Abstract
Background
The aim of this study was to investigate dose-response effects of vitamin D3 (VD3) supplementation on the early stages of diethylnitrosamine (DEN) and carbon tetrachloride (CCl4)-induced hepatocarcinogenesis in rats.
Methods
The animals were randomly allocated into six experimental groups (10 rats each) treated as follows: group 1: no treatment; groups 2–6: single intraperitoneal injection of N-diethylnitrosamine; groups 2–6: intragastric CCl4; groups 3–6: intragastric VD3 at 10,000, 20,000, 40,000, and 60,000 IU/kg b.w., respectively.
Results
Serum 25-hydroxyvitamin D (25-OHD) levels in the VD3-supplemented groups were significantly higher than those in the control groups (G1 and G2, p < 0.001). Serum levels of phosphate were higher in the groups supplemented with VD3 at 10,000 and 60,000 IU/kg (G3 and G6, p < 0.005). VD3 higher doses reduced cell proliferation and the number of larger placental glutathione S-transferase (GST-P)-positive hepatocellular preneoplastic lesions. Neither the DEN/CCl4 regimen nor the VD3 supplementation altered vitamin D receptor (VDR) protein expression in the liver.
Conclusion
The results indicate that high-dose VD3 supplementation reduced the development of DEN/CCl4-induced preneoplastic lesions in the liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Vitamin D (VD) deficiency is a trending global health issue [1,2,3]. According to the World Health Organization (WHO), over a billion people worldwide are VD deficient or insufficient [4, 5]. This highly prevalent condition has been associated with an increased risk of developing chronic diseases such as diabetes, obesity, and cancer [6,7,8]. Over the past few years, a growing number of epidemiological studies have reported that VD deficiency is very common in patients with chronic liver diseases [9,10,11,12]. Clinical evidence highlights the prevalence of VD deficiency among patients with chronic hepatitis C, cirrhosis, and hepatocellular carcinoma (HCC) [13,14,15,16]. Therefore, VD supplementation has become an appealing treatment in order to prevent, suppress, or ameliorate a number of chronic liver diseases [7, 17,18,19,20].
Dietary VD can be obtained through naturally food sources containing VD2 (ergocalciferol), which is found in plant sources, or VD3 (choleocalciferol), found in animal sources [21, 22]. However, the major source of VD3 comes from natural synthesis in the human skin through exposure to natural sunlight [23, 24]. The energy of ultraviolet B sun rays stimulates vitamin D synthesis in the skin from the conversion of 7-dehydrocholesterol to the secosteroid VD3. VD3 is further hydroxylated in the liver to a circulating pro-hormone 25-hydroxyvitamin D (25OHD3, calcidiol). This hydroxylation, which occurs exclusively in hepatocytes, is mediated by CYP27A1 and CYP2R1 that show different specificity and affinity for VD3 [25]. The conversion of 25OHD3 to its final active form 1,25-dihydroxyvitamin D3 (1,25OHD3, calcitriol) is subsequently achieved in the kidneys through enzymatic activity catalyzed by the mitochondrial cytochrome 1α-hydroxylase (CYP27B1) enzyme [1, 26].
The biological functions of the active form of VD3 are mediated by the nuclear vitamin D receptor (VDR), a high-affinity phosphoprotein receptor that binds to the 1,25OHD3 hormone and regulates gene expression in a number of cellular processes, including cell proliferation, differentiation, apoptosis, and immunomodulation [20, 27]. Therefore, the hepatic expression of VDR is suggested to be inversely associated with the severity of liver damage [25,26,27,28]. Low liver VDR expression has been implicated in the development of non-alcoholic steatohepatitis (NASH), fibrosis, and HCC [27].
To date, only a few rodent studies have proposed that VD3 supplementation reduces chemically induced rat hepatocarcinogenesis or liver fibrosis, with a positive outcome observed in the late stages of these diseases [17, 29, 30]. The purpose of the present study is therefore to investigate dose-response effects of VD supplementation in the early stages of diethylnitrosamine (DEN) and carbon tetrachloride (CCl4)-induced hepatocarcinogenesis in rats.
Methods
Animals
Four-week-old male Wistar rats, acquired from the São Paulo University Medical School (Ribeirão Preto, SP, Brazil), were housed in polypropylene cages under controlled temperature (22 ± 2 °C), humidity (55 ± 10%), and lighting (12 h light/12 h dark cycle) with free access to water and commercial chow (Presence®, Paraná, Brazil).
Study design
The animals were randomly assigned into six experimental groups of 10 rats each. Group 1: untreated group (sham); groups 2–6: received a single intraperitoneal injection of 200 mg/kg body weight (b.w.) of N-diethylnitrosamine (DEN, Sigma-Aldrich Co., St. Louis, CA, USA) as an initiating agent. Groups 2–6 received intragastric administrations of 1.0 ml/kg b.w. of carbon tetrachloride (CCl4, Dinâmica®, SP, Brazil), once a week, as a promoting agent for 10 weeks and stopped [31, 32]. Groups 3–6 received intragastric administration of VD3 (Dry Vitamin D3 100, BASF—Ludwigshafen, Germany) at 10,000, 20,000, 40,000 and 60,000 IU/kg b.w., respectively, on alternate days for 16 weeks. Both CCl4 regimen and VD3 supplementation started 2 weeks after DEN administration.
At the end of the experiment, the animals were euthanized by exsanguination under sodium pentobarbital anesthesia (30 mg/kg b.w.). Peripheral blood samples were collected for measuring serum aspartate amino transferase (AST, automated kinetic method, Cobas C501—Roche, USA), cholecalciferol (25OHD3, high-performance liquid chromatography—HPLC), calcium, and phosphate (Bioclin, Germany). After the sacrifice, the liver was removed and weighed. Liver tissue fragments were collected and either stored at − 80 °C for protein extraction or fixed in 10% phosphate-buffered formalin for histological and immunohistochemical analyses (Fig. 1).
Immunohistochemistry
Immunoreactivity for Ki-67 and placental glutathione S-transferase (GST-P) was detected using a universal labeled Streptavidin-Biotin system (LSAB System-HRP, DakoCytomation, Denmark). Briefly, deparaffinated 5-μm liver sections on silanized slides were sequentially treated with citrate buffer (120 °C, 5 min) in a Pascal Pressure Chamber (DakoCytomation, Denmark), 3% H2O2 in phosphate-buffered saline (PBS) (10 min), skim milk (60 min), rabbit monoclonal anti-Ki-67 (1:100 dilution, Abcam, UK) or GST-P (1:1000 dilution, Medical & Biological Laboratories, Japan) antibodies overnight (4 °C) and biotinylated universal link and streptavidin HPR (20 min each). Color development was achieved using 3,3-diaminobenzidine (DAB, Sigma-Aldrich, USA) and counterstained with Harris’s hematoxylin. The proliferative index was defined as the number of Ki-67-positive hepatocytes per microscope field (40 microscopic fields per animal at × 40 objective). Preneoplastic liver lesions (PNL) were assessed by immunohistochemical staining for GST-P, a biomarker for detection of preneoplastic and neoplastic lesions [33, 34]. GST-P-positive PNL were measured using a KS-300 image analysis software (Kontron Elektronic, Germany). Data were expressed as number of GST-P-positive PNL per liver area (cm2), classified into three different sizes: < 0.5 mm2, 0.5–1.0 mm2, and > 1.0 mm [34, 35]. Larger GST-P-positive lesions are indicative of promoting effects and higher growth rates [34].
Western blot analysis
Liver samples were homogenized in lysis buffer (1% Triton X-100 and 2 μl/100 ml protease inhibitor, Sigma-Aldrich, USA). After this procedure, the extracted material was centrifuged (4000 rpm, 4 °C, 20 min) and the supernatant collected for protein quantification by Bradford’s method. Aliquots of liver homogenates containing 70 μg of total protein were heated (95 °C, 5 min) in sample-loading buffer and, then, electrophoretically separated in a 12% SDS–PAGE gel under reducing conditions and transferred to nitrocellulose membranes (Sigma-Aldrich, USA). Membranes were blocked with skim milk in TBS-T (0.05 M Tris, 0.15 M NaCl, pH 7.2, 1% Tween-20) for 1 h. The nitrocellulose membranes were subsequently incubated with polyclonal antibodies rabbit anti-VDR (1:1000 dilution, Santa Cruz Biotechnology, CA, USA) and anti-CYP27A1 (1:1000 dilution, Santa Cruz Biotechnology, CA, USA) or goat polyclonal anti-actin (1:1000 dilution, Santa Cruz Biotechnology, USA) primary antibodies in 5% BSA solution overnight. After five wash steps with PBS-T, membranes were incubated with specific horseradish-conjugated secondary antibodies, according to the primary antibodies used, for 2 h at room temperature. Finally, after five wash steps, the membranes were submitted to immunoreactive protein signals (GE Healthcare Life Sciences, UK). Signals were captured by a G:BOXChemi system (Syngene, UK) controlled by an automatic software (GeneSys, Syngene, UK). Band intensities were quantified using densitometry analysis software (Image J software, Austria). Finally, VDR and CYP27A1 protein expression was reported as fold change according to actin protein expression, used as a normalizer.
Statistical analysis
Body weight, liver weight, food intake, as well as cell proliferation index, the number of GST-P-positive lesions, and calcium and phosphorus levels were analyzed by the ANOVA test and post hoc Tukey’s test. Statistical analysis was performed using the Jandel Sigma Stat Software (Jandel Corporation, San Rafael, CA, USA). Graphics were generated by the GraphPad Prism software (Version 6.01, La Jolla, CA). Statistical differences were considered significant when p < 0.05.
Results
Body weight, food intake, and liver weight
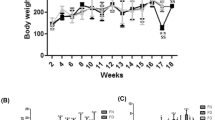
A significant reduction in body weight gain and final body weight was observed in the groups receiving VD3 supplementation at doses of 40,000 and 60,000 IU/kg b.w. (G5 and G6, p < 0.001) when compared to the untreated and DEN/CCl4-treated groups (G1 and G2, respectively). The weight loss in the VD3 high-dose groups was accompanied by a significant decrease in food intake (G5 and G6, p < 0.001) (Table 1). The relative liver weight was significantly lower in the groups supplemented with high doses of VD3 (G5 and G6, p < 0.001) than in the untreated and DEN/CCl4-treated groups (G1 and G2, respectively).
Serum levels of 25OHD3, AST, calcium, and phosphate
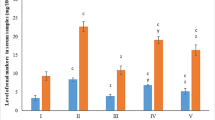
Serum 25-hydroxyvitamin D (25-OHD) levels in VD3-supplemented groups were significantly higher than those in the control groups (G1 and G2, p < 0.001) (Fig. 2).
Serum levels of 25 (OH) D. Values are means ± SD compared by one-way ANOVA followed by Tukey’s multiple comparisons test. One asterisk indicates significantly different from G1, p < 0.0001. Two asterisks indicate significantly different from G2, p < 0.0001, and three asterisks indicate significantly different from G3, p < 0.0001. G1 = untreated group; G2 = DEN + CCl4; G3 = DEN + CCl4 + VD3 10,000 IU; G4 = DEN + CCl4 + VD3 20,000 IU; G5 = DEN + CCl4 + VD3 40,000 IU; G6 = DEN + CCl4 + VD3 60,000 IU
Furthermore, serum levels of phosphate were higher in the groups supplemented with VD3 at 10,000 and 60,000 IU/kg (G3 and G6, p < 0.005) than in remaining groups. However, serum calcium and AST levels did not differ among groups (Table 2).
Cell proliferation and preneoplastic lesion development
The average for Ki-67 labeling index (Ki-67 LI%) in the DEN/CCl4-induced group (G2) was significantly higher than that in the untreated group (G1, p < 0.001). There was a reduction in cell proliferation indexes in the group that received the higher doses of VD (60,000 IU/kg (p = 0.0002, Fig. 3) when compared to the DEN/CCl4-induced group (G2).
Immunohistochemical detection of Ki-67-positive hepatocytes. a Cell proliferation index in the different groups. b Representative image with hepatocytes showing nuclear positivity for Ki-67(final magnification × 400). Values are means ± SD, compared by one-way ANOVA followed by Tukey’s multiple comparisons test. One asterisk indicates significantly different from G1, p < 0.001. Two asterisks indicate significantly different from G2, p < 0.0002. G1 = untreated group; G2 = DEN + CCl4; G3 = DEN + CCl4 + VD3 10,000 IU; G4 = DEN + CCl4 + VD3 20,000 IU; G5 = DEN + CCl4 + VD3 40,000 IU; G6 = DEN + CCl4 + VD3 60,000 IU
With regard to the number of GST-P-positive PNL per liver area, VD3 supplementation significantly reduced the number of GST-P-positive PNL larger than 1.0 mm2 when compared to the positive control group (G2, p < 0.0001) (Fig. 4).
a–c Evaluation of number of GST-P-positive PNL per liver area (mm2). d Representative image of a GST-P-positive hepatic foci (final magnification × 400). 1Values are means ± SD, compared by one-way ANOVA followed by Tukey’s multiple comparisons test. One asterisk indicates significantly different from G3, G4, G5, and G6, p < 0.0001. G1 = untreated group; G2 = DEN + CCl4; G3 = DEN + CCl4 + VD3 10,000 IU; G4 = DEN + CCl4 + VD3 20,000 IU; G5 = DEN + CCl4 + VD3 40,000 IU; G6 = DEN + CCl4 + VD3 60,000 IU
VDR and CYP27A1 protein expression
Hepatic VDR protein expression was similar in all groups, independently of the DEN/CCl4 regimen or VD3 supplementation. In contrast, CYP27A1 protein expression was significantly higher in the liver from DEN/CCl4-treated group (G2) than in the remaining groups (G1, G4, G5, and G6, p = 0.007) (Fig. 5).
a Western blotting for the hepatic CYP27A1 and b VDR proteins. Values are means ± SD. Means were compared by one-way ANOVA followed by Tukey’s multiple comparisons test. One asterisk indicates significantly different from G1, p = 0.0067. Two asterisks indicate significantly different from G2, p = 0.0067. G1 = SHAM; G2 = DEN + CCl4; G3 = DEN + CCl4 + VD3 10,000 IU; G4 = DEN + CCl4 + VD3 20,000 IU; G5 = DEN + CCl4 + VD3 40,000 IU; G6 = DEN + CCl4 + VD3 60,000 IU
Discussion
The aim of this study was to investigate dose-response effects of VD3 supplementation on the early stages of DEN/CCl4-induced hepatocarcinogenesis in rats. Our results indicate that VD3 supplementation at 40,000 and 60,000 IU/kg significantly decreased body weight gain, accompanied by a reduction in food intake. These findings are in agreement with the literature indicating that VD3 intake may contribute to weight loss [36]. Experimental studies and intervention trials have proposed that VD3-mediated weight loss may be attributed to the modulation of fat oxidation profiles, thus increasing the overall metabolism [36, 37]. VD3 has also been shown to be capable of modulating insulin sensitivity and thereby decrease hunger, improve satiety, and reduce food intake [38, 39]. Furthermore, relative liver weight was also decreased in the groups supplemented with high doses of VD3, but without no specific hepatocellular alterations or ALT levels changes. Although VD3 toxicity is low, the doses used in this study were lower than 100,000 UI because higher doses can cause vitamin D intoxication, hypercalcemia, hyperphosphatemia, and ultimately death [40, 41]. However, the possibility of long-term high-dose toxicity should be investigated.
The steroid hormone 1,25OHD3 (calcitriol) plays a crucial role in calcium and phosphorus homeostasis. The parathyroid hormone regulates conversion of 25OHD3 to its active metabolite, 1,25OHD3, which increases calcium and phosphorus levels in blood by increasing intestinal absorption [42, 43]. Serum calcium is highly regulated, promptly mobilized, and stored in the bones, maintaining serum calcium concentrations within a normal physiological range. Dietary phosphorus excess is excreted by the kidneys under the regulatory activity of the fibroblast growth factor 23 protein (FGF23), decreasing CYP27B1 expression and VD3 activation and promoting phosphorus excretion in urine [43, 44]. Although no changes in serum calcium levels were found in any of the study groups, higher levels of serum phosphate were observed in those supplemented with VD3 at 10,000 and 60,000 IU (G3 and G6, respectively) than in the other groups. These results support the hypothesis that VD3 supplementation was effective and promoted phosphorus mobilization via VD3 metabolic activation [45, 46]. However, since high dietary inorganic phosphate can initially promote but later inhibit lung cancer progression in mice [47], the increase in serum phosphate should be investigated in long-term VD3 interventions.
VD3 has been demonstrated to suppress cellular proliferation in highly cell-specific manners, via apoptosis, cell cycle progression, and differentiation [48, 49]. In our study, DEN/CCl4 regimen (G2) increased hepatic cell proliferation when compared to the untreated group (G1). However, VD3 supplementation at higher doses (40,000 and 60,000 IU/kg) suppressed cell proliferation in comparison to the DEN/CCl4 group (G2). In this regard, higher serum 25(OH)D levels might be associated with this finding, modulating hepatocyte cell proliferation in DEN/CCl4-treated groups. Cell proliferation plays an important role during critical phases of rat liver carcinogenesis, including the processes of initiation and promotion [50]. Therefore, the suppression of cell proliferation is considered an important feature for a chemopreventive candidate [51, 52].
Glutathione S-transferases (GST) comprise a group of phase II metabolic enzymes involved in cellular protection against xenobiotics, oxidative stress, and resistance against chemotherapeutic compounds [53, 54]. The rat GST-P 7-7, an isozyme of glutathione S-transferase, is abnormally expressed in the early stages of chemical hepatocarcinogenesis. Therefore, single hepatocytes expressing GST-P develop very early in carcinogen-treated rat liver and are considered suitable markers of preneoplastic lesions [55, 56]. The detection of GST-P-positive foci is an important tool for analyzing relevant carcinogenic or anti-carcinogenic responses during the initiation and promotion stages of rat liver carcinogenesis [57, 58]. It was found that VD3 supplementation reduced the number of larger GST-P-positive PNL (> 1.0 mm2) when compared to the positive control group (G2). However, the underlying mechanisms by which VD3 can suppress GST-P-positive PNL development still need to be clarified.
Active VD3 effects are mediated by VDR which regulates gene expression in the target tissues [49]. The VDR is a member of a superfamily of nuclear steroid hormone receptors which participates in VD3 biological functions, regulating calcium and phosphorus homeostasis, immune response, cell differentiation, and cell proliferation [58]. This nuclear receptor can be found in many type cells throughout the body, widely expressed in tissues such as intestines, lungs, kidneys, skin, bones, and liver [59]. Liver expression of VDR might be inversely associated with the severity of liver damage in a number of chronic diseases [27, 28]. Decreased VDR expression has been related to an increasing susceptibility to the development of carcinogen-induced cancers and may be considered a potential biomarker candidate for cancer prognostic [60, 61]. Many clinical trials have found evidence of reduced VDR expression in HCC and cholangiosarcoma alone but not in dysplastic nodules and hepatomas [27, 62]. For instance, in the present study, DEN/CCl4 regimen did not alter VDR protein expression in the experimental groups, indicating that low VDR expression is associated with the progression phase of liver disease rather than early hepatocarcinogenesis [63]. Besides, VD3 treatment did not increase VDR levels, which is consistent with a previous study indicating that dietary VD3 did not affect VDR gene expression in azoxymethane (AOM)-induced PNL in mice [64]. The results also showed that hepatic CYP27A1 protein expression was significantly higher in the DEN/CCl4-treated group (G2) than in the remaining groups (Fig. 5). Although mitochondrial CYP27A1 has been shown to activate 25-hydroxilate VD3, this enzyme has a relatively low affinity and plays a minor role in VD3 hydroxylation. CYP27A1 is a bifunctional enzyme also involved in bile acid and cholesterol metabolism [65]. Therefore, the increased CYP27A1 expression seen in the positive control group might be related to the CCl4-induced regimen that leads to mitochondrial dysfunction and oxidative metabolism impairment [31]. In fact, VD3 supplementation increased total glutathione levels and GSH-Px activity, as well as diminished lipid hydroperoxide levels in the liver [66], indicating a possible protective mechanism of VD3 against DEN/CCl4-induced hepatocarcinogenesis.
A limitation of this study is the short time of CCl4 exposure, which most likely did not allow observing the extensive liver lesions expected to occur with its use. Thus, studies including longer periods of CCl4 exposure are required to further investigate the molecular mechanisms and potential protective role of vitamin D against liver tumorigenesis.
Conclusions
Dietary factors have been in the spotlight of scientific interest since that they can exert preventive activities against human chronic diseases, including cancer [67]. It is currently known that vitamins play an important role in the prevention and treatment of precancerous and cancerous conditions [68], but until now, no conclusive results were obtained. Therefore, the findings the present study support the hypothesis that VD3 supplementation can reduce the early development of hepatocellular PNL in rats, but only in higher doses. However, the possibility of long-term VD3 high-dose toxicity should be investigated.
References
Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88:720–55.
Wacker M, Holick MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol. 2013;5:51–108.
Hilger J, Goerig T, Weber P, Hoeft B, Eggersdorfer M, Carvalho NC, et al. Micronutrient intake in healthy toddlers: a multinational perspective. Nutrients. 2015;7:6938–55.
Chagas CEA, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4:52–67.
Iruzubieta P, Terán Á, Crespo J, Fábrega E. Vitamin D deficiency in chronic liver disease. World J Hepatol. 2014;6:901–15.
Naeem Z. Vitamin D deficiency—an ignored epidemic. Int J Health Sci. 2010;4(Qassim):V–VI.
Holick MF. Vitamin D, sunlight and cancer connection. Anti Cancer Agents Med Chem. 2013;13:70–82.
Carlberg C. What do we learn from the genome-wide perspective on vitamin D3? Anticancer Res. 2015;35:1143–51.
Malham M, Jørgensen SP, Ott P, Agnholt J, Vilstrup H, Borre M, et al. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011;17:922–5.
Wang X, Li W, Zhang Y, Yang Y, Qin G. Association between vitamin D and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: results from a meta-analysis. Int J Clin Exp Med. 2015;8:17221–34.
Dongiovanni P, Lanti C, Riso P, Valenti L. Nutritional therapy for nonalcoholic fatty liver disease. J Nutr Biochem. 2016;29:1–11.
Ko BJ, Kim YS, Kim SG, et al. Relationship between 25-hydroxyvitamin D levels and liver fibrosis as assessed by transient elastography in patients with chronic liver disease. Gut Liver. 2016;10:818–25.
Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–8.
Finkelmeier F, Kronenberger B, Köberle V, Bojunga J, Zeuzem S, Trojan J, et al. Severe 25-hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma—a prospective cohort study. Aliment Pharmacol Ther. 2014;39:1204–12.
Luo Y, Wu X, Ling Z, Cheng Y, Yuan L, Xiang C. Association between serum vitamin D and severity of liver fibrosis in chronic hepatitis C patients: a systematic meta-analysis. J Zhejiang Univ Sci B. 2014;15:900–6.
Finkelmeier F, Kronenberger B, Zeuzem S, Piiper A, Waidmann O. Low 25-hydroxyvitamin D levels are associated with infections and mortality in patients with cirrhosis. PLoS One. 2016;10:e0132119.
Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, Brazowski E, et al. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–37.
Malham M, Peter Jørgensen S, Lauridsen AL, Ott P, Glerup H, Dahlerup JF. The effect of a single oral megadose of vitamin D provided as either ergocalciferol (D2) or cholecalciferol (D3) in alcoholic liver cirrhosis. Eur J Gastroenterol Hepatol. 2012;24:172–8.
Fernández Fernández N, Linares Torres P, Joáo Matias D, Jorquera Plaza F, Olcoz Goñi JL. Vitamin D deficiency in chronic liver disease, clinical-epidemiological analysis and report after vitamin d supplementation. Gastroenterol Hepatol. 2016;39:305–10.
Li R, Guo E, Yang J, Li A, Yang Y, Liu S, et al. 1, 25 (OH) 2 D3 attenuates hepatic steatosis by inducing autophagy in mice. Obesity. 2017;25:561–71.
Jones G. Extrarenal vitamin D activation and interactions between vitamin D2, vitamin D3, and vitamin D analogs. Annu Rev Nutr. 2013;33:23–44.
Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55:1193–205.
Macdonald HM. Contributions of sunlight and diet to vitamin D status. Calcif Tissue Int. 2013;92:163–76.
Holick MF. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016;36:1345–56.
Barchetta I, Carotti S, Labbadia G, et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180–7.
Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin N Am. 2010;39:243–53.
Eliades M, Spyrou E. Vitamin D: a new player in non-alcoholic fatty liver disease? World J Gastroenterol. 2015;21:1718–27.
Petta S, Grimaudo S, Tripodo C, et al. The hepatic expression of vitamin D receptor is inversely associated with the severity of liver damage in genotype 1 chronic hepatitis C patients. J Clin Endocrinol Metab. 2015;100:193–200.
Basak R, Basu M, Chatterjee M. Combined supplementation of vanadium and 1alpha, 25-dihydroxyvitamin D (3) inhibit diethylnitrosamine-induced rat liver carcinogenesis. Chem Biol Interact. 2000;128:1–18.
Guo J, Ma Z, Ma Q, et al. 1, 25 (OH) 2D3 inhibits hepatocellular carcinoma development through reducing secretion of inflammatory cytokines from immunocytes. Curr Med Chem. 2013;20:4131–41.
Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Ver. 2007;25:185–209.
Wang GS, Eriksson LC, Xia L, Olsson J, Stål P. Dietary iron overload inhibits carbon tetrachloride-induced promotion in chemical hepatocarcinogenesis: effects on cell proliferation, apoptosis, and antioxidation. J Hepatol. 1999;30:689–98.
Bannasch P, Haertel T, Su Q. Significance of hepatic preneoplasia in risk identification and early detection of neoplasia. Toxicol Pathol. 2003;31:134–9.
Tsuda H, Fukushima S, Wanibuchi H, et al. Value of GST-P positive preneoplastic hepatic foci in dose-response studies of hepatocarcinogenesis: evidence for practical thresholds with both genotoxic and nongenotoxic carcinogens. A review of recent work. Toxicol Pathol. 2003;31:80–6.
Kishima MO, Barbisan LF, Estevão D, Rodrigues MA, Viana de Camargo JL. Promotion of hepatocarcinogenesis by hexachlorobenzene in energy-restricted rats. Cancer Lett. 2000;152:37–44.
Slusher AL, McAllister MJ, Huang C-J. A therapeutic role for vitamin D on obesity-associated inflammation and weight-loss intervention. Inflamm Res. 2015;64:565–75.
Marcotorchino J, Tourniaire F, Astier J, et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J Nutr Biochem. 2014;25:1077–83.
Ji L, Gupta M, Feldman BJ. Vitamin D regulates fatty acid composition in subcutaneous adipose tissue through Elovl3. Endocrinology. 2016;157:91–7.
Tzotzas T, Papadopoulou FG, Tziomalos K, et al. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J Clin Endocrinol Metab. 2010;95:4251–7.
Shephard RM, DeLuca HF. Plasma concentrations of vitamin D3 and its metabolites in the rat as influenced by vitamin D3 or 25-hydroxyvitamin D3 intakes. Arch Biochem Biophys. 1980;202(1):43–53.
Ozkan B, Hatun S, Bereket A. Vitamin D intoxication. Turk J Pediat. 2012;54(2):93–8.
Sisley SR, Arble DM, Chambers AP, et al. Hypothalamic vitamin D improves glucose homeostasis and reduces weight. Diabetes. 2016;65:2732–41.
Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011;25:531–41.
DeLuca HF. Vitamin D: historical overview. Vitam Horm. 2016;100:1–20.
Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Ver. 2012;92:131–55.
Wacker M, Holick MF. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111–48.
Lee S, et al. High inorganic phosphate intake promotes tumorigenesis at early stages in a mouse model of lung cancer. PLoS One. 2015;10:e0135582.
Khundmiri SJ, Murray RD, Lederer E. PTH and vitamin D. Compr Physiol. 2016;6:561–601.
Chiang K-C, Yeh C-N, Chen M-F, Chen TC. Hepatocellular carcinoma and vitamin D: a review. J Gastroenterol Hepatol. 2011;26:1597–603.
Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6:793–804.
Pitot HC. Adventures in hepatocarcinogenesis. Annu Rev Pathol. 2007;2:1–29.
Mori H, Sugie S, Yoshimi N, Hara A, Tanaka T. Control of cell proliferation in cancer prevention. Mutat Res. 1999;428:291–8.
Strange RC, Jones PW, Fryer AA. Glutathione S-transferase: genetics and role in toxicology. Toxicol Lett. 2000;112–113:357–63.
Noguti J, Barbisan LF, Cesar A, Dias Seabra C, Choueri RB, Ribeiro DA. Review: in vivo models for measuring placental glutatione-S-transferase (GST-P 7-7) levels: a suitable biomarker for understanding cancer pathogenesis. In Vivo. 2012;26:647–50.
Sakai M, Muramatsu M. Regulation of glutathione transferase P: a tumor marker of hepatocarcinogenesis. Biochem Biophys Res Commun. 2007;357:575–8.
Dias MC, Rodrigues MAM, Reimberg MCH, Barbisan LF. Protective effects of Ginkgo biloba against rat liver carcinogenesis. Chem Biol Interact. 2008;173:32–42.
Tsuda H, Futakuchi M, Fukamachi K, et al. A medium-term, rapid rat bioassay model for the detection of carcinogenic potential of chemicals. Toxicol Pathol. 2010;38:182–7.
Matsuda S, Kitagishi Y. Peroxisome proliferator-activated receptor and vitamin d receptor signaling pathways in cancer cells. Cancers (Basel). 2013;5:1261–70.
Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011;1814:186–99.
Jóźwicki W, Brożyna AA, Siekiera J, Slominski AT. Expression of vitamin D receptor (VDR) positively correlates with survival of urothelial bladder cancer patients. Int J Mol Sci. 2015;16:24369–86.
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408.
Han S, Chiang JYL. Mechanism of vitamin D receptor inhibition of cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes. Drug Metab Dispos. 2009;37:469–78.
Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–33.
Hummel DM, Thiem U, Höbaus J, et al. Prevention of preneoplastic lesions by dietary vitamin D in a mouse model of colorectal carcinogenesis. J Steroid Biochem Mol Biol. 2013;136:284–8.
Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology. 2015;62:635–43.
Romualdo GR, et al. Vitamin D3 supplementation attenuates the early stage of mouse hepatocarcinogenesis promoted by hexachlorobenzene fungicide. Food Chem Toxicol. 2017;107:27–36.
Mysuru Shivanna L, Urooj A. A review on dietary and non-dietary risk factors associated with gastrointestinal cancer. J Gastrointest Cancer. 2016;47:247–54.
Janakiram NB, Mohammed A, Madka V, Kumar G, Rao CV. Prevention and treatment of cancers by immune modulating nutrients. Mol Nutr Food Res. 2016;60:1275–94.
Acknowledgements
This article is dedicated to the memory of professor Carlos Eduardo Andrade Chagas, acknowledging his significant and important contributions in this experimental study.
Funding
Mariana B. Tablas and Renata L. Goto were recipients of a fellowship from FAPESP (2012/03964-8) and CAPES (DS 2013-2016), respectively. Luis F. Barbisan was a recipient of support research from FAPESP (2012/03628-8).
Availability of data and materials
Please contact the author for data requests.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed according to the Ethical Principles for Animals Research adopted by the Brazilian College of Animal Experimentation (COBEA) and approved by the Institution’s Ethics Review Board (403-CEUA).
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tablas, M.B., Goto, R.L., Caetano, B.F.R. et al. Vitamin D3 suppresses the early stages of chemically induced hepatocarcinogenesis in rats: a dose-response analysis. Nutrire 43, 12 (2018). https://doi.org/10.1186/s41110-018-0065-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-018-0065-2