Abstract
Background
Iron deficiency anemia and feeding difficulties (FD) are common issues in childhood, reinforcing the concern about the risk of micronutrient deficiencies. FD do not necessarily reflect nutritional deficiencies, since they may or may not relate to specific nutrient sources. The objective of the study is to describe the prevalence of iron depletion and iron deficiency anemia in children with FD and to seek associations with diagnosis and its markers.
Methods
This is a cross-sectional study with 68 patients (convenience sample). The following data were assessed through medical records: age (months), gender, exclusive breastfeeding duration (months), birth weight (kg), iron supplementation, hemoglobin (Hb), ferritin, and C-reactive protein (CRP) levels, repertory of foods consumed (food inventory and 3-day food record analysis), and diagnosis of FD. Data were classified according to references for age and were analyzed using correlation tests, Student’s t test, ANOVA and chi-square test, or its nonparametric equivalents. A significance level of 5% was considered.
Results
Iron depletion and anemia were identified in 10.1 and 6% of children, respectively. Picky eating was diagnosed in 35.3%. Food repertory consisted on average of 21 foods, with null correlation to Hb and ferritin. The average fortified milk intake was 517 ml/day, with null correlation to Hb. There was no effect of diagnosis of FD on Hb (p = 0.18) or ferritin (p = 0.52). The same was verified in the children without supplementation, to both Hb (p = 0.54) and ferritin (p = 0.08).
Conclusions
No evidence of association between diagnosis of FD or repertory of foods to anemia or iron depletion was found, which could be a reassuring factor for caregivers. Reproduction in large scale as well as inclusion of dietary intake variables is suggested for further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Iron deficiency and iron deficiency anemia are considered the most common nutritional disorders around the world [1, 2]. Data from WHO [3] on the global prevalence of anemia states that around 24.8% of the population is anemic. In preschool-age children, anemia prevalence is 47.4% and in pregnancy, around 41.8%. Other studies estimate that up to 43% of the children under 5 years old and 38% of pregnant women are affected in the world [4]. In Brazil, official data from 2014 describe the prevalence of anemia in children under 5 years of age of 20.9% and global prevalence above 40% [1]. Other national studies suggest a global prevalence between 45 and 50% [5]. Repercussions on growth and development are associated with high morbidity and mortality in children under 2 years old [4, 5]. Among prevention measures, nutritional education towards proper eating habits is the priority. However, when dealing with a high prevalence of anemia, other ways of intervention should also be established, such as preventive supplementation as well as enriching foods with iron [5].
Biologically, there are three phases of iron deficiency. The first phase—iron depletion—takes place when the dietary offer of iron is inadequate, with reduction of its deposits (characterized by reduced serum ferritin, without functional alterations). If this negative balance persists, there comes the second phase—the iron deficient erythropoiesis—characterized by decrease in serum iron, low saturation of transferrin, and elevation of free erythrocyte protoporphyrin (possibly with the diminishing of labor capacity and the appearance of cognitive alterations). In the third and final phase—anemia—hemoglobin goes down to a level below standards and is characterized by the appearance of microcytosis and hypochromia [6]. Iron depletion is substantially more prevalent than anemia itself. It is estimated that for each person with anemia, there is at least one other person with iron deficiency [1, 4]. Hence, in a population like the one in Brazil, both iron stocks and anemia are relevant topics for concern.
Parallel to anemia, feeding difficulties (FD), a group of several eating complaints common during early childhood [7, 8], affect approximately 25% of healthy children and up to 80% of children with gastrointestinal and developmental issues [9, 10]. According to Kerzner et al. [7], FD can be divided into groups of similar behaviors, such as:
-
“Children with limited appetite”: ranges from children who eat appropriately but appear to eat too little (misperception), to those with overt organic disease that impact on appetite.
-
“Agitated children”: children with limited appetite or food refusal, generally caused by lack of interest in food, and more on environmental stimuli.
-
Phobia: characterized by great resistance to feeding (such as screaming, intense crying, back arching, tachycardia, cold sweating, and fussiness), including feeding equipment, and severe food refusal. In general, children who have been put through invasive procedures, such as intubation or tube feeding, as well as accidents or severe choking may present such behaviors.
-
Misperception of caregivers: when caregivers believe their children eat too little, when, in fact, they achieve nutritional requirements.
-
“Picky eating”: children who eat appropriately for their stage of development, but present sensory-related aversions, with partial or total refusal of foods according to its texture, color, smell, consistency, or taste.
-
Organic causes: relevant conditions (structural, gastrointestinal, cardiorespiratory, neural and metabolic, food allergy, celiac disease, esophagitis, gastroesophageal reflux, and others) that may impair the process of feeding and appetite.
Inadequate eating habits during this cycle of life can impact children’s current and future health conditions negatively, causing even growth impairment or anemia [11,12,13,14]. Additionally, there is still a striking prevalence of nutritional problems in Brazil, mainly in pre-school children, whose primary determinant factor is inadequate dietary habits [13]. Clinical perception of families’ concern and common sense is specifically that the child with FD is necessarily at risk of micronutrient deficiency, which worsens parents’ anxiety and attempts to correct the behavior. The same happens to pediatricians, who usually associate the child who does not eat with biochemical deficiencies. Despite this risk, FD may not be necessarily connected to anemias and other micronutrient disorders, since the child’s eating restrictions may or may not be related to their main sources of food.
There is lack of population studies on this theme, especially in Brazil. Hence, the objective of this article is to describe the prevalence of iron depletion and iron deficiency anemia in children diagnosed with FD and to investigate possible associations with the diagnosis and other traits of FD.
Methods
Study design and sampling
The study has a cross-sectional design and was conducted in the Center of Feeding Difficulties (CFD), an outpatient private model service and investigation center dedicated to the support of children and adolescents between 0 and 19 years old with complaints of feeding difficulties in general, part of the PENSI Institute—Sabará Children’s Hospital/José Luiz Egydio Setúbal Foundation (São Paulo, Brazil). Sampling was planned due to convenience, with the inclusion of all the patients followed in service up to the moment of the gathering of data, by the end of 2015 (n = 68). All patients have presented written consent forms signed by parents or guardians (allowing use of information in medical charts), as well as the approval of the CFD research project by the PENSI Institute ethics and research committee.
Data collection
Data was gathered through medical chart analysis, from which the following variables were selected: age (months), gender, duration of exclusive breastfeeding (EB, in months), birth weight (kg), current iron supplementation, levels of hemoglobin (Hb) and ferritin, biomarker of infection “C-reactive protein” (CRP), quantity of foods the child usually accepts and total volume of milk intake (ml/day)—both assessed by the nutritionists through a food inventory and 3-day food record and whose protocol is described elsewhere [15]—and the multidisciplinary diagnosis of FD done by the team (pediatricians and nutrologists, nutritionists, and speech therapists), according to the criteria proposed by Kerzner et al. [7]. Levels of Hb and ferritin were classified according to references for age [1]: children from 6 to 59 months of age (11 g/dl), children from 5 to 11 years old (11.5 g/dl), children from 12 to 14 years old and older female adolescents (12 g/dl), and males older than 14 years old (13 g/dl). Ferritin levels followed criteria of 12 mcg/l in children with less than 5 years of age and of 15 mcg/l in children with 5 years of age or older, regardless of gender. In the presence of infection, cutoff points were considered as depletion when below 30 mcg/l [16]. To assess CRP levels, results were considered suggestive of infection/inflammation when higher than 1.0 mg/dl, according to WHO [17] recommendations.
Statistical analysis
After the evaluation of consistency of the data, statistical analysis was conducted by the software SPSS v21. Descriptive analysis was conducted through distribution frequencies (%) to category variables (gender, birth weight, presence of iron supplementation, classification of anemia and iron depletion, and diagnosis of FD) and average and/or median ± standard deviation (sd) to continuous variables (age, duration of EB, levels of iron and ferritin, and food repertoire).
The continuous variables were tested as to their normality and homogeneity, and Pearson or Spearman’s correlation was conducted to test associations between milk intake/food repertoire and biochemical levels. To analyze the impact of FD diagnosis on levels of Hb and ferritin, multivariate general linear model (GLM) and chi-squared tests were used and comparison between biochemical levels and their respective references for age was conducted through Student’s t test or Wilcoxon (one sample). Additionally, a sub-sample was created to compare the profiles between p25 and p75, through nonparametric test of Mann-Whitney. A 5% level of significance was considered.
Results
The general characteristics of the sample are described in Table 1. Children were mostly male (67.6%) and younger than 5 years old (76.5%), with a median of 33 months of age (p25% 18.8 months; p75% 57 months). Low birth weight (LBW) has been identified in approximately 16% of children, and EB lasted for 2 months (median). The most frequent FD diagnosis was picky eating (35.3%), food repertoire usually accepted by children consisted of—on average—21 foods, and daily milk intake reported was, on average, 516.9 ml ± 257.8. Around 59% of the children were not currently under any iron supplementation. Hb levels were significantly higher than references for age in children younger than 5 years old (t = 12.4; dif. 1.92; 95% CI 1.60–2.23; p = 0.000) and between 5 and 11 years of age (p = 0.017; Wilcoxon test), but no differences were found in older children (p = 0.18; Wilcoxon test). Around 10% of the sample presented low levels of ferritin, and only 6% were considered anemic (Fig. 1). No correlation was found between milk intake and Hb (r Pearson = 0.014; p = 0.92).
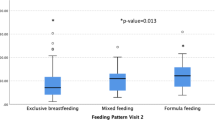
Figure 2 compares FD diagnosis with Hb and ferritin levels. The GLM multivariate test shows no significant effect from FD diagnosis on the levels of Hb (F = 1.54; p = 0.18; eta2 = 0.21) or ferritin (F = 0.89; p = 0.52; eta2 = 0.13). Similar results were found when children receiving iron supplementation were excluded from tests, both to Hb (F = 0.81; p = 0.57; eta2 = 0.21) and ferritin (F = 2.01; p = 0.12; eta2 = 0.40). Moreover, no association between ferritin levels adjusted for PCR levels and types of FD was found (p = 0.22). In Table 2, the Mann-Whitney test shows no relation between Hb levels and type of FD, even when extremities of the samples (p25% and p75%) are compared. Correlations between food repertoire and biochemical data (Table 3) were considered null for Hb and weak for ferritin, demonstrating lack of association between these variables.
Discussion
Results found show low rates of anemia and iron depletion in children with FD complaints, as well as no association between iron status and the type of FD diagnosed or the repertoire of foods consumed, even when controlled for the extreme quartiles or confusing factors. These results suggest a healthy iron status profile in the current sample.
In Brazil, a review of regional studies in 2009 [2] showed a high prevalence of iron deficiency anemia (53%) in children below 5 years old from all social and economic levels. When the prevalence of anemia in these national studies [2] is analyzed according to age older than 24 months old (similar to the present sample), the mean prevalence of anemia is 11%, diminishing the comparison to results herein (6%). However, it is highlighted that the difference is still 1.83 times bigger. Vieira and Ferreira [18], in a systematic review of Brazilian studies from different regions, showed that the prevalence of anemia reduced according to children’s growth, with a mean prevalence of 40% nationwide.
The frequency of anemia described in the present sample resembles data compatible with developed countries, as described by Cairo et al. [19], which show a prevalence between 4.3 and 20% in children from developed countries in general. Desalegn et al. [20] mention a prevalence of anemia in the same group of children of around 5.9%, and Batrouni et al. [21] have also found significant difference of prevalence of anemia between high and low social and economic levels (36 and 8%, respectively) in a population of a total anemia prevalence of 42%. A possible explanation for this resemblance could be the characteristic social and economic level of the sample, which would allow more access to information, adequate supplementation of iron during the first 24 months of life, and acquisition of fortified foods. Despite the fact that the present study did not evaluate the social and economic level of the sample, the profile of patients attended at the hospital and in the private outpatient service is characterized essentially by social classes of a higher purchasing power.
According to the WHO report mentioned previously, it is estimated that there is one case of iron depletion for each case of anemia [2], similar results herein, that shows a relation between low levels of ferritin and anemia of 1:1.6. Studies that analyze hemoglobin together with ferritin levels were not found to evaluate the nutritional state of iron, which makes comparisons more difficult. Cairo et al. [19] emphasize that 95% of the cases of iron deficiency anemia are related to a diet poor in iron food sources, which makes the investigation of stock levels extremely relevant to prevention strategies. For Sirdah et al. [22], the non-association of serum ferritin in the researches about anemia in countries most affected translates into an important limitation in the real evaluation of this deficiency.
It is known that children with FD notoriously compensate dietary restriction with milk [23] and that the use of formulas and fortified dairy products in the prophylaxis of anemia is effective with children who are 2 years old or younger, if breastfeeding is impossible [6, 24]. Hence, consumption of fortified dairy products may also have played a role in the prevention of anemia and in the maintenance of good stocks of iron in the current sample, despite the unexpressive relation between the consumed volume (with an average of 517 ml/day) and biochemical showed in the results.
As to FD diagnosis, other studies that associate types of FD to the levels of iron in children are scarce, which impair comparisons. Nevertheless, this lack of association may be due to the specific repertoire of foods consumed by children and not the type of FD itself, since foods rejected may not necessarily be the ones that are sources of iron. However, this variable was not available at the moment of data collection and could not be analyzed. As well as the type of FD, the repertoire (quantity) of foods consumed by the children probably does not predict iron depletion or anemia either, according to the present results. Studies that evaluate impact of picky eating on daily intake of iron have not been found. Therefore, it is possible that the restriction found (average of 21 foods) is sufficient for the adequate supply of macro- and micronutrients for these children. As the correlation between this variable and the Hb and ferritin in the extreme quartiles of the sample were average (r = 0.50), it is suggested that the test is replicated in studies with a wider sample and the inclusion of other dietary variables (such as food types and groups) to provide a deeper investigation. Nevertheless, the total repertoire of ingested food by the child does not seem suggestive of the risk of anemia.
The study has limitations, such as the restricted sample size (insufficient to prove hypothesis) and the absence of a control group and the limited frequency of anemia and iron depletion found, which hampers comparisons, besides the variable of the dietary consumption of sources of iron in the analysis, or the variable “consumed food repertoire” stratified into food groups. The fact that the sample consists of patients from high socioeconomic level is also considered a limitation. Results, therefore, cannot be extrapolated to the overall population. However, the fact that it is a study done exclusively with children showing complaints of feeding difficulties is considered a strong trait, which adds to the discussion that the inadequate food intake is not always followed by biochemical alterations, and contributes to the scenario of scarce scientific published evidences internationally.
Conclusions
There is no evidence that the type of feeding difficulty diagnosis or the repertoire of foods children consumed are associated with anemia or iron depletion in the sample, even when controlled for the extreme quartiles of the sample or for age, birth weight, duration of exclusive breastfeeding, and iron supplementation. The low prevalence of anemia found was similar to data from developed countries and could be related to the higher social and economic level of the studied population, as well as to the intake of fortified dairy products characteristic of the sample. These results could be a soothing factor for caregivers during the child’s clinical follow-up for FD. A reproduction of this study is suggested with representative samples, with the inclusion of variables of dietary consumption of iron, for the confirmation of these hypotheses.
References
Brasil: Ministério da Saúde. Portaria SAS/MS 1247. Protocolo clínico e diretrizes terapêuticas: anemia por deficiência de ferro.2014.
Jordão RE, Bernardi JLD, Barros Filho AA. Prevalência de anemia ferropriva no Brasil: uma revisão sistemática. Rev Paul Pediatr. 2009;27(1):90–8.
WHO. World Health Organization. Global epidemiology of hemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):417–96.
Pasricha SR, Draskesmith H. Iron deficiency anemia: problems in diagnosis and prevention at the population level. Hemtol Oncol. 2016;30(2):309–25.
Braga JAP, Vitalle MSS. Deficiência de ferro na criança. Rev Bras Hematol Hemot. 2010;32 Suppl 2:38–44.
Lamounier J, Capanema FD, Rocha DS, Oliveira JED. Nutrologia pediátrica: Anemia ferropriva. São Paulo: Manole; 2016.
Kerzner B, Milano K, MacLean WC, Berall G, Stuart S, Chatoor I. A practical approach to classifying and managing feeding difficulties. Pediatrics. 2015;135(2):344–53.
Chatoor I. Sensory food aversions in infant and toddlers. Zero to three. 2009;29(3):43–9.
Fisberg M, Maximino P. Nutrologia Pediátrica: Dificuldades alimentares. São Paulo: Manole; 2016.
Estrem HH, Pados BF, Thovre S, Knafl K, McComish C, Park J. Concept of pediatric feeding problems from the parents’ perspective. Am J Matern Child Nurs. 2016;41(4):212–20.
Brusco TR, Delgado SE. Caracterização de desenvolvimento da alimentação de crianças nascidas pré-termo entre três e doze meses. Rev CEFAC. 2014;16(3):917–28.
Chatoor I, Ganiban J. Food refusal by infants and young children: diagnosis and treatment. Cogn Behav Pract. 2003;10(2):138–46.
Gontijo LM, de Paula GF, Weffort VRS, Pedrosa AK. Distúrbios alimentares na infância e adolescência. Rev Med Minas Gerais. 2011;21(3):S1–144104.
Green RJ, Samy G, Miqdady MS, Salah M, Sleiman R. How to improve eating behaviour during early childhood. Pediatr Gastroenterol Hepatol Nutr. 2015;18(1):1–9.
Maximino P, Machado RHV, Junqueira P, Ciari M, Tosatti AM, Ramos dC C, et al. How to monitor children with feeding difficulties in a multidisciplinary scope? Multidisciplinary care protocol for children and adolescents. J Hum Growth Dev. 2016;26(2): p. ahead of print.
WHO. World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Geneva: WHO/NMH/NHD/MNM/11.2, Vitamin and mineral nutrition information system; 2011.
WHO. World Health Organization. C-reactive protein concentrations as a marker of inflammation or infection for interpreting biomarkers of micronutrient status. Geneva: Vitamin and Mineral Nutrition Information System; 2014.
Vieira RCS, Ferreira HS. Prevalência de anemia em crianças brasileiras, segundo diferentes cenários epidemiológicos. Rev Nutr Campinas. 2010;23(3):433–44.
Cairo RCA, Silva LR, Bistani NC, Marques DF. Iron deficiency anemia in adolescents: a literature review. Nutr Hosp. 2014;29:1240–9.
Desalegn A, Mossie A, Gedefaw L. Nutritional iron deficiency anemia: magnitude and its predictors among school age children, southwest Ethiopia: a community based cross-sectional study. PLoS One. 2014;9(12):e114059.
Batrouni LN, Frassoni NA, Eandi AM, Dasbul G, Piran AMF. Hierro hematico y desarrollo en ninos de 6 a 24 meses de edad, segun su origen social, Córdoba, Argentina. Rev Fac Cien Med Univ Nac Cordoba. 2006;63(3):9–16.
Sirdah MM, Yaghi A, Yaghi AR. Iron deficiency anemia among kindergarten children living in the marginalized areas of Gaza Strip, Palestine. Rev Bras Hematol Hemoter. 2014;36(2):132–8.
Almeida CAN, Melo ED, Maranhão HS, Vieira MC, Barros R, Fisberg M. Dificuldades alimentares na infância: revisão da literatura com foco nas repercussões à saúde. Pediatr Mod. 2012;48:9.
Moraes MB. Deficiência de ferro nas afecções gastrointestinais da criança. Rev Bras Hematol Hemoter. 2010;32(2):62–9.
Acknowledgements
We thank Ms. Carolina dos Santos Scarpa, who participated in the data collection.
Funding
The study was supported by the PENSI Institute (Brazil). The funder has contributed to the study conduction of the study, but not to the analysis of the data, interpretation of the findings, or the preparation of the manuscript.
Availability of data and materials
The data that support the findings of this study were used under license for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of PI Prof. Dr. Mauro Fisberg.
Authors’ contributions
JSS is the main researcher, carried out the study, and participated in the design, data analysis, and preparation of the manuscript. RHVM carried out the study and participated in the design, data analysis, and preparation of the manuscript. ABB, AMT, CCR, and PM assisted in carrying out the study and preparation of the manuscript. MF supervised and assisted in all phases of the study (project PI). All authors read and approved the final manuscript.
Competing interests
The PI of the project (Mauro Fisberg) conferences in events such as Abbott, CPW, EMS, Danone, Nestlé, Nutrociencia, PICME, Sanofi, Wyeth; is a scientific board member of Danone Institute International, Danone Research, Mondelez; and supports research projects at Abbott, CNPq, Coca-Cola, CPW, Danone Institute International, Danone Research, Fapesp, Fap Unifesp, and Nestlé.
Priscilla Maximino consults for Hyproca Nutrition Nutrição Infantil Ltda.
The authors’ declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the PENSI Institute Ethical Committee (Brazil) under the code 32939314.0.0000.5567. Written informed consent was obtained from all patients’ parents or legal guardians.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Soares, J.S., Maximino, P., Machado, R.H.V. et al. Feeding difficulties are not associated with higher rates of iron deficiency anemia in Brazilian children and adolescents—cross-sectional study. Nutrire 42, 4 (2017). https://doi.org/10.1186/s41110-016-0027-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-016-0027-5