org/10.1016/S1473-3099(19)30572-9.
Omulo S, Thumbi SM, Njenga MK, Call DR. A review of 40 years of enteric antimicrobial resistance research in Eastern Africa: what can be done better? Antimicrob Resist Infect Control. 2015;4(1):1–13 Available from: https://link.springer.com/articles/10.1186/s13756-014-0041-4 [cited 2020 Oct 4].
Gomila A, Shaw E, Carratalà J, Leibovici L, Tebé C, Wiegand I, et al. Predictive factors for multidrug-resistant gram-negative bacteria among hospitalised patients with complicated urinary tract infections 11 Medical and Health Sciences 1108 Medical Microbiology. Antimicrob Resist Infect Control. 2018;7(1):111 Available from: https://aricjournal.biomedcentral.com/articles/10.1186/s13756-018-0401-6 [cited 2020 Nov 25].
Lindsay B, Ochieng JB, Ikumapayi UN, Toure A, Ahmed D, Li S, et al. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J Clin Microbiol. 2013;51(6):1740–6. https://doi.org/10.1128/JCM.02713-12.
Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–301. https://doi.org/10.1016/S0140-6736(16)31529-X.
Acknowledgements
The authors thank all study participants and the study staff of the icddr,b Dhaka Hospital for their help and support
Funding
Funding for data collection was provided through grants from the NIH National Institute for Diabetes and Digestive and Kidney Diseases (DK116163). The funders had no role in the study design, data collection, data analysis, interpretation of data, or in the writing or decision to submit the manuscript for publication. TC is supported by Institutional Development Award Number U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds Advance Clinical and Translational Research (Advance-CTR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
SCG: Study concept and design, data analysis, data interpretation, and manuscript writing; ACL: Study concept, data analysis, interpretation, and manuscript writing; TC, KQ, and CHS: Statistical analysis and data interpretation; MG, MAB, and SK: Literature review, data analysis, and manuscript writing; SN and NHA: Patient enrollment and data collection; EJN and DTL: Subject matter expertise, data interpretation, and manuscript writing. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not required for this secondary analysis of de-identified data. Ethical approval for the NIRUDAK Study was obtained from the icddr,b Ethical Review Committee and Rhode Island Hospital Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the views of NIH or any governmental bodies or academic organizations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Clinical, historical and socio-environmental variables and presence of multidrug-resistant organisms stratified by age group.
Additional file 2.
Sensitivity analysis of multiple logistic regression analysis excluding patients with Aeromonas spp. only isolated.
Additional file 3.
Sensitivity analysis of multiple logistic regression analysis excluding patients with unknown prior medication use.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garbern, S.C., Chu, TC., Gainey, M. et al. Multidrug-resistant enteric pathogens in older children and adults with diarrhea in Bangladesh: epidemiology and risk factors. Trop Med Health 49, 34 (2021). https://doi.org/10.1186/s41182-021-00327-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-021-00327-x