Abstract
Background
The last decade has been marked by increasing data regarding gastroinstestinal diseases, specially gastritis and ulcer. In order to prevent or treat these diseases, many studies have demonstrated the potential of medicinal plants. The aim of this study was to evaluate the phytochemical profile and the gastroprotective activity of the methanolic extract of Myrcianthes pungens whole fruit, peel, pulp, seeds, and leaves.
Methods
The methanolic extracts were analyzed by thin layer chromatography (TLC) to detect the presence of phenolic compounds by direct comparison with an authentic sample. To evaluate the gastroprotective activity, two experimental models were used: acute ulcer model induced by ethanol/HCl and acute ulcer model induced by nonsteroidal anti-inflammatory drug (indomethacin). Animals were divided in different groups (n = 6) and pretreated orally with the methanolic extracts of M. pungens at doses of 50, 125, and 250 mg/kg, the positive control (cimetidine 100 mg/kg) and negative control (distilled water).
Results
The TLC analysis indicated the presence of the flavonoids quercetin and quercitrin in the leaves, quercetin in the peel, and catechin and epicatechin in the leaves and seeds of M. pungens. The extracts of leaves, peel, and pulp showed significant gastroprotective potential regarding the relative area of the lesion observed only in acute ulcer model induced by ethanol. The extracts of whole fruit, peel, pulp, seeds, and leaves showed significant gastroprotective potential observed in acute ulcer induced by indomethacin model.
Conclusions
The gastroprotective activity can be related with the presence of some phenolic compounds identified in phytochemical analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Peptic ulcer is a multifactorial chronic disease, and Helicobacter pylori infection, use of nonsteroidal anti-inflammatory drug (NSAIDs), and alcohol abuse are the main causative agents. It can prejudice any part of the gastrointestinal tract, characterized by ulceration of the mucosa, affecting more often the gastric and duodenal mucosa [1, 2].
The use of medicinal plants has been encouraged in public health because of the large number of people affected by diseases that generate economic impact. Furthermore, most synthetic drugs for treatment are associated with undesirable side effects [3, 4]. Several studies indicate the efficacy of medicinal plants for the peptic ulcer treatment, because of the presence of bioactive phytochemicals such as flavonoids, alkaloids, terpenes, tannins, saponins, phenolic acids, and others [2, 5].
The species Myrcianthes pungens, native of Rio Grande do Sul, belonging to the Myrtaceae family and popularly known as “guabiju” [6, 7], is used in folk medicine for possessing antidiarrheal properties [8], and its leaves have been described as diuretic and for the ability to reduce stomach disorders [9].
M. pungens fruits have high dietary fiber content, sugar, and protein, with low acidity and sweet taste. It has anthocyanins and high level of carotenoids and polyphenols, with antioxidant capacity [10, 11]. In a recent study, the peel, pulp, and leaves of M. pungens presented a significant antinociceptive activity. The presence of triterpenes and flavonoids were evidenced and may be responsible for the antinociceptive activity [12]. The aim of this study was to evaluate the phytochemical profile and the gastroprotective activity of the methanolic extract of M. pungens whole fruit, peel, pulp, seeds, and leaves.
Methods
Plant material
M. pungens was collected at Urussanga, in the state of Santa Catarina (Brazil), and identified by Prof. Oscar B. Iza (UNIVALI). A voucher specimen was deposited at the Barbosa Rodrigues Herbarium (Itajaí-SC) under number VC Filho152.
Preparation of extracts and phytochemical analysis
Fresh parts (whole fruit, peel, pulp, seeds, and leaves) of M. pungens were cut into small pieces and macerated with methanol at room temperature for approximately 7 days, providing the methanol extract from each part after solvent evaporation.
To verify the presence of phenolic compounds, aliquots of methanolic extracts were examined, using authentic samples as a parameter, by thin layer chromatography (TLC). The solvents chloroform and methanol (90:10) were used as mobile phase, and ferric chloride was used as a specific reactive of phenolic compounds.
Gastroprotective activity in vivo
Female Swiss mice (20–35 g) were provided by the Central Animal House of the Universidade do Vale do Itajaí (UNIVALI) (Itajaí, SC, Brazil). The animals were housed in groups of five, in standard cages, at room temperature (22 ± 2 °C) with 12-h dark/12-h light cycles, and received food and water ad libitum.
Twelve hours prior to the experiments, they were transferred to the laboratory and given only water ad libitum. In all experiments, the animals were kept in cages with wide-mesh raised floors to prevent coprophagy. The animals used in the present study were housed and cared for in accordance with the Federal Government legislation on animal care. The experiments were authorized by the Ethical Committee for Animal Care of UNIVALI (process number 005/14).
Ethanol/HCl-induced ulcer
The experiment was performed according to the method described by Mizui and Doteuchi [13], with some modifications. After 12 h of fasting, the animals were randomly divided into different groups of six animals each and pre-treated orally with cimetidine (positive control—100 mg/kg), vehicle (negative control—distilled water), and the methanolic extracts from each part of the plant at doses of 50, 125, and 250 mg/kg.
One hour after treatment, all animals received 0.1 mL/10 g (body weight) of a 0.3 mol/L HCl in 60% ethanol solution (ethanol/HCl) to induce gastric ulcer. Another hour later, the animals were sacrificed by cervical dislocation, and the stomachs removed and opened along the greater curvature.
Nonsteroidal anti-inflammatory drug-induced ulcer
Experiments were carried out according to the method described in Rainsford [14], with a few modifications. After 12 h of fasting, the animals were randomly divided into different groups of six animals each and pre-treated orally with cimetidine (positive control—100 mg/kg), vehicle (negative control—distilled water), and the methanolic extracts from each part of the plant at doses of 50, 125, and 250 mg/kg.
One hour after treatment, they received indomethacin (100 mg/kg body weight) to induce gastric ulcer. Twelve hours after treatment with indomethacin, the animals were sacrificed by cervical dislocation, and the stomachs removed and opened along the greater curvature.
Evaluation of gastroprotective activity
After completion of the two methods described above, the stomachs stretched on glass plates were scanned and analyzed using image analysis software EARP to determine the number and size of the lesions. The results are expressed as total lesion area (mm2) and relative lesion area (%). The data are reported as mean ± standard error of the mean (SEM) and were compared using one-way analysis of variance (ANOVA), followed by Dunnett’s pairwise test, and p values <0.05 were considered significant. The GraphPad INSTAT software was used for statistical analysis.
Results
The results obtained by the phytochemical analysis by TLC indicated the presence of quercetin and quercitrin in the leaves, quercetin in the peel, and catechin and epicatechin in the leaves and seeds.
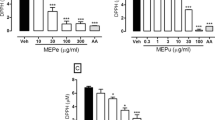
The methanolic extracts of peel and leaves at all concentrations and pulp at concentrations of 125 and 250 mg showed defense capability of the gastric mucosa against ethanol, reducing the relative lesion area significantly when compared to the negative control, as well as cimetidine, which proved its gastroprotective effect (Table 1).
In relation to indomethacin model, all M. pungens extracts presented defense capability of the gastric mucosa, reducing significantly total and relative lesions areas compared to the negative control (Table 2).
Discussion
This study evidenced the presence of phenolic compounds in different parts of M. pungens. Nesello et al. [12] verified the presence of terpenes/steroids and phenolic compounds at the same evaluated parts. Desoti et al. [15] evaluated the methanol crude extract of M. pungens fruits and identified tannins, flavonoids, steroids, and alkaloids in its composition. Andrade et al. [10] identified anthocyanins, polyphenols, and flavonoids and also showed antioxidant activity. Nora [11] identified anthocyanins and carotenoids (β-carotene), as well as antioxidant activity of methanolic fruit extract.
The reason why seed extracts (all concentrations) and the whole fruit (concentrations of 125 and 250 mg) extracts were not as effective as other extracts/concentrations was not fully investigated in this study. A possible explanation would be the presence of irritant compounds in seeds and/or because their primary mechanism of action is maintaining or increasing the production of prostaglandins, mucus, and bicarbonate [16, 17].
There are no reports in the literature regarding the gastroprotective potential of M. pungens, but a study by Desoti et al. [15] showed that ethyl acetate and hexane extracts of M. pungens fruits showed bactericidal properties. The same authors also identified antifungal activity of ethyl acetate and methanol extracts and low cytotoxic effect of these extracts. Silveira et al. [6] identified acetylcholinesterase activity related to the reduction of gastric secretion in the ethanol extracts of green and ripe fruits of M. pungens.
In a recent study, the peel, pulp, and leaves of M. pungens inhibited the acetic acid-induced contractions in mice. The leaves were also active in the formalin, capsaicin, and glutamate models. The triterpenes alpha-amyrin and beta-amyrin and the flavonoids quercitrin and rutin were the major components of M. pungens leaves and may be responsible for the antinociceptive activity evidenced. The results demonstrate the efficacy of M. pungens, specially the leaves, as a potentially novel and effective analgesic agent for pain control [12].
The mechanism for the gastroprotective action of M. pungens in this study may be related to the presence of flavonoids evidenced in phytochemical analysis, which have been considered the responsible for attenuating the oxidative effects induced by indomethacin [18]. According to Klein-Junior et al. [19], flavonoids demonstrate gastroprotective activity in the induction model with indomethacin, reducing gastric lesions caused by these NSAIDs. Brzozowski et al. [20] also reported gastroprotective activity, as well as antibacterial and antioxidant action against ethanol-induced damages, observing increasing of vasodilation and prostaglandin related to the activity of NO, possibly stimulated by the phytochemical compound in discussion.
The results obtained from both models demonstrated that the methanolic extracts of different parts of M. pungens have gastroprotective activity, which suggests that the phenolic compounds present in the extract favor anti-inflammatory and antioxidants mechanisms, possibly acting in the maintenance of prostaglandin and NO, favoring the protection mechanisms and injury repair [21, 22].
Conclusions
M. pungens seeds and whole fruit extracts demonstrated no gastroprotective activity on ulcer model induced by ethanol. The remaining extracts (peel, pulp, and leaves) showed gastroprotective effect which can be explained by the presence of various phenolic compounds evidence in phytochemical analysis.
Further studies are necessary to confirm these effects and to elucidate the mechanisms of action of gastroprotective activity. The phytochemical studies are in progress to isolate/identify other bioactive compounds.
References
Ferreira SAS. Evolução na abordagem fármaco - terapêutica da úlcera péptica. 2013. 65 f. In: Dissertação (Mestrado em Ciências Farmacêuticas). Porto: Universidade Fernando Pessoa; 2013.
Paguigan ND, Castillo DHB, Chichioco-Hernandez CL. Atividade anti-úlcera de plantas leguminosas. Ar Gastroenterol. 2014;51:64–8.
Carvalho AST. Úlcera péptica. J Pediatr (Rio J). 2000;76:127–34.
Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84:102–13.
Braz DC, Oliveira LRS, Viana AFSC. Atividade antiulcerogênica do extrato aquoso da Bryophyllum pinnatum (Lam.) Kurz. Rev Bras Plantas Med. 2013;15:86–90.
Silveira S, Lucena EV, Pereira TF, Garnés FLS, Romagnolo MB, Takemura OS, Laverde JA. Atividade anticolinesterásica dos frutos de Myrcianthes pungens (O.Berg) D.Legrand (Myrtaceae). Arq Ciênc Saúde UNIPAR. 2011;15:127–33.
Apel AM, Sobral M, Henriques AT. Composição química do óleo volátil de Myrcianthes nativas da região sul do Brasil. Rev Bras Farmacogn. 2006;16:402–7.
Pio CM. Dicionário das plantas úteis do Brasil e das exóticas cultivadas. Rio de Janeiro: Imprensa Nacional; 1984.
Martínez-Crovetto R. Las plantas utilizadas en medicina popular en el Noroeste de Corrientes (República Argentina). Texas: Ministerio de Cultura y Educación, Fundación Miguel Lillo; 1981.
Andrade JMM, Aboy AL, Apel MA, Raseira MCB, Pereira JFM, Henriques AT. Phenolic composition in different genotypes of guabiju fruits (Myrcianthes pungens) and their potencial as antioxidant and antichemotactic agents. J Food Sci. 2011;76:1181–7.
Nora CD. Caracterização, atividade antioxidante "in vivo" e efeito do processamento na estabilidade de compostos bioativos de araçá vermelho e guabiju. 2012. 90 f. In: Dissertação (Mestrado em Ciência e Tecnologia de Alimentos). Porto Alegre: Universidade Federal do Rio Grande do Sul; 2012.
Nesello LAN, Campos A, Capistrano K, de Campos BF, Cechinel FV. Chemical composition and antinociceptive activity of Myrcianthes pungens leaves. Int J Appl Res Nat Prod. 2016;9:14–9.
Mizui T, Doteuchi M. Effect of polyamines on acidified ethanol-induced gastric lesion on rats. Jpn J Pharmacol. 1983;33:939–45.
Rainsford KD. Biochemical gastroprotection from acute ulceration induced by aspirin and related drugs. Biochem Pharmacol. 1980;29:1281–9.
Desoti VC, Maldaner CL, Carletto MS, Heinz AA, Coelho MS, Piati D, Tiuman TS. Triagem fitoquímica e avaliação das atividades antimicrobiana e citotóxica de plantas medicinais nativas da região oeste do estado do Paraná. Arq Ciênc Saúde UNIPAR. 2011;15:3–13.
Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33:224–34.
Madalosso RC, Oliveira GC, Martins MT, Vieira AE, Barbosa J, Caliari MV, Castilho RO, Tagliati CA. Campomanesia lineatifolia Ruiz & Pav. as a gastroprotective agent. J Ethnopharmacol. 2012;139:772–9.
Adhikary B, Yadav SK, Bandyopadhyay SK, Chattopadhyay S. Epigallocatechin gallate accelerates healing of indomethacin-induced stomach ulcers in mice. Pharmacol Rep. 2011;63:527–36.
Klein-Júnior LC, Santin JR, Niero R, de Andrade SF, Cechinel FV. The therapeutic lead potential of metabolites obtained from natural sources for the treatment of peptic ulcer. Phytochemistry Rev. 2012;11:567–616.
Brzozowski T, Konturek PC, Drozdowicz D, Konturek SJ, Zayachivska O, Pajdo R, Kwiecien S, Pawlik WW, Hahn EG. Grapefruit-seed extract attenuates ethanol-and stress-induced gastric lesions via activation of prostaglandin, nitric oxide and sensory nerve pathways. World J Gastroenterol. 2005;11:6450–8.
Hamaishi K, Kojima R, Ito M. Anti-ulcer effect of tea catechin in rats. Biol Pharm Bull. 2006;29:2206–13.
Lemos M, Santin JR, Klein-Júnior LC, Niero R, de Andrade SF. Gastroprotective activity of hydroalcoholic extract obtained from the leaves of Brassica oleracea var. acephala DC in different animal models. J Ethnopharmacol. 2011;138:503–7.
Acknowledgements
This work was supported by grants from the Brazilian institutions Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Apoio a Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC). The authors give thanks to Prof. Oscar Iza for plant identification.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to this research article and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiments were authorized by the Ethical Committee for Animal Care of UNIVALI (process number 005/14).
Consent for publication
Not applicable.
Competing interests
We wish to confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
de Almeida, A.L., Beleza, M.L.M.L., Campos, A. et al. Phytochemical profile and gastroprotective potential of Myrcianthes pungens fruits and leaves. Nutrire 42, 24 (2017). https://doi.org/10.1186/s41110-017-0040-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-017-0040-3