Abstract
Background
Preventing the development of iron deficiency anemia during infancy requires the appropriate complementary foods with high energy, nutrient density, and adequate iron content, as well as high nutrient bioavailability. We aimed to evaluate iron intake, bioavailability, and absorption from foods, in healthy infants and toddlers at a Well Child Clinic.
Methods
This observational, cross-sectional, descriptive study evaluated 96 consecutive infants and toddlers, 6 to 12 months of age (group I) and 13 to 36 months of age (group II) that were brought for regular pediatric visits and introduced to complementary foods. Quantitative 24-h dietary recalls were obtained, and iron intakes quantified for lunch and dinner. Iron bioavailability and absorption were calculated and analyzed by Monsen’s and FAO/WHO’s methods according to enhancing factors: meat, poultry, and fish (MPF) and vitamin C.
Results
There were no significant differences in demographic, clinical, and anthropometric variables between groups. Vitamin C intake was not different between groups, but MPF was significantly lower in group I. The proportion of children with recommended RDA iron intake was lower (p < 0.05) in group I (16 %) than that in group II (47 %). Group I had lesser MPF intake and iron absorption and a higher proportion of children with low bioavailability in lunch and dinner when compared to group II (p < 0.05).
Conclusions
Inclusion of low-cost meat, especially chicken meat and vitamin C-rich foods, at the same meal, both in lunch and dinner, would be of particular advantage to ensure an adequate intake of bioavailable iron during complementary feeding.
Similar content being viewed by others
Background
Adequate nutrition during infancy and early childhood is essential to ensure the full potential of growth and development [1]. The transition from breastfeeding to complementary foods and family foods represents a phase of nutritional vulnerability for infants and toddlers. The World Health Organization (WHO) emphasized exclusive breastfeeding until 6 months of age instead of 4 to 6 months as recommended previously [2].
Iron is essential in human nutrition whereas iron deficiency is the most prevalent micronutrient deficiency in the world [3]. Adequate iron status in infancy is important because of the adverse effects of its insufficiency on child health and development such as cognitive impairment, decreased activity, and death from severe anemia [4]. In Brazil, a systematic review based on 53 studies evaluated 256 publications from 1996 to 2007 and data of 20,952 children under 5 years pointed out 53 % of anemia prevalence, and anemia was strongly associated to age under two years [5]. This data is considered a high prevalence rate by the WHO and contrast with 39.5 % in all of Latin America.
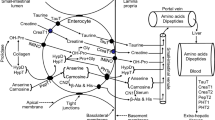
Infants and toddlers are especially susceptible to iron deficiency anemia because exogenous iron requirements increase rapidly during the second half of the first year. Preventing the development of iron deficiency anemia during this early phase of life requires the appropriate complementary foods with high energy, nutrient density, and adequate iron content, as well as high nutrient bioavailability. Different approaches are suggested to improve iron bioavailability from complementary foods, e.g., by increased consumption of vitamin C-rich fruits and vegetables and meat. In particular, red meat is an excellent source of highly bioavailable heme iron, in addition to significantly increased non-heme iron bioavailability [6]. Therefore, meat intake during complementary foods is very powerful for maintaining iron stores [7] and appropriate complementary foods should be explored and adapted to local conditions.
On the basis of these observations, the aim of this study was to evaluate iron intake, bioavailability, and absorption from foods, in healthy infants and toddlers at a Well Child Clinic. We hypothesize that the complementary feeding of children with normal growth and oriented for the recommended “Ten Steps for Healthy-Eating Children Under Age Two” [8] is adequate on macronutrients but is low in iron intake and bioavailability.
Methods
Design and subjects
This is an observational, cross-sectional, descriptive, and quantitative study. The sample comprised consecutive infants and toddlers, aged 6 to 12 months (group I) and 13 to 36 months (group II) who were attended at the Well Child Clinic between September 2009 and December 2010. For selection of the subjects, inclusion criteria were as follows: children from mothers who had early and regular antenatal care, gestational age of 37–41 weeks, birth weight within the 10th–90th percentiles, and with regular pediatric visits to the Well Child Clinic since 1 month of age. All of them had been introduced to home-prepared complementary foods and oriented for the recommended Ten Steps for Healthy-Eating Children Under Age Two by two of the authors [8]. Exclusion criteria were as follows: birth defects or congenital malformation, long-term diseases, and any food allergies. The study was approved by the Ethics Committee of the Botucatu Medical School/UNESP. All legal parent or guardian of the children studied gave written consent to participate in the study under the “Ethics, consent and permissions” heading and had consent to publish the results.
Procedures
A questionnaire was developed specifically for this project, and the data were obtained from the mothers and notes of the patients. Data was collected, coded, and processed by all the authors with adequate precautions to ensure patient confidentiality.
Anthropometry
All anthropometric measurements were made using calibrated equipment and standardized techniques according to WHO guidelines [9] and included weight recorded by an electronic scale (Microelectronic Filizola scales, model E-150/3P) accurate to 5 g whereas the recumbent length and height were measured by an infantometer and anthropometer, respectively. The z scores for weight-for-age, length/stature for-age, and BMI-for-age were calculated via the computer program Anthro from the WHO Multicentre Growth Reference Study, and a z score between −2z and 2z was considered normal [10].
Dietary intake
Dietary interviews, conducted by a nutritionist, obtained quantitative 24-h dietary recalls of the day preceding the outpatient clinic visit. Intake was estimated through common household measures referred by mothers and the composition described on the brand of the products [11]. The dietary data collected were breastfeeding and bottle/cup feeding (number of times per day), number and size of foods, and composition of meals. Iron and vitamins from medicinal supplements were not included in this analysis. The methodology of Drewett et al. [12] was used to estimate breast milk intake. By this method, the volume of milk consumed was based on the duration and frequency of feedings, quantity (in kilocalories) of complementary feeding, and the child’s age. The software DietPro 5.0 (Nutritional Support Program) and a Brazilian food composition table [13], with additional information obtained from food manufacturers about products not included in the tables, were used to calculate the nutritional values of the diets (energy, macronutrients, total iron, heme, non-heme iron, and vitamin C). The nutrient intakes were evaluated in two ways: g/kg of body weight and by energy intakes (g/1000 kcal). The dietary intakes of iron were quantified separately for lunch and dinner.
Iron bioavailability and absorption
The analysis of iron absorption was calculated for lunch and dinner by Monsen’s and FAO/WHO’s methods. Monsen’s method was used to estimate iron bioavailability according to enhancing factors calculated as the sum of meat, poultry, and fish (MPF) in grams and vitamin C in milligrams, consumed in the same meal. Monsen’s method [14, 15] based on algorithms suggests that heme iron bioavailability is 23 % while non-heme is 3 % in low, 5 % in intermediate, and 8 % in a high bioavailability diet. Also, the FAO/WHO [16] method is based on daily total dietary intakes adjusted for heme and non-heme iron and ascorbic acid intake. In this method, the bioavailability of heme iron is 25 %, and non-heme iron is 5 % in a low, 10 % in an intermediate, and 15 % in a high bioavailability diet.
Recommended Dietary Allowances for iron
The Recommended Dietary Allowance (RDA) for iron according to age is 11 mg/day for infants from 7 to 12 months and 7 mg/day for toddlers from 1 to 3 years of age [17]. The daily recommended nutrient intakes for iron based on dietary iron bioavailability for high (15 %), intermediate (10 %), and low (5 %) bioavailability diets are, respectively, 6.2, 9.3, and 18.6 mg for 6–12 months and 3.9, 5.8, and 11.6 mg for 13–36 months [18].
Statistical analysis
Data management and analysis was performed using GraphPad Prism Version 5.0, 2005 (GraphPad Software Inc. San Diego, CA, USA). Descriptive statistics were used for the participants’ characteristics and nutrient intake, presented as mean, standard error, and confidence interval of the mean (95 %).Comparisons between the two groups were made using unrelated t tests for continuous variables and chi-square test for categorical variables, considering p < 0.05 significant.
Results
Characteristics of the participants
This study evaluated 107 children between 6 and 36 months of age, of which 96 were included for analysis and were divided into two groups. Eleven children were excluded due to refusal to participate and/or incomplete 24-h recall data. Sociodemographic, anthropometric, weaning, and nutritional variables of the subjects and their parents are presented in Table 1. By design, there was a highly significant difference in the mean age of children between groups, and there were no significant differences in other variables. z scores <−2 of weight-for-age, length/stature for-age, and BMI-for-age were observed in 3.9, 5.4, and 3.9 %, respectively, with no difference between groups.
Dietary daily intake
Table 2 presents the results obtained from the daily dietary intakes in g/kg and g/1000 kcal of selected nutrients. There were no differences in nutrient intakes between the groups except for MPF in g/kg and g/1000 kcal, and group I was significantly lower than group II (p < 0.05). There was no difference in proportion of heme iron (16 %) and non-heme iron (84 %) intake in group I and heme iron (18 %) and non-heme iron (82 %) in group II. The proportion of children with RDA for iron intake was lower in group I (16 %) than that in group II (47 %) and statistically significant (p = 0.0013). There was no difference in proportion of children with medicinal iron intake: 38 % for group I and 39 % for group II.
Iron bioavailability and absorption
Table 3 presents total iron intake, vitamin C, MPF, iron bioavailability, and absorption from lunch and dinner by age groups. In summary, group I had lower MPF intake and iron absorption from lunch and dinner according to FAO/WHO’s method (p < 0.05) and lower MPF intake from Monsen’s in lunch but not in dinner. The proportion of children with low bioavailability was higher in group I both for lunch and dinner than that in group II (p < 0.05).
Discussion
The present study was designed to determine the intake, bioavailability, and absorption of iron from foods of 6–12-month- and 13–36-month-old children. The main results of macronutrients and iron intake supported the study hypothesis and demonstrated the low intake of MPF of the 6–12-month-old group. In this group, the bioavailability of iron is of special concern, because requirements for absorbed iron are high in relation to their energy intake and body size [18]. A limitation of this study is that the inhibitory factors of iron absorption as phytic acid and calcium were not evaluated. Strength of this study included the degree of homogeneity between groups in sociodemographic, clinical, and nutritional data.
In Brazil, the traditional homemade complementary foods are based on fruits and fruit juices during the first step and cereals, legumes, and meat in the second step [8]. As observed, vitamin C intake is adequate as an enhancing factor in both groups, and MPF intake is low in the second half of the first year. Body iron tends to change mainly during the first 6 to 12 postnatal months when iron stores have been depleted. Additional dietary iron needs to be supplied for the rapidly expanding blood volume and replacement of iron losses. Therefore, iron requirements from exogenous sources are particularly important after 6 months. A study on food intakes of infants showed that they were eating vegetables and fruits and very few consumed meat or meat mixtures [19].
In developing countries, the role of animal source foods was highlighted, specially meat in complementary feeding for improving infant health [20]. Meat intake is associated with increased weight gain and psychomotor development in infants [21] and prevents a decrease in hemoglobin, possibly by enhancing iron absorption from less bioavailable food sources [22]. Also, introduction of meat as a complementary food for exclusively breastfed infants is feasible and associated with potential benefits [23]. Fortunately, foods rich in vitamin C and heme iron food sources such as chicken meat are annually available and not expensive for the population evaluated, being both powerful enhancers of iron absorption when consumed within a meal. Another good alternative would be the regular distribution of infant formula or infant cereal enriched with iron [24].
Conclusions
The findings of this study have serious implications for future practice, like the inclusion of chicken meat and vitamin C at the same meal, both in lunch and dinner, as first complementary foods, inclusive for breastfed infants.
Abbreviations
BMI, body mass index; FAO, Food and Agriculture Organization; MPF, meat, poultry, and fish; RDA, Recommended Dietary Allowances; WHO, World Health Organization
References
WHO. The global burden of disease: 2004 update. Geneva: World Health Organization; 2008.
Baker RD, Greer FR, Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126:1040.
DeBenoist B, McLean E, Egli I, et al. Worldwide prevalence of anemia 1993-2005: WHO global database on anemia. Geneva: World Health Organization; 2008. Available at: http://apps.who.int/iris/bitstream/10665/43894/1/9789241596657_eng.pdf. Accessed 09 Nov 2015.
Beard JL, Stoltzfus RJ. Iron deficiency anemia: reexamining the nature and magnitude of the public health problem. J Nutr. 2001;131:561–703.
Jordão RE, Bernardi JLD, Barros Filho AA. Prevalência de anemia ferropriva no Brasil: uma revisão sistemática. Rev Paul Pediatr. 2009;27:90–8.
Davidsson L. Approaches to improve iron bioavailability from complementary foods. J Nutr. 2003;133:1560–2.
Vitolo MR, Bortolini GA. Biodisponibilidade do ferro como fator de proteção contra anemia entre crianças de 12 a 16 meses. J Pediatr. 2007;83:33–8.
Brasil/Ministério da Saúde/Organização Pan-Americana da Saúde. Guia alimentar para crianças menores de 2 anos. Serie A. Normas e manuais técnicos no 107. Brasília: Ministério da Saúde; 2002.
WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. Geneva: World Health Organization; 1995.
WHO. Anthro for personal computers. Version 2. 2007: Software for assessing growth and development of the world’s children. World Health Organization: Geneva; 2007. Available at: http://www.who.int/childgrowth/software/en. Accessed 16 July 2015.
Pinheiro ABV, Lacerda EMA, Benzecry EH, Gomes MCS, Costa VM. Tabela para avaliação de consumo alimentar em medidas caseiras. 5ath ed. Atheneu: São Paulo; 2004.
Drewett RF, Woolridge MW, Jackson DA, Imong SM, Mangklabruks A, Wongsawasdii L, et al. Relationships between nursing patterns, supplementary food intake and breast milk intake in a rural Thai population. Early Hum Dev. 1989;20:13–23.
TACO. Tabela brasileira de composição de alimentos, 4a ed. rev. e ampl. NEPA-UNICAMP: Campinas; 2011. Available at: www.unicamp.br/nepa/taco/. Accessed 1 July 2011.
Monsen ER, Balintfy JL. Calculating dietary iron bioavailability: refinement and computerization. J Am Diet Assoc. 1982;80:307–11.
Monsen ER, Hallberg L, Layrisse M, Hegsted DM, Cook JD, Mertz W, et al. Estimation of available dietary iron. Am J ClinNutr. 1978;31:134–41.
FAO, WHO. Requirements of vitamin A, iron, folate and vitamin B12. Report of a joint FAO/WHO expert consultation. FAO Food Nutr. Series no. 23. Rome: FAO; 1988.
Baker RD, Greer FR. Clinical report—diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics. 2010;126:1040–50.
FAO/WHO. Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation. WHO technical report series: 916. Geneva: World Health Organization; 2003.
Siega-Riz AM, Deming DM, Reidy KC, Fox MK, Condon E, Briefel RR. Food consumption patterns of infants and toddlers: where are we now? J Am Diet Assoc. 2010;110(12 Suppl):S38–51.
Gibson RS, Yeudall F, Droast N, Mtitimini BM, Cullinan TR. Experiences of a community based dietary intervention to enhance micronutrient adequacy of diets low in animal source foods and high in phytate: a case study in rural Malawian children. J Nutr. 2003;133:3992S–9.
Morgan J, Taylor A, Fewtrell M. Meat consumption is positively associated with psychomotor outcome in children up to 24 months of age. J Pediatr Gastroenterol Nutr. 2004;39:493–8.
Hurrell RF, Reddy MB, Juillerat M, Cook JD. Meat protein fractions enhance nonheme iron absorption in humans. J Nutr. 2006;136:2808–12.
Krebs NF, Westcott JE, Butler N, Robinson C, Bell M, Hambidge KM. Meat as first complementary food for breastfed infants: feasibility and impact on zinc intake and status. J Pediatric Gastroenterol Nutr. 2006;42:207–14.
Davidsson L, Kastenmayer P, Szajewska H, Hurrell RF, Barclay D. Iron bioavailability in infants from an infant cereal fortified with ferric pyrophosphate or ferrous fumarate. Am J Clin Nutr. 2000;71:1597–602.
Funding
There was no financial support.
Authors’ contributions
All the authors had contributed to conceptualizing, designing, analyzing, and interpreting the data and to writing the manuscript. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interest.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Botucatu Medical School—UNESP (Protocol number 3290/2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Faleiros, F.T.V., da Silva, V.N., de Assis Carvalho, M. et al. Intake, bioavailability, and absorption of iron in infants aged 6 to 36 months: an observational study in a Brazilian Well Child Clinic. Nutrire 41, 10 (2016). https://doi.org/10.1186/s41110-016-0011-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-016-0011-0