Abstract
Background
Currently, the synthesis pathway of metal nuclide-labeled radiopharmaceuticals is mainly divided into two steps: first, connecting the chelator with the target molecule, and second, labeling the metal nuclide to the chelator. However, the second step of the reaction to label the metal nuclide requires high temperature (90–100 °C), which tends to denature and inactivate the target molecule, leading to loss of biological activities, especially the targeting ability. A feasible solution may be the click chemistry labeling method, which consists of reacting a metal nuclide with a chelating agent to generate an intermediate and then synthesizing a radiopharmaceutical agent via the click chemistry intermediate and the target molecule-alkyne compound. In this study, through the click chemistry of 177Lu-DOTA-N3 with prostate-specific membrane antigen (PSMA)-alkyne compound, 177Lu-labeled PSMA-targeted molecular probe was synthesized and evaluated for its potential to be cleared from the bloodstream and rapidly distributed to tissues and organs, achieving a high target/non-target ratio. 177Lu-PSMA-617 was utilized as an analogue for comparison in terms of synthesizing efficiency and PSMA-targeting ability.
Results
A novel 177Lu-labeled PSMA radioligand was successfully synthesized through the click chemistry of 177Lu-DOTA-N3 with PSMA-alkyne compound, and abbreviated as 177Lu-DOTA-CC-PSMA, achieving a radiochemical yield of 77.07% ± 0.03% (n = 6) and a radiochemical purity of 97.62% ± 1.49% (n = 6) when purified by SepPak C18 column. Notably, 177Lu-DOTA-CC-PSMA was characterized as a hydrophilic compound that exhibited stability at room temperature and commendable pharmacokinetic properties, such as the superior uptake (19.75 ± 3.02%ID/g at 0.5 h) and retention (9.14 ± 3.16%ID/g at 24 h) within xenografts of 22Rv1 tumor-bearing mice. SPECT/CT imaging indicated that radioactivity in both kidneys and bladder was essentially eliminated after 24 h, while 177Lu-DOTA-CC-PSMA was further enriched and retained in PSMA-expressing tumors, resulting in the high target/non-target ratio.
Conclusion
This study demonstrated the potential of click chemistry to unify the synthesis of metal radiopharmaceuticals, and 177Lu-DOTA-CC-PSMA was found for rapid clearance and appropriate chemical stability as a PSMA-targeted radioligand.
Similar content being viewed by others
Background
The main aim of radiopharmaceutical therapy is to form targeted radiopharmaceuticals by linking therapeutic radioisotopes to targeting molecules that precisely identify tumor cells and bind to certain receptors of the tumor cells (Sgouros et al. 2020; Dhoundiyal et al. 2024; Salerno et al. 2023). The radioisotope accumulates and decays at the tumor site, releasing a certain amount of ionizing radiation, which destroys the tumor tissue; meanwhile, the precise positioning allows for targeted therapy with minimal potential impact on surrounding healthy tissues. Prostate-specific membrane antigen (PSMA), a type II transmembrane glycoprotein consisting of 750 amino acids, is commonly overexpressed in almost all prostate cancer cells (Kiess et al. 2015; Haberkorn et al. 2016; Osborne et al. 2013). In contrast, it is expressed at low levels in normal tissues such as kidneys, salivary glands, and small intestine (He et al. 2022); thus, PSMA-targeted radioligand therapy (PRLT) has become a popular therapy for the treatment of prostate cancer (Cimadamore et al. 2018; Wang et al. 2022, 2023).
The synthesis of metal nuclide-based radiopharmaceuticals is generally divided into two steps: first, connecting the chelating agent with the target molecule to form a precursor (e.g., PSMA-617, PSMA-HYNIC, and FAPI-04, which are common in clinic), and then, labeling the radioisotope on the precursor. The target molecules, especially antibodies and some heat-sensitive peptides with complex spatial structures, are prone to denaturation and inactivation at high temperatures or rigor reaction conditions. However, the second step of the reaction usually requires high temperatures or weak acidic conditions, leading to problems such as loss of targeting. A possible way to overcome these problems is to bring forward the labeling reaction of metal radionuclides as a pre-reaction of radio-labeled bioconjugate to bioactive molecules. The click chemistry, a successful method for constructing novel pharmacophores through a series of dependable chemical reactions, has played a significant role in the discovery and optimization of drug leads (Kolb et al. 2001). In 2006, researchers have (Marik and Sutcliffe 2006) pioneered the introduction of Cu-catalyzed azide alkyne cycloaddition (CuAAC) reaction into the synthesis and labeling of radiopharmaceuticals, thereby introducing a novel approach for radionuclide labeling. As the development of radiopharmaceuticals progressed, an increasing number of radionuclides have been employed for click chemistry labeling (Zhong et al. 2023), including both radiodiagnostic radionuclides such as 99mTc, 18F, and 68Ga and radiotherapeutic radionuclides such as 188Re and 177Lu (Colombo and Bianchi 2010; Mindt et al. 2008; Choy et al. 2017; Evans et al. 2014; Quigley et al. 2022; Wang et al. 2012). In addition, the synthesis of radiotracers targeting PSMA receptors for use as PRLT by click chemistry has been widely used, e.g., Verena i Böhmer synthesised and in vivo evaluated F-18 labeled PSMA-targeted “18F-PSMA-MIC” radiotracers by copper(I)-catalyzed azide-alkane cycloaddition and demonstrated that the binding affinity could be improved by copper(I)-catalyzed azide-alkane cycloaddition to promote alkane binding in PSMA (Böhmer et al. 2020); James Kelly developed high-affinity PSMA inhibitors labeled with F-18 by click chemistry, and showed that the radiosynthesis of 18F-labeled triazoles is simple and highly productive, with high PSMA affinity and specific uptake (Kelly et al. 2017).
In this study, the potential of click chemistry in simplifying and unifying the synthesis of metal radiopharmaceuticals was proposed and explored using an 177Lu-labeled PSMA-targeting molecular probe. The 177Lu-labeled PSMA ligand was achieved under mild conditions by incorporating a triazole ring into the molecular structures of DOTA and PSMA using click chemistry, named 177Lu-DOTA-CC-PSMA. The effect of this triazole ring on the overall drug properties of the complex was preliminarily investigated, and its biological properties were studied in mice with PSMA-positive tumors.
Materials and methods
Main instruments
The main equipment used in this study included a 1260 Infinity II high-performance liquid chromatography (HPLC) (Agilent Technologies, Inc., USA), an AR2000 thin-layer chromatography (TLC) scanner (Eckert & Ziegler Radiopharma, Inc., USA), a Flow-RAM radioactivity detector (LabLogic Systems Ltd., UK), a Wizard 2470 gamma counter (Perkin-Elmer Instruments Inc., USA), and a X-cube and γ-cube integrated SPECT/CT scanner (Molecubes, Belgium).
Radiosynthesis of 177Lu-DOTA-CC-PSMA and 177Lu-PSMA-617
Detailed methods and results on the cold labeling of 175Lu-DOTA-CC-PSMA are presented in the Supplementary Data (Figs. S1 and S2). The radiosynthesis scheme of 177Lu-DOTA-CC-PSMA is shown in Scheme 1. 177LuCl3 was obtained from Isotopia Molecular Imaging Ltd (Petach Tikva, Israel). Briefly, DOTA-N3 (0.41 mmol/L, 150 µL, 62 nmol) was added to 177LuCl3 (25 µL, 0.67 GBq) in a sealed vial. The reaction was allowed to proceed at 95 °C for 15 min. The labeling rate was evaluated by HPLC method [A: H2O-0.1% trifluoroacetic acid (TFA), B: CH3CN-0.1% TFA, 1-1-10-10-1-1% B (0-5-10-14-15–17 min), 1 mL/min]. A solution of PSMA-alkyne (0.93 mmol/L, 95 µL, 89 nmol), CuSO4 (20.00 mmol/L, 43 µL, 860 nmol), and sodium ascorbate (94.40 mmol/L, 45 µL, 4250 nmol) was added to the freshly prepared 177Lu-DOTA-N3 mixture with final concentration. The resulting mixture was heated at 37 °C for 60 min. The tracer mixture was passed through a Sep-Pak cartridge (Waters, Sep-Pak C18 column) and rinsed with sterile water for injection to remove impurities and salts, and the tracer was eluted with ethanol. Finally, the solution was diluted with 0.5 M HAc-NaAc buffer solution (pH 5.2) and filtered through a 0.22-µm disposable sterile filter to obtain an injectable solution [< 8% (v: v) ethyl alcohol (EtOH)]. The radiochemical purity (RCP) was evaluated by the HPLC method [A: H2O-0.1% TFA, B: CH3CN-0.1% TFA, 20-40-40-100-20-20% B (0-20-22-27-30–35 min), 1 mL/min].
177Lu-PSMA-617 was prepared by successively adding 177LuCl3 solution (5 µL, 40.7 MBq), PSMA-617 (0.22 mmol/L, 30 µL, 6.7 nmol; prepared in 0.5 M HAc-NaAc buffer, pH 5.2), and gentisic acid (65.00 mmol/L, 40 µL, 2600 nmol) in a reaction flask. The mixture was mixed evenly and incubated for 15 min at 95 °C. Finally, the solution was cooled and diluted with 930 µL of sodium ascorbate (0.28 M) solution and filtered through a 0.22-µm disposable sterile filter to obtain an injectable solution. The RCP was evaluated by the HPLC method [A: H2O-0.1% TFA, B: CH3CN-0.1% TFA, 20-40-40-100-20-20% B (0-20-22-27-30–35 min), 1 mL/min].
Stability test
To evaluate the stability of 177Lu-DOTA-CC-PSMA, samples were mixed with HAc-NaAc buffer solution or serum and incubated at 25–37 °C. Samples were taken at 0, 1, 4, 24, 48, 72, 120 and 144 h (n = 3) and analysed with radio-HPLC [A: H2O-0.1% TFA, B: CH3CN-0.1% TFA, 20-40-40-100-20-20% B (0-20-22-27-30–35 min), 1 mL/min] or radio-TLC [iTLC-SA (Agilent), 0.9% NaCl/HCl, 100:0.5] and further plotted in OriginPro 2019b.
Determination of the partition coefficient
The partition coefficient was determined by mixing the labeled compound 177Lu-DOTA-CC-PSMA or 177Lu-PSMA-617 (10 µL) with n-octanol (600 µL) and phosphate buffer (590 µL, 0.1 M, pH 7.4) in an Eppendorf microcentrifuge tube. The mixture was vigorously stirred for 2 min at room temperature and was centrifuged at 8,000 rpm for 3 min. Samples in triplicate from n-octanol and aqueous layer were obtained, and were counted by γ-counter (n = 6). The partition coefficients were calculated using the following equation: logD7.4 = log (activity concentration in n-octanol)/(activity concentration in aqueous layer).
Pharmacokinetics
177Lu-DOTA-CC-PSMA (3.7 MBq in 100 µL) or 177Lu-PSMA-617 (3.7 MBq in 100 µL) was administered intravenously via the tail into BALB/c male mice (n = 6), and a venous blood sample (5 µL) was collected from the tail end of each mouse at different time points after injection (2, 4, 6, 10, 15, 30, 60, 120, and 240 min). The radioactivity of the blood samples was measured by γ-counter, and the pharmacokinetics data were obtained using the DAS 2.0 software.
Tumor model
All experiments were approved by the Committee on the Management and Use of Laboratory Animals of Shanghai Vista Pharmaceutical Technology Co. Ltd. (Institutional Animal Care and Use Committee number Vista-IA-2-1-2306-01), and all methods were carried out in accordance with relevant guidelines and regulations. The reporting in this manuscript adhered to the recommendations in the ARRIVE guidelines.
For in vivo studies, 6-week-old athymic nu/nu CDX (22Rv1) model male mice (subcutaneously inoculating 1 × 106 22Rv1 cells at the base of the right forelimb) were obtained from Nanchang Royo Biotech Co. Ltd. The mice were kept in individually ventilated cages under standard conditions with food and water provided ad libitum. Housing conditions were as following: dark/light cycle 12/12 h, ambient temperature around 21–22 °C and humidity between 40 and 70%. Biodistribution and SPECT/CT imaging were performed 3–4 weeks after cell inoculation (when the tumor reached a size of approximately 400 mm3). 99mTc-HYNIC-PSMA SPECT/CT imaging was performed to verify PSMA-avidity of CDX models before the start of the experiment. Details on SPECT/CT acquisition process and PSMA-avidity assessment are provided in Supplementary Data (Fig. S3).
In vivo biodistribution studies
The 22Rv1 tumor-bearing mice were injected in the lateral tail vein with 177Lu-DOTA-CC-PSMA (n = 4/group, 3.7 MBq in 100 µL per mouse) or 177Lu-PSMA-617 (n = 4/group, 3.7 MBq in 100 µL per mouse) under isoflurane anesthesia (1-2% isoflurane) and sacrificed by cervical dislocation 0.5, 1, 4, and 24 h later. The mice were kept under persistent anesthesia and warm throughout the injection period. Blood (177Lu-DOTA-CC-PSMA), organs (177Lu-DOTA-CC-PSMA), and tumors (177Lu-DOTA-CC-PSMA and 177Lu-PSMA-617) were collected and weighed. For the tumor specimens, liquid necrotic areas were discarded, and only solid tumor parts were examined. Activity content was assessed by γ-counter. Tissue counts and injected dose for individual mice were decay-corrected to the time of euthanasia. Tissue uptake was expressed as the percentage injected dose per gram of tissue (% injected dose/gram tissue, %ID/g). Values of tumor uptake were divided by muscle (blood, kidneys) uptake for each animal to calculate the tumor-to-muscle (blood, kidneys) [T/M (B, K)] ratio. The biodistribution data and target/non-target (T/NT) ratios were reported as mean values with the standard deviation (SD).
SPECT/CT imaging
A total 4 mice with subcutaneous 22Rv1 tumors were scanned as exemplary cases for in vivo SPECT/CT imaging. Under isoflurane anesthesia (1-2% isoflurane) 177Lu-DOTA-CC-PSMA (18.5 MBq in 100 µL per mouse) injection and whole-body imaging was performed 0.5, 1, 2, 4, 6 and 24 h after injection with modular SPECT/CT scanner. The mice were placed in the supine position on the scanning bed post-injection, respectively, and were scanned using modular high-resolution CT (image acquisition parameters: CT, tube voltage: 50 kV; tube current: 30 µA), which was anaesthetised for 5 min using 1% isoflurane. After the CT acquisition, the mice were placed supine on the scanning bed and scanned using modular high-resolution SPECT (image acquisition parameters: SPECT, algorithm: maximum likelihood algorithm; peak: 140 keV; isometric voxel size: 500 μm), and anaesthesia was applied with 1% isoflurane for 28 min. Images were constructed using maximum likelihood algorithm (50 iterations) and the data were corrected for attenuation. Image processing and analysis were performed using VivoQuant software (Invicro) to form hybrid SPECT/CT images in transverse, coronal and sagittal planes.
Data analysis and statistics
All statistical analyses were performed using SPSS 23.0 software (SPSS Inc.), and graphs were plotted using OriginPro 2019b (OriginLab Inc.). Quantitative data are expressed as the mean ± SD. Independent samples of tumor uptake for each organ, as well as for tumor tissue and T/M (T/B, T/K) ratio, tracer uptakes at each time point were compared with Kruskal Wallis H test, and Mann-Whitney-U test was used as post-hoc test in pairwise comparisons of variables with significant results. Two-sided p-values of < 0.05 were considered statistically significant.
Results
Radiochemistry
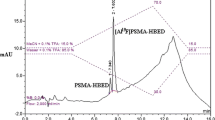
Radiolabeling of the precursor was accomplished by first reacting 177LuCl3 with DOTA-N3, followed by a click-chemistry step to assemble the intact 177Lu-DOTA-CC-PSMA (Scheme 1). 177LuCl3 and DOTA-N3 were incubated in a sodium acetate buffer (pH 5.2) at 95 °C for 15 min. DOTA-N3 incorporated 177LuCl3 readily with a labeling rate of 86.56% ± 1.26% (n = 6), as determined by radio-HPLC (Fig. 1A), and the CUAAC was initiated by adding PSMA-alkyne into the 177Lu-DOTA-N3 mixture at 37 °C for 60 min. The yield of 177Lu-DOTA-CC-PSMA was 77.07% ± 0.03% (n = 6), as determined by radio-HPLC (Fig. 1B); the RCP was 97.62% ± 1.49% (n = 6) after the purification according to radio-HPLC analysis (Fig. 1C); and the molar activity was 5.0 MBq/nmol. As shown in Table 1, the reaction temperature and the RCP of 177Lu-DOTA-CC-PSMA were superior to those of 177Lu-PSMA-617. The stabilities of 177Lu-PSMA-617 and 177Lu-DOTA-CC-PSMA in HAc-NaAc buffer (25 °C) and serum (37 °C) were investigated, and the results showed that the RCP (> 95%) of 177Lu-DOTA-CC-PSMA was superior to that of 177Lu-PSMA-617 over an observation period of 144 h (Fig. 1D-I), which indicates that 177Lu-DOTA-CC-PSMA is of high stability.
The logD7.4 values were − 3.99 ± 0.32 for 177Lu-DOTA-CC-PSMA and − 4.21 ± 0.08 for 177Lu-PSMA-617, suggesting that 177Lu-DOTA-CC-PSMA was highly hydrophilic and could be rapidly eliminated from blood.
Radio-HPLC profile of (A) 177Lu-DOTA-N3, (B) 177Lu-DOTA-CC-PSMA, and (C) purified 177Lu-DOTA-CC-PSMA. Multi-temporal superimposed Radio-HPLC profile of (D) 177Lu-DOTA-CC-PSMA, (E) 177Lu-PSMA-617, and (F) RCP of 177Lu-DOTA-CC-PSMA and 177Lu-PSMA-617 measured within 144 h in HAc-NaAc buffer. Multi-temporal superimposed Radio-TLC profile of (G) 177Lu-DOTA-CC-PSMA, (H) 177Lu-PSMA-617, and (I) RCP of 177Lu-DOTA-CC-PSMA and 177Lu-PSMA-617 measured within 144 h in serum
Biodistribution and tumor uptake
In vivo biodistribution data showed highly specific tumor uptake of 177Lu-DOTA-CC-PSMA in PSMA-positive tumors (Table 2; Fig. 2A).
Tumor uptake of 177Lu-DOTA-CC-PSMA peaked at 30 min (19.75 ± 3.02%ID/g). However, 177Lu-DOTA-CC-PSMA showed the highest tumor-to-muscle (blood) ratio at 24 h (T/M: 801.21 ± 299.73; T/B: 296.30 ± 71.82), compared with the time point of the tumor uptake peak (T/M: 22.70 ± 6.07; T/B: 6.29 ± 1.46), indicating fast initial uptake followed by slow tumor activity washout paralleled by a stronger washout in the muscle (blood) (Table 2). Still, the T/M (B) ratio was greater than 1 at all of the examined time points (Table 1). 177Lu-DOTA-CC-PSMA was excreted via the renal route. Among normal organs, the kidneys showed the highest uptake (1.58 ± 0.70%ID/g at 24 h after injection), while others showed very low radioactivity accumulation and rapid elimination (Fig. 2A).
The blood drug concentration-time curves of the probe 177Lu-PSMA-617 and 177Lu-DOTA-CC-PSMA in healthy mice are shown in Fig. 2B. The curves showed that the pharmacokinetics of 177Lu-DOTA-CC-PSMA and 177Lu-PSMA-617 were essentially the same (P > 0.05), with rapid clearance from the blood and rapid distribution to all tissues and organs in the body. In addition, we calculated the blood clearance half-life of the radiopharmaceuticals synthesised by the two methods, which was shorter at 15.16 min for 177Lu-DOTA-CC-PSMA compared to 177Lu-PSMA-617 (17.18 min).
In addition, we compared the tumor uptake of 177Lu-DOTA-CC-PSMA (click chemistry method) and that of 177Lu-PSMA-617 (conventional labeling method) and found that the tumors had significantly higher uptake of the click chemistry-synthesized 177Lu-DOTA-CC-PSMA (Fig. 2C, P < 0.05 within 24 h).
(A) Biodistribution of 177Lu-DOTA-CC-PSMA. Data were obtained 0.5, 1, 4, and 24 h after 177Lu-DOTA-CC-PSMA injection. Values are expressed as %ID/g for organs and tissues. Values are shown as mean ± SD; n = 4. (B) Blood drug concentration-time curves for 177Lu-PSMA-617 and 177Lu-DOTA-CC-PSMA. Values are shown as mean ± SD; n = 6. (C) Tumor uptake of 177Lu-PSMA-617 and 177Lu-DOTA-CC-PSMA over time. Values are shown as mean ± SD; n = 4
SPECT/CT imaging
SPECT/CT images (Fig. 3) showed high tumor uptake in 22Rv1 tumor-bearing mice at all time points, peaking at 2 h, and tumor uptake even after 24 h, indicating that 177Lu-DOTA-CC-PSMA has a high tumor affinity with good accumulation and retention capacity. The uptake of 177Lu-DOTA-CC-PSMA was moderate in the kidneys and low in other normal organs, with a gradual decrease in uptake over time, and the radioactivity essentially disappeared from the kidneys and bladder after 24 h, whereas 177Lu-DOTA-CC-PSMA was further enriched and retained in the PSMA-positive tumors. High T/M ratios and high T/K ratios indicated good non-target organ clearance efficiency and high T/NT ratios. Thus, targeting molecular probes synthesized by click chemistry with good tumor uptake and rapid clearance shows significant potential in simplifying and unifying the synthesis of metal radiopharmaceuticals.
In vivo 177Lu-DOTA-CC-PSMA of a PSMA-positive tumor model. Whole-body scans at 0.5, 1, 2, 4, 6, and 24 h after injection of 177Lu-DOTA-CC-PSMA (22Rv1 tumor-bearing mouse) are shown, with clearly visible renal accumulation, low uptake in other normal organs, and tumor uptake peaking at 2 h and remaining at 24 h. The tumor is marked with a white arrow
Discussion
This study explored the potential of click chemistry in simplifying and unifying the synthesis of metal radiopharmaceuticals using 177Lu-labeled PSMA-targeting molecule probe as an example. 177Lu has a long half-life and can release γ-rays, and these γ-rays in SPECT imaging and external dose assessment have the potential for early diagnosis of tumors, recurrence, and metastatic foci (Liu and Chen 2019). As the pairing metal nuclide of 177Lu, the positronic nuclide 68Ga has a similar labeling chemistry and can often be labeled with the same drug precursor, and thus it can be prepared for diagnosis and treatment integration of radiopharmaceuticals (Chen et al. 2018). In a similar way, the proposed protocol is suited for 68Ga-labeling of bioactive molecules. This study achieves a significant reduction in time compared to other click chemistry methods (Choy et al. 2017), which is not significant for Lu-177 labeling, but as a methodological study is significant for the improvement of the radiochemical yield of other short half-life nuclides, e.g. Ga-68.
The synthesis of 177Lu-DOTA-CC-PSMA is straightforward and expands the scope of PSMA-617 as a labeling precursor, which simplifies the labeling process by avoiding high-temperature reaction conditions compared with conventional 177Lu labeling. In this study, the click chemistry method was employed to incorporate a triazole ring into both the DOTA and PSMA molecular structure. The effect of this triazole ring on the overall drug properties of the complex was subsequently investigated, and its biological properties were studied in mice with PSMA-positive tumors. The results showed that 177Lu-DOTA-N3 was successfully labeled with PSMA-alkyne compounds after using click chemistry, and the labeling process was simple and efficient, with mild conditions and high RCP. The 177Lu-DOTA-CC-PSMA in this study showed good in vitro and in vivo stability, which was still maintained at more than 90% within 144 h, which is more consistent with the stability of 177Lu-PSMA-617 (> 90% within 144 h). 177Lu-DOTA-CC-PSMA could also maintain stability in vivo, indicating that it can be used for in vivo studies.
177Lu-DOTA-CC-PSMA is a PSMA-targeted nuclear drug with translational therapeutic promise for PRLT. 177Lu-DOTA-CC-PSMA is of good hydrophilic and low lipid-soluble, so it does not easily cross the blood-brain barrier, and the brain tissue is exposed to less radiation. Our pharmacokinetic results showed that 177Lu-DOTA-CC-PSMA could be cleared from the blood and rapidly distributed to all tissues and organs in the body, providing a lower blood background signal during imaging, which was conducive to the formation of a high T/B ratio and clear SPECT images. The biodistribution and SPECT/CT imaging results in the 22Rv1 tumor-bearing mice, especially the significant renal excretion, suggested that 177Lu-DOTA-CC-PSMA was efficiently cleared from the blood and other organs, which is an important aspect of in vivo imaging with targeted molecular probes. Meanwhile, the results of the biodistribution and SPECT/CT imaging showed that 177Lu-DOTA-CC-PSMA exhibited superior uptake and retention within the tumor, good non-target organ clearance efficiency, and high T/NT ratio. 177Lu-DOTA-CC-PSMA is of the advantage of achieving high accumulation within the tumour, with potential therapeutic value.
In conclusion, the labeling of 177Lu-DOTA-CC-PSMA synthesized by click chemistry is simple and easy, with mild labeling conditions, qualified quality control, and high labeling rate; 177Lu-DOTA-CC-PSMA has ideal biodistribution, most of which is excreted via the kidneys; and it offers rapid blood clearance and good stability, demonstrating the potential of click chemistry to unify the synthesis of metal radiopharmaceuticals. However, only the popular 177Lu-labeled PSMA-targeting molecular probe was used as an example in this study, and more studies on metal nuclide-labeled targeting molecules were not carried out. The potential of click chemistry to unify the synthesis of metal radiopharmaceuticals has yet to be verified for more metal nuclides through the click-chemistry-labeled targeting molecular probes.
Conclusion
In this study, we proposed a scheme to avoid the loss of targeting properties during the degeneration and inactivation of targeting molecules in the process of producing metal nuclide-conjugated radiopharmaceuticals and validated it with 177Lu-labeled PSMA-targeting molecules. A novel 177Lu-labeled PSMA-targeted molecular probe, designated as 177Lu-DOTA-CC-PSMA, was successfully synthesized through the click chemistry of 177Lu-DOTA-N3 with PSMA-alkyne compound. The stability and tumor uptake of 177Lu-DOTA-CC-PSMA synthesized by click chemistry were higher and with consistent pharmacokinetics, compared with 177Lu-PSMA-617 synthesized by conventional methods. Consequently, metal nuclide-coupled radiopharmaceuticals, represented by 177Lu-DOTA-CC-PSMA, demonstrate the potential of click chemistry to unify the synthesis of metal radiopharmaceuticals.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Abbreviations
- PSMA:
-
Prostate-specific membrane antigen
- PRLT:
-
PSMA-targeted radioligand therapy
- SPECT:
-
Single-photon emission computed tomography
- CT:
-
Computed tomography
- HPLC:
-
High performance liquid chromatography
- TLC:
-
Thin-layer chromatography
- ROI:
-
Region of interest
- T/M:
-
Tumor to muscle
- T/K:
-
Tumor to kidney
- T/B:
-
Tumor to blood
- T/NT:
-
Target/non-target
- RCP:
-
Radiochemical purity
- %ID/g:
-
% injected dose/gram tissue
- CuAAC:
-
Cu-catalyzed azide alkyne cycloaddition
- TFA:
-
Trifluoroacetic acid
- EtOH:
-
Ethyl alcohol
References
Böhmer VI, Szymanski W, van den Berg KO, Mulder C, Kobauri P, Helbert H, et al. Modular Medical Imaging agents based on Azide-Alkyne Huisgen cycloadditions: synthesis and pre-clinical evaluation of 18F-Labeled PSMA-Tracers for prostate Cancer imaging. Chemistry. 2020;26:10871–81. https://doi.org/10.1002/chem.202001795.
Chen L, Zou S, XH Z. Targeted imaging and therapy of prostate canceer by radionuclide labeled small molecule inhibitors of prostate specific membrane antigen. Chin J Nucl Med Mol Imaging. 2018;38:53–8. https://doi.org/10.3760/cma.j.issn.2095-2848.2018.01.014.
Choy CJ, Ling X, Geruntho JJ, Beyer SK, Latoche JD, Langton-Webster B, et al. (177)Lu-Labeled phosphoramidate-based PSMA inhibitors: the Effect of an Albumin Binder on Biodistribution and therapeutic efficacy in prostate tumor-bearing mice. Theranostics. 2017;7:1928–39. https://doi.org/10.7150/thno.18719.
Cimadamore A, Cheng M, Santoni M, Lopez-Beltran A, Battelli N, Massari F, et al. New prostate Cancer targets for diagnosis, imaging, and therapy: focus on prostate-specific membrane Antigen. Front Oncol. 2018;8:653. https://doi.org/10.3389/fonc.2018.00653.
Colombo M, Bianchi A. Click chemistry for the synthesis of RGD-containing integrin ligands. Molecules. 2010;15:178–97. https://doi.org/10.3390/molecules15010178.
Dhoundiyal S, Srivastava S, Kumar S, Singh G, Ashique S, Pal R, et al. Radiopharmaceuticals: navigating the frontier of precision medicine and therapeutic innovation. Eur J Med Res. 2024;29:26. https://doi.org/10.1186/s40001-023-01627-0.
Evans HL, Nguyen QD, Carroll LS, Kaliszczak M, Twyman FJ, Spivey AC, et al. A bioorthogonal (68)Ga-labelling strategy for rapid in vivo imaging. Chem Commun. 2014;50:9557–60. https://doi.org/10.1039/c4cc03903c.
Haberkorn U, Eder M, Kopka K, Babich JW, Eisenhut M. New strategies in prostate Cancer: prostate-specific membrane Antigen (PSMA) ligands for diagnosis and therapy. Clin Cancer Res. 2016;22:9–15. https://doi.org/10.1158/1078-0432.Ccr-15-0820.
He Y, Xu W, Xiao YT, Huang H, Gu D, Ren S. Targeting signaling pathways in prostate cancer: mechanisms and clinical trials. Signal Transduct Target Ther. 2022;7:198. https://doi.org/10.1038/s41392-022-01042-7.
Kelly J, Amor-Coarasa A, Nikolopoulou A, Kim D, Williams C Jr, Ponnala S, et al. Synthesis and pre-clinical evaluation of a new class of high-affinity 18F-labeled PSMA ligands for detection of prostate cancer by PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:647–61. https://doi.org/10.1007/s00259-016-3556-5.
Kiess AP, Banerjee SR, Mease RC, Rowe SP, Rao A, Foss CA, et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q J Nucl Med Mol Imaging. 2015;59:241–68.
Kolb HC, Finn MG, Sharpless KB. Click Chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40:2004–21. https://doi.org/10.1002/1521-3773(20010601)40:11%3C;2004. :Aid-anie2004>3.0.Co;2-5.
Liu H, Chen Y. Application progress of radionuclide imaging and therapy in neuroendocrine tumors. Chin J Nucl Med Mol Imaging. 2019;39:564–7. https://doi.org/10.3760/cma.j.issn.2095-2848.2019.09.015.
Marik J, Sutcliffe JL. Click for PET: rapid preparation of [18F]fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006;47:6681–4. https://doi.org/10.1016/j.tetlet.2006.06.176.
Mindt TL, Müller C, Melis M, de Jong M, Schibli R. Click-to-chelate: in vitro and in vivo comparison of a 99mTc(CO)3-labeled N(tau)-histidine folate derivative with its isostructural, clicked 1,2,3-triazole analogue. Bioconjug Chem. 2008;19:1689–95. https://doi.org/10.1021/bc800183r.
Osborne JR, Akhtar NH, Vallabhajosula S, Anand A, Deh K, Tagawa ST. Prostate-specific membrane antigen-based imaging. Urol Oncol. 2013;31:144–54. https://doi.org/10.1016/j.urolonc.2012.04.016.
Quigley NG, Steiger K, Hoberück S, Czech N, Zierke MA, Kossatz S, et al. PET/CT imaging of head-and-neck and pancreatic cancer in humans by targeting the Cancer integrin αvβ6 with Ga-68-Trivehexin. Eur J Nucl Med Mol Imaging. 2022;49:1136–47. https://doi.org/10.1007/s00259-021-05559-x.
Salerno KE, Roy S, Ribaudo C, Fisher T, Patel RB, Mena E, et al. A primer on Radiopharmaceutical Therapy. Int J Radiat Oncol Biol Phys. 2023;115:48–59. https://doi.org/10.1016/j.ijrobp.2022.08.010.
Sgouros G, Bodei L, McDevitt MR, Nedrow JR. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. 2020;19:589–608. https://doi.org/10.1038/s41573-020-0073-9.
Wang C, Zhou W, Yu J, Zhang L, Wang N. A study of the radiosynthesis of fac-[¹⁸⁸ReCO₃(H₂O)₃]⁺ and its application in labeling 1,2,3-triazole analogs obtained by click chemistry. Nucl Med Commun. 2012;33:84–9. https://doi.org/10.1097/MNM.0b013e32834d3ba7.
Wang F, Li Z, Feng X, Yang D, Lin M. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2022;25:11–26. https://doi.org/10.1038/s41391-021-00394-5.
Wang H, Li G, Zhao J, Eiber M, Tian R. Current status of PSMA-targeted imaging and therapy. Front Oncol. 2023;13:1230251. https://doi.org/10.3389/fonc.2023.1230251.
Zhong X, Yan J, Ding X, Su C, Xu Y, Yang M. Recent advances in Bioorthogonal Click Chemistry for enhanced PET and SPECT Radiochemistry. Bioconjug Chem. 2023;34:457–76. https://doi.org/10.1021/acs.bioconjchem.2c00583.
Acknowledgements
Not applicable.
Funding
This work was supported by The Pudong New Area Clinical Characteristic Discipline Project (No. PWYts2021-01), Clinical research program of Health Industry of Shanghai Municipal Commission of Health (202150002), Pudong Hospital, Fudan University, College Level Project (YJYJRC202108/YJYJRC202101/Zdzk2020-14), National Natural Science Foundation of China (82272042).
Author information
Authors and Affiliations
Contributions
X.Z., L.Z. and X.L. contributed to the study conception and design. Data collection was performed by X.Z., S.X., Z.Z., S.J., S.H., L.J. Data analysis and interpretation was performed by X.Z., Z.L., Z.K., T.W. The first draft of the manuscript was written by X.Z. and S.X., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experiments were approved by the Committee on the Management and Use of Laboratory Animals of Shanghai Vista Pharmaceutical Technology Co. Ltd. (Institutional Animal Care and Use Committee number Vista-IA-2-1-2306-01), and all methods were carried out in accordance with relevant guidelines and regulations. The reporting in this manuscript adhered to the recommendations in the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, X., Xue, S., Zhao, Z. et al. The development of 177Lu-DOTA-CC-PSMA following a unified “Click Chemistry” protocol of synthesizing metal nuclide-conjugated radiopharmaceuticals. EJNMMI radiopharm. chem. 9, 56 (2024). https://doi.org/10.1186/s41181-024-00287-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41181-024-00287-7