Abstract
Background
The aim of this study is to test the hypothesis that obesity induced by a diet rich in saturated fats and balanced in carbohydrates is associated with the development of systemic complications and comorbidities.
Methods
Thirty-seven 60-day-old male Wistar rats were randomized into two groups: control (C, n = 18, standard diet) and obese (OB, n = 19, high-saturated fat diet), for 33 weeks. Nutritional profile: food and caloric intake, feed efficiency, body weight, and adiposity index. Complications: in plasma were analyzed dyslipidemia, insulin resistance (HOMA-IR), glucose intolerance, hyperleptinemia, hyperinsulinemia, plasmatic C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α); in the myocardial and epididymal adipose tissue were assessed IL-6 and TNF-α. Comorbidities: diabetes mellitus and systemic blood pressure (SBP). Student’s t test, ANOVA, and Bonferroni P < 0.05.
Results
The final body weight, feed efficiency, and adiposity index were higher in OB group than in control; although food intake was lower in OB group, caloric intake was similar in both groups. Specific parameters, such as LDL, cholesterol, triglycerides, HOMA-IR, CRP, TNF-α in epididymal adipose tissue, and IL-6 in the myocardium, were higher in obese rats than in controls. SBP, baseline glucose, and glucose after 2 h of overload were significantly increased in OB group; however, the severity was not enough to classify the animals as diabetic and hypertensive.
Conclusion
Obesity induced by a diet high in saturated fatty acids with balanced carbohydrates for 33 weeks in Wistar rats was effective in triggering complications but unable to develop comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Obesity is a disease resulting from chronic energy imbalance and excessive accumulation of body fat [1,2,3]. This disease can lead to complications, such as inflammation, alteration of the redox system, hyperleptinemia, hyperinsulinemia, glucose intolerance, insulin resistance, and dyslipidemia, which may result in comorbidities as atherosclerosis [4,5,6,7], nonalcoholic steatohepatitis [8], diabetes mellitus [9], and systemic blood pressure [10].
Different types of high-fat diet have been used in animal models to develop complications and comorbidities observed in human obesity [11,12,13]. It is not well defined what would be the most appropriate diet to build up the triad in experimental models: obesity complications, and comorbidities. For this purpose, several authors use diets rich in fatty acids [14,15,16,17,18]. In diets using predominantly unsaturated fat, the rodents develop obesity and different types of complications associated, whereas comorbidities are not common manifestations [19, 20]. When there is a preponderance of saturated fatty acids, both abnormalities are more constant, by raising total cholesterol and LDL cholesterol, which causes deposition in the arteries in the heart and other parts of the body besides triggering obesity and overweight [14, 21, 22]. However, diets that use high fat content result in a decrement of other nutrients, such as carbohydrate, in order to maintain an appropriate proportion of dietary constituents [6, 17, 18]. Due to the reduction in energy availability from carbohydrates, the fat becomes the main source of energy, which could cause a decrease in fat deposits and attenuates the pathogenic events associated with obesity [23]. Considering this issue as well as the harmful role of saturated fat [21, 22], here we tested the hypothesis that the obesity induced by a diet rich in saturated fats and balanced in carbohydrates is associated with the development of systemic complications and comorbidities.
Methods
Animals and experimental protocol
Thirty-seven 60-day-old male Wistar rats, from Anilab Laboratory Animal Creation and Trade Ltd. (Paulinia, São Paulo, Brazil), were kept in rodent vivarium in Experimental Research Unit of Botucatu Medical School–UNESP under the following conditions: individual cages with free access to water and food ad libitum, controlled temperature (24 ± 2 °C), humidity (55 ± 5 °C), and light (12‐h light/dark cycle). The experimental procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” published by the “US National Institutes of Health.” The rats were randomized into two groups. The control group (C, n = 18) received standard diet (SD) and the obese group (OB, n = 19) was fed diet high in saturated fatty acids (SHFD), for a 33-week period. The body weight and food intake were measured weekly.
Diet
The diets were developed in Experimental Research Unit of Botucatu Medical School–UNESP in partnership with Biotron Zootecnica® (Rio Claro, SP, Brazil) and adapted from the dietary model used previously by our research group [18]. The following ingredients were used: soybean bran, soybean hulls, corn bran, dextrin, soybean oil, palm kernel oil (as source of saturated fatty acid), and vitamin and mineral complex (adjusted in both diets for all animals receive similar amounts). The SD consisted of 24% of its kilocalorie from protein, 40% from carbohydrates, and 6% from fat; the SHFD 18% from protein, 40% from carbohydrate, and 17% from fat. The SHFD contained a higher amount of calories (SHFD = 3.85 kcal/g vs SD = 3.10 kcal/g) due to increased content of energy from fat. The proportion of saturated/unsaturated fatty acids was 61.5/38.5% in SD and 64.8/35.2% in SHFD (Table 1).
Euthanasia of animals and biological material collection
At the end of the experimental protocol, the animals were fasted for 12 h and the euthanasia was performed by decapitation after anesthesia via intraperitoneal with a mixture of ketamine (50 mg/kg) and xylazine (1 mg/kg). Then, the samples were collected and stored at −80 °C for further analysis.
Nutritional profile
The nutritional status was determined by food and caloric intake feed efficiency, body weight, and adiposity index. Dietary intake and body weight were measured weekly. The caloric intake was determined by the following formula: weekly food intake multiplied by the energy value of each diet (g × kcal). To analyze the animal’s capacity to convert consumed food energy in body weight, feed efficiency was calculated by dividing the total body weight gain (g) by the total energy intake (kcal). The amount of total body fat was obtained by the sum of epididymal, retroperitoneal, and visceral deposits; the adiposity index was calculated by the total body fat normalized by the final body weight and multiplied by 100.
Complications
The presence of complications was divided into two sections: (1) metabolic/hormonal which were assessed by dyslipidemia, insulin resistance, glucose intolerance, hyperleptinemia, hyperinsulinemia, and (2) inflammatory, analyzed by C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). The plasmatic triglyceride, total cholesterol, and high‐ (HDL) and low‐density lipoprotein (LDL) were determined using specific kits (BIOCLIN®, Belo Horizonte, MG, Brazil) and analyzed by automated enzymatic colorimetric method (Chemistry Analyzer BS‐200, Mindray Medical International Limited, Shenzhen, China); the nonesterified fatty acids (NEFA) levels were evaluated by colorimetric kit (WAKO Pure Chemical Industries Ltd., Osaka, Japan). Insulin and leptin were analyzed by enzyme‐linked immunosorbent assay (ELISA) method (EMD Millipore Corporation, Billerica, MA, USA). The reading was performed using a microplate reader (Spectra MAX 190, Molecular Devices, Sunnyvale, CA, USA). Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) calculated by the formula: HOMA-IR = fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5 [8]. The glucose intolerance was assessed through the glucose tolerance test and area under the 2-h blood glucose response curve (AUC). After a period of 6 h fasting, the blood samples were collected in the caudal artery at baseline and 15, 30, 60, 90, and 120 min after intraperitoneal administration of glucose 30% (Sigma-Aldrich, ®St Louis, MO, USA), equivalent to 2.0 g/kg body weight. The measurement of blood glucose level was performed using a handheld glucometer (Accu-Chek Go Kit, Roche Diagnostic Brazil Ltda, São Paulo, Brazil). TNF-α and IL-6 (R&D Systems Inc., Minneapolis, MN, USA) levels were analyzed in the plasma and myocardial and epididymal adipose tissue and C-reactive protein (CRP) (Linco Research Inc., St. Louis, MO, USA) in the plasma by ELISA according to manufacturer’s instructions. The reading was performed using a microplate reader. The cytokine (TNF-α and IL-6) results in adipose tissue and myocardium were corrected per gram of total protein.
Comorbidities
The following comorbidities were evaluated: diabetes mellitus (DM) and systemic arterial hypertension (SAH). The diagnoses of DM and SAH were determined using the same criteria used by the World Health Organization (WHO) [24] to humans, i.e., DM: fasting blood glucose higher than 126 and ≥200 mg/dL 2 h after a glucose load and SAH: arterial pressure ≥140/90 mmHg. The glycemic values were determined during the glucose tolerance test. In this experiment, only the systolic blood pressure was measured by tail plethysmography using electro-sphygmomanometer, Narco Bio-System®, model 709-0610 (International Biomedical, Austin, TX, USA); this method does not assess diastolic blood pressure. These two criteria of comorbidities were established in human references by the facts that criteria for rats are close to those for humans [25].
Statistical analysis
All results are presented as mean ± standard deviation or median (minimum and maximum value) and were subjected to Student’s t test for independent samples or Mann-Whitney. The level of significance for all variables was 5%.
Results
Nutritional profile
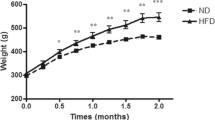
Table 2 shows the nutritional profile in C and OB animals. The final body weight was greater in OB group as well as the adiposity index. The caloric intake was similar in both groups; however, the obese rats had higher feed efficiency, even with a lower food intake.
Complications
The complications due to obesity are presented in Table 3. The obese group showed a significant increase in the concentrations of cholesterol triglycerides, LDL, HOMA-IR, plasma CRP, TNF-α in the epididymal adipose tissue, and IL-6 in the myocardium when compared to control group.
Comorbidities
The levels of systolic blood pressure baseline glucose and glucose after 2 h of overload were significantly higher in the OB group. However, the levels were not high enough to classify the animals as diabetic and hypertensive according to the established criteria (Table 4).
Discussion
The aim of this study was to determine whether the obesity induced by a diet rich in saturated fatty acids and appropriated (balanced) amounts of carbohydrates is associated with the development of complications and comorbidities. We observed that despite the SHFD-fed animals having eaten a lower amount of food and a similar amount of calories to control, they showed a significant increase in body weight and adiposity index, whereas they were classified as obese. The effectiveness of dietary model is attributed to the greater feed efficiency in obese animals probably due to the thermal effect of fat, since this nutrient requires less energy (2–3%) to be absorbed and digested, making it easily stored as triglycerides in adipocytes [6, 17, 26].
The current study showed that obese animals had numerous complications and absence of comorbidities. These results are in agreement with some authors [9, 10, 20,21,22,23,24,25] that did not find comorbidities, only complications, and in disagreement with other studies that did not find such complications in obesity induced by diet with a predominance of saturated fatty acids [27,28,29,30]. Although blood glucose 2 h after the glucose tolerance test and blood pressure were significantly higher than those in the control group, they did not reach the levels considered to classify animals as having diabetes mellitus and systemic hypertension, according to the World Health Organization [1]. All changes, metabolic/hormonal/inflammatory, viewed in obese rats probably result from adipose tissue dysfunction; this imbalance in adipose tissue is characterized by adipocyte hypertrophy followed by reduced capacity of triglyceride storage and increased production of inflammatory adipokines, oxidative stress, and fatty acid releasing, by lipolysis, that cause systemic deleterious effects [31, 32]. Though, even with double fat index, obese animals have not reached a magnitude capable of triggering the comorbidities proposed in this experiment. One of the explanations for the absence of comorbidities was the use of complex carbohydrates in the hyperlipid diet, and literature reports that simple carbohydrates are more deleterious and frequently used in the induction of obesity, complications, and comorbidities [33, 34]. However, in this study, this type of carbohydrate was not used, since this would not allow knowing if the manifestation of the complications and comorbidities would be exclusively due to the increase of the amount of fat or its association with a carbohydrate with harmful capacity. It is believed that other factors may also have influenced this result as time of exposure to diet, fat percentage, and type (saturated or unsaturated) and source (animal or vegetable) of fatty acids and animal model [6, 17, 18, 30, 33, 35,36,37].
Conclusion
In conclusion, obesity induced by a diet rich in saturated fatty acids with equilibrated amount of carbohydrates was effective in triggering complications but unable to develop comorbidities analyzed. A relevant aspect, based on our results, would be that a diet rich in saturated fatty acids, with balance of other nutrients, does not trigger comorbidities. Another would be that this model of dietary obesity can be used in research involving only complications arising from the increase in fat mass.
Abbreviations
- C:
-
Control
- CRP:
-
C-reactive protein
- DM:
-
Diabetes mellitus
- FBW:
-
Final body weight
- HDL:
-
High-density lipoprotein
- HOMA-IR:
-
Insulin resistance was calculated using the index of homeostasis model assessment
- IL-6:
-
Interleukin-6
- LDL:
-
Low-density lipoprotein
- NEFA:
-
Nonesterified fatty acids
- OB:
-
Obese
- SAH:
-
Systemic arterial hypertension
- SBP:
-
Systolic blood pressure
- SD:
-
Standard diet
- SHFD:
-
Saturated high-fat diet
- TNF-α:
-
Tumor necrosis factor-alpha
References
World OH. World Health Organisation obesity and overweight fact sheet. Who. 2016;1(1):2016.
Cotillard A, Poitou C, Torcivia A, Bouillot J, Dietrich A, Klöting N, et al. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99(8):1466–70.
Skurk T, Alberti-huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2016;92(3):1023–33.
Friedman J. Obesity: causes and control of excess body fat. Nature. 2009;342(May):340–2.
Lopaschuk GD, Folmes CDL, Stanley WC. Cardiac energy metabolism in obesity. Circ Res. 2007;101:335–47.
Junio A, Ferron T, Jacobsen BB, Grippa P, Ana S. Cardiac dysfunction induced by obesity is not related to β-adrenergic system impairment at the receptor-signalling pathway. PLoS One. 2015;10:1–18
Dupuy AM, Jaussent I, Lacroux A, Durant R, Cristol JP, Delcourt C. Waist circumference adds to the variance in plasma C-reactive protein levels in elderly patients with metabolic syndrome. Gerontology. 2007;53(6):329–39.
Anders LC, Yeo H, Kaelin BR, Lang AL, Bushau AM, Douglas AN, et al. Role of dietary fatty acids in liver injury caused by vinyl chloride metabolites in mice. Toxicol Appl Pharmacol. 2016;(in press).
Erdei N, Tóth A, Pásztor E, Papp Z, Edes I, Koller A, et al. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol. 2006;291:H2107–15.
Panchal SK, Poudyal H, Waanders J, Brown L. Coffee extract attenuates changes in cardiovascular and hepatic structure and function without decreasing obesity in high-carbohydrate, high-fat diet-fed male rats. J Nutr. 2012;142(4):690–7.
Johnson AR, Wilkerson MD, Sampey BP, Troester MA, Hayes DN, Makowski L. Cafeteria diet-induced obesity causes oxidative damage in white adipose. Biochem Biophys Res Commun. 2016;473(2):545–50.
Li H, Lelliott C, Håkansson P, Ploj K, Tuneld A, Verolin-johansson M, et al. Intestinal, adipose, and liver inflammation in diet-induced obese mice. Metabolism [Internet]. 2008;57(12):1704–10. Available from: http://dx.doi.org/10.1016/j.metabol.2008.07.029.
Gomez-smith M, Karthikeyan S, Jeffers MS, Thomason LA, Stefanovic B, Corbett D. A physiological characterization of the cafeteria diet model of metabolic syndrome in the rat. Physiol Behav [Internet]. 2016;16: Available from: http://dx.doi.org/10.1016/j.physbeh.2016.09.029
Ruiz-núñez B, Dijck-brouwer DAJ, Muskiet FAJ. ScienceDirect. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J Nutr Biochem [Internet]. 2016;36:1–20. Available from: http://dx.doi.org/10.1016/j.jnutbio.2015.12.007
Lee J, Lee J, Cho W, Yim N, Kim H, Yun B, et al. KBH-1, an herbal composition, improves hepatic steatosis and leptin resistance in high-fat diet-induced obese rats. BMC Complement Altern Med [Internet]. 2016;16:1–12.
Kelly KB, Kennelly JP, Ordonez M, Nelson R, Leonard K, Stabler S, et al. Weight gain, and adipose tissue inflammation in high fat diet-fed rats. Nutrients. 2016;8:2–13.
Lima-leopoldo AP, Leopoldo AS, Silva DCT, Nascimento AF, De Campos DHS, Luvizotto RAM, et al. Long-term obesity promotes alterations in diastolic function induced by reduction of phospholamban phosphorylation at serine-16 without affecting calcium handling. J Appl Physiol. 2014;117(6):669–78.
Nascimento AF, Sugizaki MM, Leopoldo AS, Lima-Leopoldo AP, Luvizotto RAM, Nogueira CR, et al. A hypercaloric pellet-diet cycle induces obesity and co-morbidities in wistar rats. Arq Bras Endocrinol Metabol. 2008;52(6):968–74.
Zock PL, Blom WAM, Nettleton JA, Hornstra G. Progressing insights into the role of dietary fats in the prevention of cardiovascular disease. Curr Cardiol Rep. 2016;18(11):111.
Mayor S. Eating more unsaturated fats and less carbohydrate reduces blood glucose, study finds. BMJ. 2016;4053(July):i4053.
Johns DJ, Lindroos A-K, Jebb SA, Sjöström L, Carlsson LMS, Ambrosini GL. Dietary patterns, cardiometabolic risk factors, and the incidence of cardiovascular disease in severe obesity. Obesity. 2015;23(0):1063–70.
Cabello-Saavedra E, Bes-Rastrollo M, Martinez JA, Diez-Espino J, Buil-Cosiales P, Serrano-Martinez M, et al. Macronutrient intake and metabolic syndrome in subjects at high cardiovascular risk. Ann Nutr Metab. 2010;56(2):152–9.
Sawin EA, Stroup BM, Murali SG, O’Neill LM, Ntambi JM ND. Differential effects of dietary fat content and protein source on bone phenotype and fatty acid oxidation in female C57Bl/6 mice. 2016. p.:e0163234.
Gakidou E, Mallinger L, Abbott-klafter J, Guerrero R, Villalpando S. Murray. Management of diabetes and associated cardiovascular risk factors in seven countries: a comparison of data from national health examination. 2011;89(3):172–83. http://www.who.int/bulletin/volumes/89/3/10-080820/en/.
Wang Z, Yang Y, Xiang X, Zhu Y, Men J HM. Estimation of the normal range of blood glucose in rats. Vol. 133, Wei Sheng Yan Jiu. 2010. p. 2010.
Després J-P. Abdominal obesity and cardiovascular disease: is inflammation the missing link? Can J Cardiol. 2012;28(6):642–52.
Henrique D, De Campos S, Leopoldo AS, Lima-leopoldo AP, Ferreira A, De Oliveira-junior SA, et al. Obesity preserves myocardial function during blockade of the glycolytic pathway. Arq Bras Cardiol. 2014;103:330–7.
Relling DP, Esberg LB, Fang CX, Johnson WT, Murphy EJ, Carlson EC, et al. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J Hypertens. 2006;24(3):549–61.
Mazidi M, Heidari-Bakavoli A, Khayyatzadeh SS, Azarpazhooh MR, Nematy M, Safarian M, et al. Serum hs-CRP varies with dietary cholesterol, but not dietary fatty acid intake in individuals free of any history of cardiovascular disease. Eur J Clin Nutr. 2015;2016:1–4.
Stark AH, Timar B, Madar Z. Adaptation of Sprague Dawley rats to long-term feeding of high fat or high fructose diets. Eur J Nutr. 2000;39(5):229–34.
Meshkani R, Vakili S. Clinica Chimica Acta. Tissue resident macrophages: key players in the pathogenesis of type 2 diabetes and its complications. Clin Chim Acta [Internet]. 2016;462:77–89.
Dispirito JR, Mathis D. Seminars in Immunology. Immunological contributions to adipose tissue homeostasis. Semin Immunol [Internet]. 2015;27(5):315–21.
Panchal SK, Poudyal H, Iyer A, Nazer R, Alam MA, Diwan V, et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol. 2011;57(5):611–24.
Panchal SK, Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol. 2011;2011(351982):14.
Vileigas DF, de Deus AF, da Silva DCT, de Tomasi LC, de Campos DHS, Adorni CS, et al. Saturated high‐fat diet‐induced obesity increases adenylate cyclase of myocardial β ‐adrenergic system and does not compromise cardiac function. Physiol Rep. 2016;4(17):e12914.
Silvares RR, Pereira ENGDS, Flores EEI, Estato V, Reis PA, Silva IJD, et al. Combined therapy with metformin and insulin attenuates systemic and hepatic alterations in a model of high-fat diet-/streptozotocin-induced diabetes. Int J Exp Pathol. 2016;97(3):266–77.
Poudyal H, Panchal SK, Ward LC, Waanders J, Brown L. Chronic high-carbohydrate, high-fat feeding in rats induces reversible metabolic, cardiovascular, and liver changes. Am J Physiol Endocrinol Metab. 2012;312:1472–82.
Acknowledgements
The authors thank Artur Junio Togneri Ferron for the reference corrections, Damiana Tortolero Pierine for the cytokine analyses, and Dijon Henrique Salomé de Campos for the developing the diets.
Funding
Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP-2012/25094-5)
Author information
Authors and Affiliations
Contributions
Authors’ contributions
CSA conducted the experiment and participated in writing the paper, CRC participated in the outline of the experiment and writing the paper, DFV participated in the preparation of the experiment and interpretation of the results, DHSC participated in the implementation of diet and CRP performed the statistical treatment, IOM, participated in the study of the paper and ACC delineated the experiment and participated in writing the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The protocol was approved by the Ethics Committee on Animal Experiments of the Botucatu Medical School–UNESP (protocol: 993/2012).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Adorni, C.S., Corrêa, C.R., Vileigas, D.F. et al. The influence of obesity by a diet high in saturated fats and carbohydrates balance in the manifestation of systemic complications and comorbidities. Nutrire 42, 16 (2017). https://doi.org/10.1186/s41110-017-0042-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-017-0042-1