Abstract
Background
Increased vascular permeability is an early sign of vascular damage and can be measured with the transcapillary escape rate of albumin (TERalb). Although TERalb has a multi-exponential kinetic model, most published TERalb data are based on mono-exponential kinetic models with variation in blood sampling schemes. Aim of this posthoc study was to evaluate the influence of variation in blood sampling schemes and the impact of mono- or bi-exponential analyses on the calculation of TERalb. Study participants were part of a cross-over intervention study protocol, investigating effects of sodium loading on blood pressure, endothelial surface layer and microcirculation. Multiple blood samples were drawn between 3 and 60 min after injection of radioactive iodide labeled human serum albumin (rHSA).
Results
In total 27 male participants with 54 measurements were included. For all participants the maximum serum radioactivity was reached within 20 min, while 85% of the participants had their maximum serum activity within 10 min. The TERalb calculated with the subsequently chosen T20–60 min reference scheme (6.19 ± 0.49%/h) was significantly lower compared to the TERalb of the T3–60 min, T5–60 min, and Tmax – 60 min schemes. There was no significant difference between the T20–60 min reference scheme and the T10–60 min and T15–60 min schemes. Bi-exponential kinetic modeling did not result in significant different observations compared to the mono-exponential kinetic analysis.
Conclusions
As there is variation in the timing of the maximum serum radioactivity of rHSA, blood sampling schemes starting before 10 min after administration of rHSA will result in a significant overestimation of TERalb. In addition, variation in kinetic modeling did not result in significant changes in TERalb. Therefore, we emphasize the need to standardize TERalb and for practical and logistical reasons advocate the use of a mono-exponential model with blood sampling starting 20 min after rHSA administration.
Similar content being viewed by others
Background
Diabetes mellitus and hypertension are characterized by an increased risk of vascular complications. An early sign of vascular damage is increased vascular permeability, which can be determined by the transcapillary escape rate of albumin (TERalb) (Broekhuizen et al., 2010).
TERalb is the rate in which intravenous albumin escapes from the intravascular to the extravascular volume in the first hour after injection of radioactive iodide labeled human serum albumin (rHSA) (Parving, 1975). The pharmacokinetics of rHSA could be described as the sum of three exponential components, with respective half-life’s of 6.8 h, 1.29 days and 19.4 days (Berson et al., 1953; Bauman et al., 1955; CIS bio International, 2010). The disappearance of rHSA, in the first hour after injection, could be described as a bi-exponential decay curve with a inflection point after approximately 10 min (Margarson & Soni, 2002).
Despite the fact that rHSA has a multi-compartment kinetic model, all published TERalb data analyses are based on a mono-exponential kinetic model. This mono-exponential TERalb model has four assumptions: the rHSA behaves like endogenous albumin; the albumin metabolism is in steady state during the TERalb test; rHSA has a mono-exponential blood pool elimination during the first hour after injection, with a rate constant equal to that of time zero; the initial blood pool elimination reflects extravasation and is not influenced by the rHSA metabolism rate (Parving & Gyntelberg, 1973).
The original protocol of Parving et al. describes that a small amount of I-125 or I-131 labeled rHSA is injected in an arm vein, and eight venous blood samples were drawn from the contralateral arm at 10, 15, 20, 30, 40, 50, 55, and 60 min after the injection. The radioactivity of the rHSA in each blood sample was measured in duplicate. The TERalb was calculated and expressed as the percentage decline of radioactivity during the first hour (%/h) (Parving & Gyntelberg, 1973).
However most studies using TERalb values show variation in sampling schemes ranging from 3 to 13 blood samples (Margarson & Soni, 2002; Jensen et al., 1992). Some of the schemes started already 1 min after the injection of rHSA, while others started blood sampling 20 min after the administration of rHSA (Margarson & Soni, 2002; Norberg et al., 2015).
This variation in sampling schemes does impact the calculated TERalb. Sampling schemes that started 5 min after administration found TERalb in the range of 6.9–9.1%/h (Dell'omo et al., 2006; Dell'Omo et al., 2000; Haskell et al., 1997; Pedrinelli et al., 2000; Pedrinelli et al., 1999; van Eijk et al., 2005; Rorije et al., 2018). While studies which started sampling 10 min after injection found a lower TERalb of approximately 5.5%/h (Parving, 1975; Jensen et al., 1992; Nannipieri et al., 1997; Jensen, 1995; Nannipieri et al., 1995; Staberg et al., 1982; Zietse et al., 1995). These differences in TERalb were not related to differences in patient population, but are in line with the multi-exponential kinetics of rHSA.
As the use of TERalb for clinical research seems to gain in popularity, standardization of the technique is essential: i.e. reducing variation in performing the test and thereby reducing variation in the test result. As we observed large variations between different publications in TERalb sampling schemes and most likely thereby variation in TERalb results, we therefore aimed to study the influence of different sampling schemes and the use of a mono- or bi-exponential analysis on the calculation of TERalb.
Methods
Study population and study design
Selected participants of this post hoc study were part of a cross-over intervention study protocol investigating whether an acute intravenous sodium load, as compared to a chronic dietary sodium load, differs in its effects on blood pressure, the endothelial surface layer and microcirculation (Rorije et al., 2018). Participants included healthy men, and both male type 1 diabetes mellitus and hereditary multiple exostosis patients (i.e., patients with, respectively, acquired and genetically determined glycocalyx changes) (Mooij et al., 2014). Exclusion criteria were hypertension (≥ 140/90 mmHg), obesity (body mass index (BMI) ≥ 30 kg/m2), history of primary hyperlipoproteinemia, coagulation disorders, and renal or cardiovascular diseases. All participants were randomized to a low sodium diet (LSD, < 50 mmol Na+ daily) or to a high sodium diet (HSD, > 200 mmol Na+ daily) for 8 days, separated by a crossover period of at least 1 week. The study was performed at the Amsterdam UMC, location AMC, Amsterdam, The Netherlands. All participants provided written informed consent and approval was obtained from the local ethics committee. The trial is registered in the Netherlands Trial Register (NTR4095 and NTR4788).
Transcapillary escape rate of rHSA
An intravenous (IV) bolus of saline solution with rHSA labeled with 100 kBq I-125 was administered in a cubital vein. Blood samples were drawn from the contralateral arm at baseline and between 3 and 60 min after injection of rHSA. Radioactivity in plasma was measured in duplicate with a Wizard2 2480 automatic gamma counter (PerkinElmer, Waltham, Massachusetts, USA) with a coefficient of variation of < 3%. The routine quality controls of the gamma counter were performed according to the standard GLP features of PerkinElmer, including detector energy resolution, background, absolute - and relative detector efficiency, detector stability probability and calibration.
The TERalb was calculated with PKSolver, a free Microsoft Excel add-in for pharmacokinetic (PK) and pharmacodynamic (PD) data analysis (Zhang et al., 2010). PKSolver has been validated and has been used in different PK/PD studies (Zhang et al., 2010; Kulo et al., 2017; de Velde et al., 2016; Nezic et al., 2014; Balakumar et al., 2013; Wenstedt et al., 2020).
TERalb was expressed as percentage decline in plasma radioactivity per hour (%/h). The TERalb calculation with PKSolver was performed for an IV bolus administration. The formula used for the calculation of TERalb was:
The predicted activity of rHSA at T0 min (A 0 min) and at T60 min (A 60 min) were calculated by PKSolver (Microsoft Excel 2016) based on a mono- and bi-exponential kinetic model. This program also calculated the correlation coefficient (R) between the observed and predicted data.
Sampling schemes
After acquiring the PK curves of rHSA, we calculated the TERalb according the following simulated blood sampling schemes:
-
T3–60 min: 3, 4, 5, 10, 15, 20, 30, 45, and 60 min
-
T5–60 min: 5, 10, 15, 20, 30, 45, and 60 min
-
T10–60 in: 10, 15, 20, 30, 45, and 60 min
-
T15–60 min: 15, 20, 30, 45, and 60 min
-
T20–60 min: 20, 30, 45, and 60 min
-
Tmax – 60 min: from individual A max till 60 min
All blood samples before A max of the PK curves were excluded for the calculation of TERalb, irrespective of the sampling scheme.
Statistics
The effect of different blood sampling schemes on the TERalb values were analyzed by fitting a mixed model as implemented in IBM SPSS Statistics (version 26, IBM, USA). This mixed model uses a compound symmetry covariance matrix and is fitted using maximum likelihood. In the absence of missing values, this method results in the same p values as multiple comparisons tests (e.g. repeated measures ANOVA) that are less able to deal with missing values. Therefore, in the presence of missing values, the results can be interpreted like repeated measures ANOVA (Harrison et al., 2018). We used Bonferroni correction as post hoc test and p values < 0.05 were considered statistically significant. Results were reported as mean ± standard error of the mean (SEM). Bland-Altman plots were used to evaluate the level of agreement between two different blood sample schemes.
Results
Patient demographics
In total 27 men were included resulting in 54 PK curves (27 linked to the LSD and 27 linked to the HSD), based on 486 (54*9 samples) blood sample analyses. The study population consisted of 12 healthy volunteers, 8 diabetes mellitus type I patients, and 7 patients with hereditary multiple exostoses. All volunteers were between 18 and 38 years old with a median age of 24 (range 18–38 years). Other characteristics of study participants are displayed in Table 1.
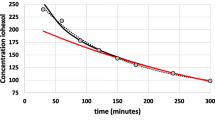
The blood serum disappearance of rHSA of the study participants is shown in Fig. 1. The pharmacokinetic graphic shows a bi-exponential slope of decay curve with inflexion point at 15–20 min.
Tmax after rHSA administration
The Tmax after rHSA administration showed a large inter-individual variability (Fig. 2). The mean Tmax was 6.9 ± 0.6 min. In 85% of the participants Amax of rHSA was reached within 10 min, while Tmax was reached at 20 min after administration for all participants without an effect of subject category (HME, DM type 1 or healthy volunteer) or the diet followed (LSD vs HSD). Therefore T20–60 min was used as the reference scheme. The mean TERalb values of the other time schemes were compared to the reference scheme T20–60 min based on mono-exponential kinetic analysis.
TERalb based on mono-exponential kinetic analysis
The reference T20–60 min scheme included 54 of the 54 PK curves. The mean TERalb of the Tmax – 60 min scheme resulted in the highest calculated TERalb: 8.30 ± 0.49%/h (Fig. 3). The TERalb calculated with the T20–60 min reference scheme (6.19 ± 0.49%/h) was significantly lower compared to the TERalb of the T3–60 min (mean difference = − 1.63%/h, CI = − 2.65 – − 0.61%/h, p < 0.001), T5–60 min (mean difference = − 1.32%/h, CI = − 2.27 – − 0.36%/h, p = 0.001), and Tmax – 60 min (mean difference = − 2.11%/h, CI = − 3.04 – − 0.48%/h, p < 0.001) schemes. There were no significant difference between the mean TERalb of the T20–60 min reference scheme and the T10–60 min and T15–60 min scheme.
TERalb based on bi-exponential kinetic analysis
Using a bi-exponential analysis according to the T20–60 min scheme did not result in significant different TERalb values when compared to the mono-exponential analysis based T20–60 min reference scheme (respectively 6.19 ± 0.46%/h vs. 6.05 ± 0.46%/h, p = 1.000). The mean TERalb of the reference T20–60 min scheme was significantly lower compared to the mean TERalb of the bi-exponential kinetic analysis of the T3–60 min (mean difference = − 1.49%/h, CI = − 2.68 – − 0.29%/h, p = 0.004), T5–60 min (mean difference = − 1.09%/h, CI = − 2.18 – − 0.01%/h, p = 0.050), and Tmax – 60 min (mean difference = − 1.87%/h, CI = − 2.95 – − 0.80%/h, p < 0.001) schemes. There were no significant difference between the mean TERalb of the T20–60 min reference scheme and the T10–60 min and T15–60 min schemes based on bi-exponential kinetic analysis (Fig. 4).
Figure 5 shows the Bland-Altman plot with agreement between the bi-exponential analysis based on T20–60 min scheme and T20–60 min reference scheme. The TERalb showed a bias of − 0.1%/h between the different time schemes without a significant trend over the data range (1.7–12.8%/h) and with a consistent variability over the data range.
Discussion
To our knowledge, this study is the first to examine the influence of different blood sampling schemes and the impact of mono- or bi-exponential analyses on the calculation of TERalb. Our findings emphasize the necessity to standardize TERalb calculations.
We found that the TERalb became lower when blood sample collection started later. This phenomenon has been reported previously (Margarson & Soni, 2002). In this context it is remarkable that the majority of published studies used a fixed time sampling scheme with the first blood sampling within 10 min (Dell'omo et al., 2006; Dell'Omo et al., 2000; Haskell et al., 1997; Pedrinelli et al., 2000; Pedrinelli et al., 1999; van Eijk et al., 2005; Rorije et al., 2018). This practice will have caused a overestimation of the reported TERalb. In addition, this makes the reported findings based on TERalb difficult to reproduce and troublesome to extrapolate. Especially when TERalb values of different sampling schemes are compared with each other.
Although the blood serum disappearance of rHSA should be described as a bi-exponential kinetic model, as shown in Fig. 1, the mean TERalb values between mono- and bi-exponential analysis were not significant different. Therefore, we concluded that the mono-exponential kinetic analysis, which is common used for TERalb analysis, is a robust and easy to use approach to calculate the TERalb in the daily practice.
Our data showed that biodistribution of rHSA seems to be complete after 15–20 min. Apparently rHSA may need up to 20 min to reach an equilibrium. This inter-individual variation may be explained by the rate of lymphatic return or redistribution into the hepatic and splenic interstitium (Margarson & Soni, 2002; Henriksen & Schlichting, 1981). To minimize the number of blood samples, we advocate the use a mono-exponential model with blood sampling starting 20 min after rHSA administration for the daily practice. For scientific purposes, we suggest to use the Tmax scheme to correct for the inter- and intra-individual variability. It should be noted that these TERalb values are significant higher compared to the daily practice scheme.
This study has several limitations that need to be addressed. First, we pooled data, because no differences between healthy participants, type 1 diabetes mellitus and hereditary multiple exostosis patients were detected. One would have expected higher TERalb values in type 1 diabetes mellitus patients. However, our study included young male with uncomplicated type 1 diabetes without albuminuria. Likely, at this stage of the disease, TERalb is still unaffected. Secondly, we did not collect any blood samples after T60 min. Blood sampling for longer time periods after administration, for example up to 24 h after rHSA injection, could have helped in better understanding the kinetics of rHSA blood clearance.
Conclusions
To our knowledge, this study examined for the first time whether different blood sampling schemes impact TERalb values. We found significant differences between the blood sampling schemes which will cause bias in reporting TERalb and makes it difficult to reproduce and extrapolate outcomes of TERalb.
As there is a large variation in the timing of the maximum serum radioactivity of rHSA, blood sampling schemes starting before 10 min after administration of rHSA will result in a significant overestimation of TERalb. In addition, variation in mono- or bi-exponential kinetic modeling did not result in significant changes in TERalb. Therefore, we emphasize the need to standardize TERalb and for practical and logistical reasons advocate the use of a mono-exponential model with blood sampling starting 20 min after rHSA administration.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- rHSA:
-
radioactive iodide labeled human serum albumin
- TERalb:
-
Transcapillary escape rate of albumin
- Tmax:
-
Time to peak drug concentration
- Cmax:
-
Peak drug concentration
- I-125:
-
Iodine-125
- I-131:
-
Iodine-131
- BMI:
-
Body mass index
- LSD:
-
Low sodium diet
- HSD:
-
High sodium diet
- GLP:
-
Good Laboratory Practice
References
Balakumar K, Raghavan CV, Selvan NT, Prasad RH, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B Biointerfaces. 2013;112:337–43.
Bauman A, Rothschild MA, Yalow RS, Berson SA. Distribution and metabolism of I131 labeled human serum albumin in congestive heart failure with and without proteinuria. J Clin Invest. 1955;34(9):1359–68.
Berson SA, Yalow RS, Schreiber SS, Post J. Tracer experiments with I131 labeled human serum albumin: distribution and degradation studies. J Clin Invest. 1953;32(8):746–68.
Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(12):2646–55.
CIS bio international. Summary of product characteristics of SERALB-125. France: CIS bio international; 2010.
de Velde F, de Winter BC, Koch BC, van Gelder T, Mouton JW. Consortium C-N. non-linear absorption pharmacokinetics of amoxicillin: consequences for dosing regimens and clinical breakpoints. J Antimicrob Chemother. 2016;71(10):2909–17.
Dell’Omo G, Bandinelli S, Penno G, Pedrinelli R, Mariani M. Simvastatin, capillary permeability, and acetylcholine-mediated vasomotion in atherosclerotic, hypercholesterolemic men. Clin Pharmacol Ther. 2000;68(4):427–34.
Dell'omo G, Penno G, Pucci L, Lucchesi D, Fotino C, Del Prato S, et al. ACE gene insertion/deletion polymorphism modulates capillary permeability in hypertension. Clin Sci. 2006;111(6):357–64.
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, et al. A brief introduction to mixed effects modelling and multi-model inference in ecology. Peer J. 2018;6:e4794.
Haskell A, Nadel ER, Stachenfeld NS, Nagashima K, Mack GW. Transcapillary escape rate of albumin in humans during exercise-induced hypervolemia. J Appl Physiol. 1997;83(2):407–13.
Henriksen JH, Schlichting P. Increased extravasation and lymphatic return rate of albumin during diuretic treatment of ascites in patients with liver cirrhosis. Scand J Clin Lab Invest. 1981;41(6):589–99.
Jensen EW, Bryde Andersen H, Nielsen SL, Christensen NJ. Long-term smoking increases transcapillary escape rate of albumin. Scand J Clin Lab Invest. 1992;52(7):653–6.
Jensen JS. Renal and systemic transvascular albumin leakage in severe atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15(9):1324–9.
Kulo A, Smits A, Maleskic S, Van de Velde M, Van Calsteren K, De Hoon J, et al. Enantiomer-specific ketorolac pharmacokinetics in young women, including pregnancy and postpartum period. Bosn J Basic Med Sci. 2017;17(1):54–60.
Margarson MP, Soni NC. Effects of albumin supplementation on microvascular permeability in septic patients. J Appl Physiol. 2002;92(5):2139–45.
Mooij HL, Cabrales P, Bernelot Moens SJ, Xu D, Udayappan SD, Tsai AG, et al. Loss of function in heparan sulfate elongation genes EXT1 and EXT 2 results in improved nitric oxide bioavailability and endothelial function. J Am Heart Assoc. 2014;3(6):e001274.
Nannipieri M, Penno G, Rizzo L, Pucci L, Bandinelli S, Mattei P, et al. Transcapillary escape rate of albumin in type II diabetic patients. The relationship with microalbuminuria and hypertension. Diabetes Care. 1997;20(6):1019–26.
Nannipieri M, Rizzo L, Rapuano A, Pilo A, Penno G, Navalesi R. Increased transcapillary escape rate of albumin in microalbuminuric type II diabetic patients. Diabetes Care. 1995;18(1):1–9.
Nezic L, Derungs A, Bruggisser M, Tschudin-Sutter S, Krahenbuhl S, Haschke M. Therapeutic drug monitoring of once daily aminoglycoside dosing: comparison of two methods and investigation of the optimal blood sampling strategy. Eur J Clin Pharmacol. 2014;70(7):829–37.
Norberg A, Rooyackers O, Segersvard R, Wernerman J. Albumin kinetics in patients undergoing major abdominal surgery. PLoS One. 2015;10(8):e0136371.
Parving HH. Microvascular permeability to plasma proteins in hypertension and diabetes mellitus in man--on the pathogenesis of hypertensive and diabetic microangiopathy. Dan Med Bull. 1975;22(6):217–33.
Parving HP, Gyntelberg F. Transcapillary escape rate of albumin and plasma volume in essential hypertension. Circ Res. 1973;32(5):643–51.
Pedrinelli R, Dell'Omo G, Bandinelli S, Penno G, Mariani M. Transvascular albumin leakage and forearm vasodilatation to acetylcholine in essential hypertension. Am J Hypertens. 2000;13(3):256–61.
Pedrinelli R, Penno G, Dell’Omo G, Bandinelli S, Giorgi D, Di Bello V, et al. Microalbuminuria and transcapillary albumin leakage in essential hypertension. Hypertension. 1999;34(3):491–5.
Rorije NMG, Olde Engberink RHG, Chahid Y, van Vlies N, van Straalen JP, van den Born BH, et al. Microvascular permeability after an acute and chronic salt load in healthy subjects: a randomized open-label crossover intervention study. Anesthesiology. 2018;128(2):352–60.
Staberg B, Worm AM, Rossing N, Brodthagen H. Microvascular leakage of plasma proteins after PUVA and UVA. J Invest Dermatol. 1982;78(4):261–3.
van Eijk LT, Pickkers P, Smits P, van den Broek W, Bouw MP, van der Hoeven JG. Microvascular permeability during experimental human endotoxemia: an open intervention study. Crit Care. 2005;9(2):R157–64.
Wenstedt EFE, Rorije NMG, Olde Engberink RHG, van der Molen KM, Chahid Y, Danser AHJ, et al. Effect of high-salt diet on blood pressure and body fluid composition in patients with type 1 diabetes: randomized controlled intervention trial. BMJ Open Diabet Res Care. 2020;8(1):e001039.
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft excel. Comput Methods Prog Biomed. 2010;99(3):306–14.
Zietse R, Derkx FH, Weimar W, Schalekamp MA. Effect of atrial natriuretic peptide on renal and vascular permeability in diabetes mellitus. J Am Soc Nephrol. 1995;5(12):2057–66.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YC and HV analyzed and interpreted the data. YC, SB and HV were major contributors in writing the manuscript. NR, LV and RM have substantively revised the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants provided written informed consent and approval was obtained from the local ethics committee. The trial is registered in the Netherlands Trial Register (NTR4095 and NTR4788).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chahid, Y., Rorije, N.M.G., el Boujoufi, S. et al. Transcapillary escape rate of 125I-albumin in relation to timing of blood sampling: the need for standardization. EJNMMI radiopharm. chem. 6, 9 (2021). https://doi.org/10.1186/s41181-021-00125-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41181-021-00125-0