Abstract
Background
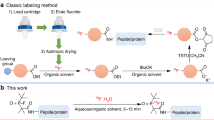
Copper-mediated radiofluorination is a straightforward method to produce a variety of [18F]fluoroarenes and [18F]fluoroheteroarenes. To minimize the number of steps in the production of 18F-labelled radiopharmaceuticals, we have developed a short and efficient azeotropic drying-free 18F-labelling method using copper-mediated fluorination. Our goal was to improve the copper-mediated method to achieve wide substrate scope with good radiochemical yields with short synthesis time.
Results
Solid phase extraction with Cu (OTf)2 in dimethylacetamide is a suitable activation method for [18F]fluoride. Elution efficiency with Cu (OTf)2 is up to 79% and radiochemical yield (RCY) of a variety of model molecules in the crude reaction mixture has reached over 90%. Clinically relevant molecules, norepinephrine transporter tracer [18F]NS12137 and monoamine transporter tracer [18F]CFT were produced with 16.5% RCY in 98 min and 5.3% RCY in 64 min, respectively.
Conclusions
Cu (OTf)2 is a suitable elution agent for releasing [18F]fluoride from an anion exchange cartridge. The method is fast and efficient and the Cu-complex is customizable after the release of [18F]fluoride. Alterations in the [18F]fluoride elution techniques did not have a negative effect on the subsequent labelling reactions. We anticipate this improved [18F]fluoride elution technique to supplant the traditional azeotropic drying of [18F]fluoride in the long run and to concurrently enable the variations of the copper-complex.
Similar content being viewed by others
Background
Positron emission tomography (PET) and related hybrid methods (PET/MRI and PET/CT) are essential tools for in vivo imaging. Among radionuclides used for PET, fluorine-18, a short-lived radioisotope of fluorine, has proven to be advantageous owing to the adequate half-life (109.8 min), clean decay process (97% β+), and low positron energy (634 keV). These properties tolerate multistep synthesis and purification. Introduction of fluorine-18 to the suitable molecular probes has remained a challenge owing to the lack of suitable 18F-labelling methods (Miller et al. 2008).
Traditionally, 18F-fluorination reactions have been divided in to two categories, electrophilic and nucleophilic radiofluorination. The electrophilic fluorination reagent, [18F]F2, is advantageous when labelling electron-rich arenes, but the produced radiotracers are always achieved with low molar activity (Am). Low Am is due to the use of added carrier-fluorine in the production of [18F]F2. Thus, nucleophilic 18F-fluorination with [18F]fluoride has been the most widely used radiofluorination method all over the world. However, the reactivity scope of the nucleophilic substitution has been confined to electron-poor arenes (Preshlock et al. 2016). The reactivity scope of the nucleophilic radiofluorination has been improved by the use of iodonium salts and ylides (Pike and Aigbirhio 1995; Ross et al. 2007; Satyamurthy and Barrio 2010) and sulfonium salts (Mu et al. 2012; Gendron et al. 2018). Recently, transition metal mediated radiofluorination with palladium (Lee et al. 2011; Kamlet et al. 2013) and nickel (Lee et al. 2012; Zlatopolskiy et al. 2015b; Hoover et al. 2016) has been introduced. Palladium-mediated fluorination has proved to be impractical due to the moisture sensitive nature of the required palladium complexes. Additionally, both of these methods involve the use of complex precursor molecules, which have proven to be challenging to synthesize. Another challenge includes the automation of the synthetic procedures of nickel- and palladium-mediated 18F-labelling. Automation of the synthesis procedures is essential for production of radiopharmaceuticals according to good manufacturing practice (GMP).
A most promising development of the radiofluorination methods was the introduction of the copper-mediated 18F-fluorination. Via this method, a wide scope of 18F-labelled arenes and heteroarenes has been synthesized starting from simple arylboronic esters (Tredwell et al. 2014), aryl boronic acids (Mossine et al. 2015), or stannylated arenes (Makaravage et al. 2016) with straightforward synthesis conditions. Copper-mediated 18F-fluorination has been widely studied in many radiochemistry laboratories around the world and it has been optimized to achieve high radiochemical yields (RCY) and good Am values by improving the reaction conditions. Copper-mediated fluorination typically involves the use of a base. For example, when [18F]fluoride is dried traditionally with conventional azeotropic drying, K2CO3 (pKa of the conjugate acid is 10.3) and cryptand, Kryprofix222, are used. Additionally, the copper-complex usually used for the radiolabelling reaction contains pyridine (Cu(OTf)2(py)4, with the pKa of the conjugate acid of pyridine being 5.3). Use of bases and/or cryptands have been blamed for suppression of the RCYs (Zlatopolskiy et al. 2015a; Antuganov et al. 2017; Mossine et al. 2017). In addition, subsequent reactions might be sensitive to bases. To avoid the use of these reagents, alternative [18F]fluoride processing methods have been reported (Mossine et al. 2017; Zischler et al. 2017). Furthermore, the use of azeotropic drying is a time consuming process and results in part of the [18F]fluoride adhering in the glass vessel walls during the drying process (Mossine et al. 2015). Consequently, the latest developments include the replacement of azeotropic drying of [18F]fluoride by solid phase extraction (SPE). The SPE process has been improved by using alcohols in the [18F]fluoride processing (Zischler et al. 2017) or pyridinium sulfonates (Antuganov et al. 2019) or dimethylaminopyridinium triflates (DMAP) as an elution agents (Zhang et al. 2019). However, these new [18F]fluoride elution methods typically involve separate evaporation steps after the elution or utilize relatively toxic chemicals, like DMAP, which might not be necessary for the radiolabelling reaction itself. In the clinical radiopharmaceutical production, it is preferred to minimize the use of different, possibly toxic reagents or chemicals to keep the final purification of the radiotracer as simple as possible.
Recently, our group improved the copper-mediated radiofluorination conditions to quickly and efficiently produce norepinephrine transporter tracer [18F]NS12137 (exo-3-[(6-[18F]fluoro-2-pyridyl)oxy]8-azabicyclo [3.2.1]octane) (Lahdenpohja et al. 2019). Herein, we introduce an azeotropic drying-free copper-mediated radiofluorination method, where the copper-complex is customizable after the drying of [18F]fluoride by SPE and the [18F]fluoride is ready to use in subsequent labelling reaction without the use of additional reagents or any other manipulations. Our aim was to study the effects of the preconditioning of different typically used SPE cartridges on trapping and elution of [18F]fluoride. We used Cu(OTf)2 and Cu(OTf)2(py)4 for the elution of [18F]fluoride, and labelled several arenes and heteroarenes, including [18F]NS12137 and monoamine transporter tracer [18F]CFT, to prove the usefulness of this method.
Methods
General
Unless otherwise stated in the supporting information, all of the reagents and solvents were used as received from commercial suppliers without further purification. The following, widely used in clinical radiotracer production, anion exchange cartridges with luers were used: Sep-Pak Accell Plus QMA Carbonate Plus Light Cartridge 46 mg; Sep-Pak Accell Plus QMA Plus Light Cartridge 130 mg and Chromafix PS-HCO3 45 mg. Additionally we used handmade 10 mg QMA cartridges to see the effect of minimized anion exchange resin mass. The columns were preconditioned with following standard preconditioning solutions: 1) 20 mL H2O; 2) 10 mL 0.5 M LiOTf and 20 mL H2O; or 3) 10 mL 0.5 M Na2SO4 and 20 mL H2O. HPLC methods are described in the Supporting information Additional file 1.
Radiochemistry studies
The full description of the elution and radiolabelling studies can be found in the supporting information. [18F]Fluoride elution and the radiolabelling reactions were performed in two hot cells with a remote-controlled synthesis devices that were built in-house. The process was not fully automated and the synthesis times of [18F]NS12137 and [18F]CFT are not optimised.
[18F]Fluoride was loaded to an anion exchange cartridge, the cartridge was washed with dimethylacetamide (DMA, 5 mL) and [18F]fluoride was eluted with Cu(OTf)2 or Cu(OTf)2(py)4 in DMA (0.5 mL). The amount of the copper-complex varied between 12 and 96 μmol to see the effect of the amount of the elution agent to the [18F]fluoride elution. In the preliminary test, LiOTf (24 μmol) was added to the elution solution. After elution with copper-complex, the cartridge was washed with DMA (0.5 mL). The elution efficiency (EE) was calculated by dividing the activity of the eluted [18F]fluoride fraction by the sum of the activity of the eluted [18F]fluoride fraction and the activity remaining in the SPE cartridge. The [18F]fluoride recovery was calculated by dividing the activity of the eluted [18F]fluoride fraction by the sum of the activity of the SPE cartridge and the waste bottle after loading the anion exchange cartridge. The EE and the [18F]fluoride recovery were non-decay-corrected because the [18F]fluoride loading, elution and the radioactivity measurements were completed within 5 min.
In the radiolabelling test, [18F]fluoride was eluted straight into a conical vial containing the precursor (8 μmol) and pyridine (50 μL) in DMA (50 μL), the total volume being 1.1 mL. The reaction solution was heated at 120 °C for 5 to 15 min under ambient air. For [18F]NS12137, a previously published deprotection method was followed (Kirjavainen et al. 2018). [18F]NS12137 and [18F]CFT were purified with semi-preparative HPLC.
[18F]Fluoride incorporation in [18F]NS12137 synthesis was determined according to SPE. The radioactivity in the reaction vial before loading the activity to the cartridge was compared to the activity eluted from the cartridge. All reported RCY values were decay-corrected to the end of bombardment (EOB). The RCY (based on radio-HPLC analysis of the crude reaction mixture) values were determined from the amount of overall radioactivity eluted from the analytical HPLC column. We verified that there was no leftover radioactivity in the injector or in the HPLC column after the analytical HPLC run. RCY (based on radio-HPLC analysis of the crude reaction mixture) values are expressed as mean ± standard deviation.
Levels of copper in [18F]NS12137 and [18F]CFT were analysed with inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer, Elan DRC Plus). Commercial multielement standard was used for instrument calibration.
Results
[18F]fluoride processing
[18F]Fluoride can be satisfactorily eluted from various SPE cartridges by using solely Cu(OTf)2(py)4 or Cu(OTf)2 as an elution agent in DMA. The term elution efficiency is used when the EE and the [18F]fluoride recovery are on the same level, which means no [18F]fluoride has been lost during trapping or washing processes. The term [18F]fluoride recovery is used when some of the [18F]fluoride is lost during the trapping and/or washing process. Preliminary [18F]fluoride elution tests showed that preconditioning of the SPE cartridge had, in some cases, a significant effect on the [18F]fluoride recovery when using copper complexes as eluting agents (see Table 1). Preconditioning of non-carbonated 130 mg QMA cartridges and carbonated 46 mg QMA cartridges with aqueous LiOTf solution resulted in higher [18F]fluoride recovery and the EE (Table 1, entries 5, 9) than with only aqueous preconditioning (Table 1, entries 7, 11). At the same time, we noticed, that using LiOTf and a copper complex together as an elution agent improved the [18F]fluoride elution when non-carbonated 130 mg QMA cartridges and carbonated 46 mg QMA cartridges were preconditioned only with water (Table 1, entries 8, 12). If these cartridges were preconditioned with aqueous LiOTf, additional LiOTf as an elution agent did not have a noteworthy effect on the EE (Table 1, entries 6, 10). With non-carbonated 10 mg QMA cartridges, the effect of the LiOTf preconditioning or use of LiOTf as an elution agent was unnoticeable (Table 1, entries 1–4). LiOTf preconditioning did not improve the [18F]fluoride elution in bicarbonated PS-HCO3 cartridges (Table 1, entries 15, 16). When using aqueous Na2SO4 solution for the preconditioning of a carbonated cartridge, [18F]fluoride recovery was only 0.6 ± 0.1% and the EE 3.5 ± 0.5% (n = 2) (Table 1, entry 14). The elution speed did not have a significant effect on the EE (Table 1, entries 11, 13). The only case when a significant difference was observed between [18F]fluoride recovery and EE was when 10 mg QMA cartridges were used (Table 1, entries 1–4).
The results from the [18F]fluoride elution studies with various cartridges, cartridge preconditioning, and amounts of the elution agent are presented in Table 2. Entries with the lowest and the highest elution efficiencies are highlighted. The EE of [18F]fluoride varied from 18.5% (Table 2, entry 7) to 79.5% (Table 2, entry 5) and the [18F]fluoride recovery ranged from 17.5% (Table 2, entry 16) to 74.7% (Table 2, entry 6). The [18F]fluoride recovery behaved in accordance with the EE. The highest elution efficiencies were achieved by using commercial non-carbonated 130 mg QMA cartridges (Table 2, entries 1–6). The PS-HCO3 cartridge behaved similarly to 130 mg QMA cartridges when comparing the amounts of the elution agent (Table 2, entries 7–12). Self-filled 10 mg QMA cartridges gave poorer results than the 130 mg QMA cartridges (Table 2, entries 13–18). Carbonated 46 mg QMA cartridges gave poor results (EE < 50%) regardless of the preconditioning of the cartridge (Table 2, entries 19–22). Increasing the molar amount of the elution agent increased the elution efficiency until a certain limit with every cartridge tested. The most significant differences in the EE were achieved with every cartridge (excluding carbonated 46 mg QMA cartridges) when the amount of the elution agent was increased from 24 μmol to 48 μmol. Cu(OTf)2 was always a better eluting agent than Cu(OTf)2(py)4, except when using a carbonated QMA cartridge.
18F-Radiolabelling
Next, we tested if the optimized elution method (Table 2, entry 5) had an effect on the radiofluorination of aryl boronic acids, aryl boronic esters, and arylstannanes. Boronic acid precursors were used to synthetize 1-[18F]fluoro-4-iodobenzene, 4-[18F]fluorobiphenyl, 4-[18F]fluorophenol, [18F]fluorobenzene 4-[18F]fluorobenzonitrile, 1-[18F]fluoro-4-nitrobenzene, 3-[18F]fluoropyridine, 2-[18F]fluoronaphthalene; boronic ester precursor was used to synthetize 4-[18F]fluoroindole and trimethylstannyl precursors were used to synthetize [18F]NS12137 and [18F]CFT. Even though our previous results (Lahdenpohja et al. 2019) showed, that a 2 min reaction at 120 °C is enough for optimal RCY (based on the HPLC analyses of the reaction solution), here we used 5 to 15 min reaction times because most related works have used 20 min reactions. Results from the 18F-labelling reactions are presented in Fig. 1. Different model molecules were produced up to 91.6% RCY (based on the HPLC analyses of the reaction solution). The lowest RCY (based on the HPLC analyses of the reaction solution) was achieved with 3-[18F]fluoropyridine and the highest with 4-[18F]fluoroindole.
Finally, as proof-of concept, we synthetized [18F]NS12137 and [18F]CFT (Fig. 2) with optimized elution conditions (Table 2, entry 5). The [18F]NS12137 intermediate was achieved with 96.3 ± 2.0% RCY (based on the HPLC analyses of the reaction solution) after a 5 min reaction at 120 °C. Incorporation of the [18F]fluoride was 37.7% according to the SPE and after the hydrolysis and semi-preparative HPLC purification, [18F]NS12137 was produced in 16.5% RCY and 100% RCP in 98 min synthesis time (n = 1). [18F]CFT was achieved in 6.9% RCY (based on the HPLC analyses of the reaction solution) after a 15 min reaction at 120 °C. After the semi-preparative purification, [18F]CFT was produced in 5.3% RCY and 98.1% RCP in an overall synthesis time of 64 min (n = 1). According to the ICP-MS analysis of the final product fractions, the amount of Cu-63 residue was 3.9 μg in [18F]NS12137 and 46.0 μg in [18F]CFT. These amounts are under the limits considered toxic according to ICH Q3D (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use n.d.).
Discussion
[18F]fluoride processing
A key step in the 18F-radiolabelling reactions is the drying of [18F]fluoride to make it more reactive, i.e., a better nucleophile. Conventionally used azeotropic distillation with MeCN is time consuming and here we have replaced it with SPE by using anion exchange cartridges. Lately, the use of SPE for the drying of [18F]fluoride has also been a widely studied method in copper-mediated 18F-radiofluorination, but typically these methods have still utilized additional evaporation steps (Zischler et al. 2017) or use of additional, sometimes toxic reagents (Antuganov et al. 2019; Zhang et al. 2019). Herein, we have described a simple method, which includes the use of Cu(OTf)2 or Cu(OTf)2(py)4 for the elution of [18F]fluoride, and is suitable for automation and clinical production of radiopharmaceuticals.
Our preliminary studies showed that the preconditioning of the 130 mg QMA cartridges and carbonated 46 mg QMA cartridges has a significant effect on [18F]fluoride recovery and elution efficiency (see Table 1). LiOTf was found to be a superior preconditioning agent providing up to 70% [18F]fluoride recovery when using non-carbonated 130 mg QMA cartridges. Besides, the addition of LiOTf to the elution solution enhanced the elution. This method proposes that the anions are effectively changed from chlorine to triflate or from carbonate to triflate inside the cartridge, and this improves the elution of [18F]fluoride. Similar enhanced elution was not detected when using PS-HCO3 cartridges or self-filled 10 mg QMA-cartridges. PS-HCO3 cartridges might have needed a higher amount of LiOTf in the preconditioning step to replace the bicarbonate. In self-filled 10 mg QMA cartridges, the amount of the anion exchange resin is minimized, which can be detected as reduced trapping efficiency of the cartridge, i.e., leakage of the fluoride during trapping and washing. This is also the reason for large difference in elution efficiency and (Ross et al. 2007)fluoride recovery (Table 1, entries 1–4). Minimized resin mass reduces the need for elution enhancer, such as LiOTf as preconditioning agent or elution agent. When using Na2SO4 solution for the preconditioning, elution efficiency was almost 0%. Such a drastic decrease in the elution compared to the standard level may likely be caused by deactivation of the eluting agent by formation of an inactive Cu(SO2)2(py)4 complex (Mossine et al. 2017).
Cu(OTf)2 was, except with carbonated cartridge, a better elution agent than Cu(OTf)2(py)4. We propose that this result is caused by the steric hindrance of Cu(OTf)2(py)4 during the coordination of [18F]fluoride. We suggest that [18F]fluoride coordinates to the copper complex in the anion exchange resin as previously suggested with alcohol-enhanced copper-mediated radiofluorination (Zarrad et al. 2017). When using large amounts of the copper-complex, the difference in the EE between these two elution agents became discreet. The optimal conditions were chosen to be 48 μmol of Cu(OTf)2 and non-carbonated 130 mg QMA cartridge even though higher amount of copper complex slightly improved the [18F]fluoride recovery. Additionally, large amounts of copper in the reaction solution might complicate the purification of the final product which supports our choice of optimal amount. Also, PS-HCO3 cartridge behaved similarly to non-carbonated 130 mg QMA cartridge, however, the QMA cartridge was chosen because of being one of the most standard anion exchange cartridges used in radiopharmaceutical chemistry laboratories. High deviations in EEs were observed with both elution agents, Cu(OTf)2 and Cu(OTf)2(py)4, as presented in Table 2. We suggest that the EE is very sensitive to the preconditioning speed. According to the results with non-carbonated 130 mg QMA cartridges (Table 1, entries 5, 8), the EE was higher with LiOTf as an additional elution agent than LiOTf as a preconditioning agent, this might be caused by negligent preconditioning.
18F-Radiolabelling
Using SPE for the drying of [18F]fluoride did not have a negative effect on the subsequent labelling reaction and negative effects of the azeotropic drying can be avoided. With time consuming azeotropic drying, some of the [18F]fluoride is typically attached to the glass vial. In the present procedure in the synthesis of [18F]NS12137 with SPE, the incorporation of the [18F]fluoride is improved with approximately 10% when comparing to the azeotropic distillation method (Lahdenpohja et al. 2019). In turn, when drying the [18F]fluoride in a traditional way, the vial is usually still warm when the subsequent reaction is started which can, in some cases, promote the reaction and shorten the reaction time slightly.
With [18F]CFT and the model molecules synthetized in this study, we noticed that increasing the reaction time from 5 to 15 min had only a minor increase in the RCY. Additionally, free [18F]fluoride was detected only in minimal amounts in all of the analyzed crude reaction mixtures according to HPLC analysis. This outcome implies that all the radioactivity in the reaction solution is in an active form. With [18F]NS12137, we observed good incorporation of the [18F]fluoride according to SPE. The lowest RCY was achieved with 3-[18F]fluoropyridine. Unsuccessful reaction is likely caused by the coordination of the precursor molecule to Cu2+ instead of with pyridine. In the setup presented here, pyridine-3-boronic acid might have coordinated to the copper complex, because the elution solution containing the copper complex Cu(OTf)2 was added to the reaction vial containing both pyridine-3-boronic acid and additional pyridine. With this method, the substrate scope is wide, but the order of the reagent additions must be taken into account, especially in the case of pyridine-containing structures.
Conclusions
In conclusion, we have described the fast and efficient copper-mediated 18F-fluorination of arylstannanes and aryl boronic acids by using SPE for the drying of the [18F]fluoride. This new method does not require azeotropic drying of [18F]fluoride or any other evaporation steps, which makes the process more simple and straightforward. We demonstrated that alterations in the [18F]fluoride elution techniques, like preconditioning of the cartridge, amount of the eluting agent, and the elution speed, can have significant effects on the [18F]fluoride recovery and thus on the RCY of the final tracer. We successfully applied the optimized elution method with Cu(OTf)2 to the semi-automated production of model molecules with high RCY and the clinically relevant molecules, [18F]NS12137 and [18F]CFT, in moderate RCY. We anticipate these improved [18F]fluoride elution techniques will supplant the traditional azeotropic drying of [18F]fluoride in clinical radiopharmaceutical production and eventually, increase the achievable RCYs. Our on-going work is concentrated on implementing the method on fully automated synthesis platform and ultimately in clinical radiopharmaceutical production.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- Am :
-
Molar activity
- DMA:
-
Dimethylacetamide
- EE:
-
Elution efficiency
- EOB:
-
End of bombardment
- EOS:
-
End of the synthesis
- HPLC:
-
High pressure liquid chromatography
- PET:
-
Positron emission tomography
- QMA:
-
Quaternary methyl ammonium
- RCP:
-
Radiochemical purity
- RCY:
-
Radiochemical yield
- SPE:
-
Solid phase extraction
- THF:
-
Tetrahydrofuran
References
Antuganov D, Zykov M, Timofeev V, Timofeeva K, Antuganova Y, Orlovskaya V, et al. Copper-mediated radiofluorination of aryl pinacolboronate esters: a straightforward protocol by using pyridinium sulfonates. Eur J Org Chem. 2019;2019:918–22.
Antuganov D, Zykov M, Timofeeva K, Antuganova Y, Orlovskaya V, Krasikova R. Effect of pyridine addition on the efficiency of copper-mediated radiofluorination of aryl pinacol boronates. ChemistrySelect. 2017;2:7909–12.
Gendron T, Sander K, Cybulska K, Benhamou L, Sin PKB, Khan A, et al. Ring-closing synthesis of dibenzothiophene sulfonium salts and their use as leaving groups for aromatic 18F-fluorination. J Am Chem Soc. 2018;140:11125–32.
Hoover A, Lazari M, Ren H, Narayanam MK, Murphy J, van Dam M, et al. A Transmetalation reaction enables the synthesis of [18F]5-fluorouracil from [18F]fluoride for human PET imaging. Organometallics. 2016;35:1008–14.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Guideline for elemental impurities Q3D(R1) (n.d.). https://www.ich.org/products/guidelines/quality/article/quality-guidelines.html ().
Kamlet AS, Neumann CN, Lee E, Carlin SM, Moseley CK, Stephenson N, et al. Application of palladium-mediated 18F-fluorination to PET radiotracer development: overcoming hurdles to translation. PLoS One. 2013;8:1–10.
Kirjavainen AK, Forsback S, López-Picón FR, Marjamäki P, Takkinen J, Haaparanta-Solin M, et al. 18F-labeled norepinephrine transporter tracer [18F]NS12137: radiosynthesis and preclinical evaluation. Nucl Med Biol. 2018;56:39–46.
Lahdenpohja S, Keller T, Rajander J, Kirjavainen AK. Radiosynthesis of the norepinephrine transporter tracer [18F]NS12137 via copper-mediated 18F-labelling. J Label Compd Radiopharm. 2019:1–6.
Lee E, Hooker JM, Ritter T. Nickel-mediated oxidative fluorination for PET with aqueous [18F]fluoride. J Am Chem Soc. 2012;134:17456–8.
Lee E, Kamlet AS, Powers DC, Neumann CN, Boursalian GB, Furuya T, et al. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science. 2011;334:639–42.
Makaravage KJ, Brooks AF, Mossine AV, Sanford MS, Scott PJH. Copper-mediated radiofluorination of arylstannanes with [18F]KF. Org Lett. 2016;18:5440–3.
Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed. 2008;47:8998–9033.
Mossine AV, Brooks AF, Ichiishi N, Makaravage KJ, Sanford MS, Scott PJH. Development of customized [18F]fluoride elution techniques for the enhancement of copper-mediated late-stage radiofluorination. Sci Rep. 2017;7:1–9.
Mossine AV, Brooks AF, Makaravage KJ, Miller JM, Ichiishi N, Sanford MS, et al. Synthesis of [18F]arenes via the copper-mediated [18F]fluorination of boronic acids. Org Lett. 2015;17:5780.
Mu L, Fischer CR, Holland JP, Becaud J, Schubiger PA, Schibli R, et al. 18F-radiolabeling of aromatic compounds using triarylsulfonium salts. Eur J Org Chem. 2012;2012:889–92.
Pike VW, Aigbirhio FI. Reactions of cyclotron-produced [18F]fluoride with diaryliodonium salts—a novel single-step route to no-carrier-added [18F]fluoroarenes. J Chem Soc Chem Commun. 1995;1995:2215–6.
Preshlock S, Tredwell M, Gouverneur V. 18F-labeling of arenes and heteroarenes for applications in positron emission tomography. Chem Rev. 2016;116:719.
Ross TL, Ermert J, Hocke C, Coenen HH. Nucleophilic 18F-fluorination of heteroaromatic iodonium salts with no-carrier-added [18F]fluoride. J Am Chem Soc. 2007;129:8018–25.
Satyamurthy N, Barrio JR. WO2010117435 A2; 2010.
Tredwell M, Preshlock SM, Taylor NJ, Gruber S, Huiban M, Passchier J, et al. A general copper-mediated nucleophilic 18F-fluorination of arenes. Angew Chem Int Ed. 2014;53:7751–5.
Zarrad F, Zlatopolskiy B, Krapf P, Zischler J, Neumaier B. A practical method for the preparation of 18F-labeled aromatic amino acids from nucleophilic [18F]fluoride and stannyl precursors for electrophilic radiohalogenation. Molecules. 2017;22:2231.
Zhang X, Basuli F, Swenson RE. An azeotropic drying-free approach for copper-mediated radiofluorination without addition of base. J Label Compd Radiopharm. 2019;62:139–45.
Zischler J, Kolks N, Modemann D, Neumaier B, Zlatopolskiy BD. Alcohol-enhanced cu-mediated radiofluorination. Chem Eur J. 2017;23:3251–6.
Zlatopolskiy BD, Zischler J, Krapf P, Zarrad F, Urusova EA, Kordys E, et al. Copper-mediated aromatic radiofluorination revisited: efficient production of PET tracers on a preparative scale. Chem Eur J. 2015a;21:5972–9.
Zlatopolskiy BD, Zischler J, Urusova EA, Endepols H, Kordys E, Frauendorf H, et al. A practical one-pot synthesis of positron emission tomography (PET) tracers via nickel-mediated radiofluorination. ChemistryOpen. 2015b;4:457–62.
Acknowledgements
We thank Prof Olof Solin from Turku PET Centre Turku, Finland for his valuable assistance and experience with 18F-labelling. We are thankful to Dr. Dan Peters of DanPET AB (Sweden) for providing the stannylated NS12137 precursor and the reference compounds. We thank Sten Lindholm from Åbo Akademi University (Turku, Finland) for executing the ICP-MS experiments.
Funding
This study was funded by the Academy of Finland, grant number 307924.
Author information
Authors and Affiliations
Contributions
SL and AKK designed the study; SL, NR, JR and AKK performed the experiments; SL and AKK analyzed the data; SL drafted the manuscript; AKK revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1.
More detailed description of analytical HPLC and SPE methods as well as radiochemistry studies are presented in the Additional file. (DOCX 1151 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lahdenpohja, S.O., Rajala, N.A., Rajander, J. et al. Fast and efficient copper-mediated 18F-fluorination of arylstannanes, aryl boronic acids, and aryl boronic esters without azeotropic drying. EJNMMI radiopharm. chem. 4, 28 (2019). https://doi.org/10.1186/s41181-019-0079-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41181-019-0079-y