Abstract
Backgrounds
Therefore, reports on the risk of HTLV-1-related diseases in organ transplantation have increased in recent years, and the management of HTLV in renal transplantation remains a challenge.
Patients and methods
We retrospectively analyzed four HTLV-1-positive recipients or donors among 89 renal transplantation cases from 2006 to 2021.
Results
Among the four HTLV-1-positive recipients, two patients developed adult T cell leukemia/lymphoma (ATL) derived from recipients at approximately 3 years (1016 days and 1195 days) after renal transplantation. Case 1 developed lymphoma-type ATL (an extranodal primary cutaneous variant), including skin and pulmonary lesions. The patient achieved CR with FK tapering and CHOP therapy following cord blood stem cell transplantation. However, the patient died 101 days after ATL development because of a severe fungal infection. Case 2 developed acute-type ATL with an unusual phenotype of CD4+8+30+. The patient was treated with FK tapering and palliative therapy because of poor PS. Notably, in case 1, histopathological findings showed high numbers of PD-1-positive TIL cells in ATL, suggesting exhausted T cells and a correlation with the early onset of ATL. Furthermore, in Case 2, histopathological findings revealed CD 30 expression in ATL cells, suggesting the importance of CD 30 in ATL development. Importantly, case 2 showed typical driver mutations, including CCR4 truncation mutations of the C-terminal, TBL1XR1 mutation, and TP53 mutation in the splice site. Notably, our present study and our previous study on renal transplantation strongly indicated that two out of two and one out of 59 “recipient” positive cases developed ATL, respectively. Furthermore, our previous nationwide study 4 out of 10 “donor” positive cases developed HAM. These findings showed that ATL may be derived from HTLV-I+ recipient cells and HAM may be derived from HTLV-1+ donor cells, although the precise mechanism remains unknown.
Conclusions
Thus, early onset and rapid progression of ATL with poor outcomes should be considered in HTLV-1 endemic areas. Furthermore, immunological or genetic mechanisms may be related to the development of ATL after renal transplantation. We believe that the mechanism of onset of ATL after transplantation may be important when considering the immune environment of ATL itself.
Similar content being viewed by others
Introduction
Since end-stage kidney disease (ESKD) has increased in recent years, renal transplantation and hemodialysis are increasingly performed [1]. Although the overall survival (OS) of renal transplantation is reported to be approximately 80–90%, the main problems are rejection of transplanted kidneys, severe infection, and post-transplant lymphoproliferative disorder (PTLD) [1, 2].
Abbas reviewed post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches [2]. Abbas reviewed two important points: the use of immunosuppressive agents, including tacrolimus (FK), and the role of Epstein–Barr virus (EBV) as the most crucial factor in PTLD [2]. However, in addition to these two factors [2], we have previously demonstrated the impact of human T cell leukemia virus type 1 (HTLV-1) on the development of adult T cell leukemia/lymphoma (ATL) after liver and renal transplantation [3,4,5].
In 2006, we described the early onset and rapid progression of three cases of ATL (6, 9, and 25 months after liver transplantation) in eight HTLV-I carriers among 164 living-donor liver transplant recipients undergoing immunosuppressive treatment with tacrolimus. These clinical findings strongly suggest that immune escape mechanisms play an important role in ATL pathophysiology. Yoshida et al. [6] reviewed immune escape mechanisms in ATL, especially PD-L1 (CD274) alterations, deletions, and mutations in genes related to major histocompatibility complex (MHC) class I, including HLA-A, HLA-B, and β2M.
In HTLV-1-negative renal transplant recipients from HTLV-1-positive donors, Yamauchi and Yamano et al. [7] reported that 4 of 10 patients (high incidence) developed HTLV-1-associated myelopathy (HAM) early after renal transplantation. Thus, previous reports on the risk of HTLV-1 infection in organ transplantation have increased, suggesting the importance of HTLV-1 testing before transplantation and clinical care with attention to the development of HTLV-1-related diseases including ATL and HTLV-1-associated myelopathy (HAM) (Tables 1 [3,4,5, 8,9,10,11,12,13,14,15,16,17,18,19,20] and 2 [21,22,23,24,25,26,27,28,29,30,31,32,33]). Furthermore, Ohshima et al. [34, 35] reported the importance of the tumor microenvironment of adult T cell leukemia/lymphoma; in particular, high numbers of programmed cell death-1-positive tumor-infiltrating lymphocytes correlate with the early onset of post-transplant lymphoproliferative disorder.
In 2022, we coincidentally experienced the early onset and rapid progression of two cases of ATL in four HTLV-I carriers of renal transplant recipients undergoing immunosuppressive treatment with tacrolimus. Thus, in this study, we retrospectively describe the clinical significance of HTLV-1-positive recipients/HTLV-1-positive donors for renal transplantation, including the pathological and genetic analysis of two ATL development cases, with a review of the literature.
Patients and methods
We retrospectively analyzed the impact of HTLV-1 infection on 89 renal transplantation cases between January 2006 and December 2021. There were four HTLV-1-positive organ transplants among the 89 renal transplantation cases. Three recipients were HTLV-1 carriers, and one HTLV-1-negative recipient was transplanted from an HLTV-1-positive donor at another hospital. Previously, we reported the impact of HTLV-1 infection on 31 renal transplantation cases between January 2006 and December 2016 [5].
Regarding renal transplantation, 89 cases comprised 80 living donors and nine cadaveric donor renal transplants. The status of HTLV-1 infection in recipients and donors is described below. All donors for renal transplantation tested negative for HTLV-1, and we excluded an HTLV-1-positive donor based on the recommendation of the Japanese Society for Clinical Renal Society and the Ministry of Health, Labour and Welfare in 2014 and the HAM guideline 2019 [36]. Thus, two HTLV-1 carrier donors were excluded as renal transplantation donors during the health checkup. However, renal transplantation from an HTLV-1-positive donor to HTLV-1-negative recipient was performed at another institution. The case (Case 4) was followed up at our institution because the residence of case 4 was in Miyazaki prefecture.
The immunosuppressive protocol to prevent graft rejection in patients after renal transplantation was as follows: induction therapy with basiliximab (20 mg daily at transplant and on postoperative day 4), followed by mycophenolate mofetil (MMF; 1000 mg twice daily, tapered to 500 mg twice daily at approximately 3 months post-transplant), tacrolimus (FK), and corticosteroids (250-mg bolus injection intraoperatively, 24 mg daily in the first week, tapered to 4 mg daily by 1-month post-transplant). Rituximab was administered one week before transplantation to patients who received repeat transplants and ABO-incompatible transplants. After renal transplantation, FK immunosuppressants were used to prevent rejection by kidney transplantation. MMF was tapered at approximately 3 months post-transplant and ceased at approximately 1 year post-transplant.
This retrospective study was conducted in compliance with the guidelines for good clinical practice and ethical principles of the Declaration of Helsinki. This study was approved by the appropriate ethics committee and institutional review board of Miyazaki Prefectural Miyazaki Hospital.
Histopathological analysis and immunohistochemical staining [35]
Evaluation of immunohistochemical staining was performed manually by experienced hematopathologists at Kurume University (H. Miyoshi. and K. Ohshima).
Antibodies used in immunohistochemistry (IHC) included anti-CD20 (L-26; Dako Cytomation, Glostrup, Denmark), anti-CD10 (56C6; Leica Microsystems, Wetzlar, Germany), anti-CD3 (F7.2.38; Dako Japan Inc., Kyoto, Japan), anti-CD30 (Ber-H2; Dako Japan Inc.), anti-FoxP3 (SP97; Abcam, Cambridge, UK), anti-PD-1 (NAT105; Abcam), and anti-PD-L1 (E1L3N; Cell Signaling Technology, Danvers, MA, USA). An Olympus BX51 microscope (Olympus, Tokyo, Japan) was used to quantify positive cells. Neoplastic cells were scored positive when ≥ 30% staining was observed. For the quantitative analysis of PD-L1 expression, cells with distinct membranous staining were scored as positive. Neoplastic and microenvironment PD-L1 + cells were evaluated separately. To assess immunohistochemically positive TILs, ≤ 10 representative high-power fields (HPFs) were randomly selected per specimen. The total number of immunohistochemically positive TILs was determined. The average number of immunohistochemically positive TILs per HPF was calculated for all patients using immunohistochemical staining. The cut-off values for the number of PD-1, CD3, CD8, CD4, FoxP3, and microenvironmental PD-L1-positive TILs were 100, 500, 150, 30, 25, and 10, respectively.
Targeted-capture sequencing [37,38,39]
Targeted-capture sequencing was performed using the custom SureSelect library (Agilent Technologies) for 235 lymphoma-associated genes in National Cancer Center Research Institute (Y. Kogure). To detect structural variations (SVs) affecting PD-L1, the sequences of coding exons, introns, and 5ʹ- and 3ʹ-UTRs were also included in the library. Sequencing data were generated using the Illumina HiSeq platform with a standard 150-bp paired-end read protocol. Sequence alignment and mutation calling were performed using Genomon pipeline (https://github.com/Genomonproject). Candidate mutations with (i) EBCall [37] p < 0.0001; (ii) > 5 variant reads in tumor samples; and (iii) a variant allele frequency > 0.05 in tumor samples were adopted and further filtered by excluding (a) synonymous SNVs, (b) variants only present in unidirectional reads, and (c) variants occurring in repetitive genomic regions. These candidate mutations were further filtered, unless they were listed ≥ 10 times in the COSMIC database version 70, by removing known germline variants (i) observed in NCBI dbSNP build 131 or (ii) observed at a frequency ≥ 0.0001 in any of the following datasets: the 1000 Genomes Project (October 2014 release); National Heart, Lung, and Blood Institute Exome Sequencing Project 6500; the Human Genome Variation Database (version 2.00); and the Exome Aggregation Consortium (ExAC) r0.3.1. Finally, all detected mutations were manually checked using Integrative Genomics Viewer (IGV) version 2.8.2.
Results
The summary of cases 1–4 (Table 3 )
Two of the four HTLV-1 carrier patients developed aggressive-type ATL after renal transplantation. Patient characteristics at the time of ATL diagnosis and treatment outcomes are summarized in Table 3. Our report provides additional information. Given the scarcity of cases, emphasis should be placed on precise patient cohort descriptions. This may add to the knowledge base. Thus, we would like to describe the patient cohort in more detail and how diagnosis and treatment were performed.
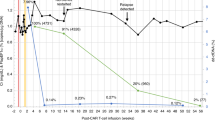
Case 1 was a 60-year-old man with ESKD of unknown etiology. Two years and nine months after renal transplantation, the patient developed a lymphoma-type ATL (an extranodal primary cutaneous variant) according to the consensus report by Tsukasaki et al. [40] (Fig. 1). Once aggressive-type ATL develops, conventional chemotherapy is not curative. To suppress the progression of ATL, immunosuppressive drugs were gradually withdrawn and chemotherapy was subsequently administered. Regarding FK trough concentration in PB just prior to develop ATL, the blood levels of FK were 5.5 ng/mL (FK 3.0 mg per day). The withdrawal of FK was performed from 3.0 mg per day into 2.0 mg per day. However, these treatments have unfortunately led to PD. Subsequently, the CHOP therapy was initiated. Fortunately, these treatments led to CR. To date, there has been only one report by Mori et al. [41] on allo-HSCT for the development of Ph1 + ALL after liver transplantation. Based on Mori's report [41], we performed cord blood stem cell transplantation (CBSCT) using the conditioning regimen of FLU/MEL/TBI and GVHD prophylaxis of FK and MMF. However, the patient died on day 101 after ATL development because of severe fungal infection.
The Clinical course of case 1. We present the clinical course of an HTLV-1-positive recipient (Case 1) who underwent renal transplantation at our institution (Table 3, Fig. 1). A 65-year-old male was diagnosed with end-stage kidney disease (ESKD; unknown etiology) in 2012 and subsequently underwent renal transplantation in July 2017. The patient was an HTLV-1 carrier prior to renal transplantation. The patient underwent a living renal transplant from his wife, who had HLA mismatch and blood type incompatibility. After the renal transplantation, immunosuppressive therapy with FK was administered. In April 2020, the skin rash had worsened throughout the body. Skin biopsy revealed ATL, suggesting monoclonal proliferation of ATL cells. Furthermore, chest CT showed glass shadows scattered in both lungs, suggesting the infiltration of ATL cells. Taking these findings together, we made a diagnosis of lymphoma-type ATL, an extranodal primary cutaneous variant, according to a report by Tsukasaki et al. [40]. Thus, case 1 developed ATL 2 years and 9 months after renal transplantation. Immunosuppressants for FK were used to prevent rejection by kidney transplantation. Regarding FK trough concentration in PB just prior to develop ATL, the blood levels of FK in the renal transplant recipient (case 1) were 5.5 ng/mL (FK 3.0 mg per day). FK withdrawal was performed from 3.0 mg per day to 2.0 mg per day. Thus, in April 2020, FK tapering and subsequent CHOP therapy were initiated. After three courses of CHOP therapy, the skin rush/lung lesions showed PR. To date, there has been only one report by Mori et al. [41] on allo-HSCT for the development of Ph1 + ALL after liver transplantation. According to Mori's report [41], we performed cord blood stem cell transplantation (CBSCT) using a conditioning regimen including FLU/MEL/TBI and graft-versus-host disease (GVHD) prophylaxis with FK plus MMF. On day 11, chest computed tomography (CT) revealed lung infiltration with a reversed halo sign in the right lung lobe and hematoma in the anterior chest and mediastinum. Rhizopus microspores and Mucor Zygomycetes from anterior chest hematomas were identified. On day 14, the patient developed septic shock and acute respiratory and renal failure. Therefore, the patient was treated with intubation and CHDF in the ICU. However, on day 16, the patient died of multiple organ failure (MOF) progression
Case 2 was a 75 year-old woman with ESKD due to nephrosclerosis. Three years and three months after a renal transplantation, the case developed acute-type ATL with abnormal multi-lobulated nuclei “flower-like” lymphocyte cells in PB, which expressed an unusual phenotype of CD4 + 8 + 30 + . Regarding FK trough concentration in PB just prior to develop ATL, the blood levels of FK were 5.7 ng/mL (FK 2.5 mg per day). The withdrawal of FK was performed from 2.5 mg per day into 1.5 mg per day. The patient was treated with FK tapering and palliative therapy (PSL) because of poor PS (Fig. 2). These treatments led the case result in PD. The patient died 5 days after the diagnosis of ATL.
The clinical course of case 2. We present the clinical course of one HTLV-1-positive recipient (case 2) who underwent renal transplantation from an HTLV-1-negative donor at our hospital (Table 3, Fig. 2A and B). In September 2018, a 75-year-old female case with nephrosclerosis underwent a living kidney transplant from her husband, who had an HLA mismatch and blood type incompatibility. Immunosuppressive therapy to prevent kidney transplantation was administered using FK. Regarding FK trough concentration in PB just prior to develop ATL, the blood levels of FK in the renal transplant recipient (case 2) were 5.7 ng/mL (FK 2.5 mg per day). FK withdrawal was performed from 2.5 mg per day to 1.5 mg per day. In December 2021, the patient showed high levels of LDH and sIL-2R, and systemic lymphadenopathy in the left cervical, mediastinal, and abdominal LNs on CT. Bone marrow examination revealed 25% large abnormal cells. Abnormal lymphocytes in the bone marrow showed an unusual phenotype of CyCD3 + CD3-4 + 8 + 30 + 25- on FCM. Furthermore, Southern blot analysis showed the monoclonal proliferation of ATL cells (Fig. 2A). Thus, the patient was diagnosed with an acute-type ATL. Consequently, the patient developed ATL 3 years and 3 months after the renal transplantation. The patient showed poor PS, ADL, and organ impairment (Fig. 2B). Regarding the treatment for ATL after renal transplantation, the patient was treated with tapering of FK and PSL therapy. However, the patient presented with MOF. Unfortunately, the patient died at day 5 after ATL diagnosis. A BM findings, FCM findings, Southern blot analysis and radiological findings (Case 2). B Clinical course of ATL development after renal transplantation (HTLV-1 carrier → Acute-type ATL) (Case 2)
Histopathological analysis by immunohistochemical staining (Table 4 , Fig. 3 )
Histopathological analysis by immunohistochemical staining. To elucidate the pathogenesis of ATL after renal transplantation, we performed histopathological analysis using immunohistochemical staining [35]. In Case 1, a high numbers of PD-1-positive TIL cells (average 20.8/HPF) were noted. Microenvironmental PD-L1 expression was also positive (50/HPF). However, Fox P3, HLA class I, HLA class II, and neoplastic PD-L1 expression was not detected. In Case 2, low numbers of PD-1-positive TIL cells (average 0.8/HPF) were observed. Fox P3, HLA class I, HLA class II, neoplastic PD-L1, and microenvironmental PD-L1 were not expressed
To elucidate the pathogenesis of ATL after renal transplantation, we performed histopathological analysis by using immunohistochemical staining [35].
In case 1, a high numbers of PD-1-positive TIL cells (average 20.8/HPF) were noted. The microenvironmental PD-L1 expression was also positive (50/HPF). However, Fox P3, HLA class I, HLA class II, and neoplastic PD-L1 were not detected. In case 2, low numbers of PD-1-positive TIL cells (average 0.8/HPF) were observed. Fox P3, HLA class I, HLA class II, neoplastic PD-L1, and microenvironmental PD-L1 were not expressed. In addition to the use of immunosuppressive treatment, in case 1, high numbers of PD-1-positive TIL may be related to ATL development, consistent with Ohshima's report [34, 35]. In Case 2, in addition to the use of FK, CD30 expression in ATL cells may be related to ATL development (Fig. 2A).
The genetic analysis
The results of the genetic analysis of cases 1 and 2 are shown in Table 5.
In case 1, we could not detect somatic alterations because of the low tumor cell fraction in the specimen. In Case 2, we found a TP53 mutation at the splice site, a CCR4 mutation truncating its C-terminus, and a TBL1XR1 mutation, which are typical ATL driver mutations [38, 39]. Thus, in case 2, consistent with previous reports [38, 39], typical driver mutations, including CCR4 truncation mutations of the C-terminal, TBL1XR1 mutation, and TP53 mutation in the splice site, may be related to the development of ATL after renal transplantation.
The comparison between previous our conducted study and present study
Regarding the incidence of ATL development after renal transplantation, Yamano’s study of 99 HTLV-1-positive renal transplantation cohorts in Japan (2000–2014 data from the Japanese Renal Transplant Registry) (Table 7) showed that the incidence of ATL development after renal transplantation was approximately 1% (1 out of 99 HTLV-1-positive recipients). Regarding the incidence of ATL development after liver transplantation, Yoshizumi’s study of 82 HTLV-1-positive liver transplantation cohorts in Japan (a multi-center study including 13 high-volume centers in Japan) (Table 9) showed that the incidence of ATL development after liver transplantation was approximately 6% (5 out of 89 HTLV-1-positive recipients). Thus, previous nationwide studies have shown that the incidence of ATL after renal/liver transplantation is rare (1–5%). Unfortunately, all six recipient-positive-derived ATL cases showed poor outcomes.
Importantly, our present study and our previous nationwide study on renal transplantation strongly indicated that two out of two ``recipient” positive cases and one out of 59 ``recipient” positive cases developed ATL (Tables 6 and 7). Furthermore, in the present study and previous nationwide study on renal transplantation, none of the “donor” positive cases and 4 out of 10 “donor” positive cases developed HAM, respectively (Tables 6 and 7). Furthermore, our present study and previous nationwide study in renal transplantation none of one “donor” positive cases and 4 out of 10 “donor” positive cases developed HAM, respectively (Tables 6 and 7). These findings showed that ATL may be derived from HTLV-I + recipient cells and HAM may be derived from HTLV-1 + donor cells, although the precise mechanism remains unknown.
Discussion
Herein, in our study, we showed the early onset and rapid progression of two cases of ATL (33 months and 39 months after renal transplantation) in four HTLV-I carriers within 89 renal transplant recipients undergoing immunosuppressive treatment with tacrolimus. Furthermore, consistent with previous reports of poor outcomes of ATL after organ transplantation, our two cases also showed poor outcomes due to ATL progression and severe infection. Notably, we first showed that immunological mechanisms or genetic mechanisms may also be related to the development of ATL after renal transplantation under immunosuppressive treatment. Furthermore, our present study and our previous nationwide study on renal transplantation may indicate that ATL is derived from HTLV-I + recipient cells and HAM is derived from HTLV-1 + donor cells, although the precise mechanism remains unknown. Thus, the origin of HTLV-1 related disease (ATL or HAM) after renal transplantation may be different.
Abbas F et al. [2] demonstrated the importance of immunosuppressive agents including FK and the role of EBV in the development of PTLD after organ transplantation. In addition to these factors, our present study and our previous nationwide study showed the significance of HTLV-1 infection in the development of PTLD after organ transplantation. However, it is unclear whether the immune and genetic mechanisms are related to ATL development after renal transplantation. Recently, PD-1 + TILs were shown to play a major role in the development of PTLD in patients [34, 35]. Furthermore, CD30 expression [42,43,44,45,46,47] or/and genetic mutations [38, 39, 48] may also play an important role in the development of tumorigenesis in PTLD.
Thus, we focused on the important pathogenesis of (i) the surface and genetic features of ATL cells and (ii) the immune characteristics of CTL cells in ATL development after renal transplantation.
The importance of CD30 for ATL [42,43,44,45,46] and PTLD [47] on the surface increasingly reported and reviewed. Ohshima et al. [42, 43] also reported that CD30 in healthy tissues plays a role in immune system surveillance and in mediating the cross-talk between B and T cells. In addition, Ohshima et al. [42,43,44,45,46] and other researchers reported that the expression CD30 in HTLV-1-positive cells plays an important role in tumorigenesis. Notably, Nakashima et al. [46] reported that increased CD30 expression may be strongly related to disease progression. Previous studies have also reported that 66% of all PTLD cases are CD30-positive [47]. Thus, these findings also highlight the importance of CD 30 in PTLD development. Consistent with previous reports [42,43,44,45,46,47], case 2 showed that CD 30 expression in ATL cells may be an important characteristic of ATL development after renal transplantation. Although the precise mechanism is unclear, CD30 expression in ATL cells and PTLD may be important for elucidating the pathogenesis of ATL and PTLD. Based on the pathogenesis of CD30 expression in ATL cells, molecular-targeted therapy may be a therapeutic option, including the anti-CD30 antibody–drug conjugate brentuximab vedotin [46, 49].
Regarding the genetic features of ATL cells in ATL development, in our study of case 2, genetic analysis revealed CCR4, TBL1XR1, and TP 53 mutations (splice site). These findings are consistent with previous reports on the genetic landscape of PTLD (T-and NK-cell PTLD) by Margolskee et al. [48] and ATL by Kogure et al. [38, 39]. In 19 PTLD patients (17 T-PTLD and 2 NK-PTLD cases), Margolskee et al. [48] reported that 377 variants with an average of 20 variants per case, mutations of epigenetic modifier genes (TET2, KMT2C, KMT2D, DNMT3A, ARID1B, ARID2, and KDM6B), and/or inactivation of TP53 (mutation and/or deletion) were the most frequent alterations. Kogure et al. [38, 39] reported that ATL is characterized by a mutation in the T cell receptor/NF-κB signaling pathway (PLCG1, PRKCB, CARD11, VAV1, CTLA4-CD28, and ICOS-CD28 fusions), molecules associated with immune surveillance (HLA-A/B, CD58, and FAS), and chemokine receptors (CCR4, CCR7, and GPR183). Among the CCR4, TBL1XR1, and TP 53 mutations (splice sites) in case 2, we focused on the TBL1XR1 mutation in ATLL. Although the TBL1XR1 mutation has been reported to be a driver of ATLL [38, 39], the function of the TBL1XR1 mutation in ATL development remains unclear. However, in DLBCL, the TBL1XR1 mutation plays a crucial role in giving rise to extranodal ABC-DLBCLs derived from memory B cells because the TBL1XR1 mutation skews the humoral immune response to produce memory B cells [50]. Furthermore, TBL1XR1 has been reported as an important partner in the translocation of TP63 [51]. Thus, the role of TBL1XR1 mutation may differ between DLBCL and PTCL. In ATLL, considering the normal function and pathogenesis of DLBCL and PTCL, the TBL1XR1 mutation may be speculated to be closely related to an unknown or undiscovered transcription factor in the pathogenesis of ATL development.
Regarding the immune characteristics of CTL cells for ATL, firstly, Ohshima et al. [34, 35] reported the importance of the tumor microenvironment of ATL; in particular, high numbers of programmed cell death-1-positive tumor-infiltrating lymphocytes correlate with early onset of PTLD. Notably, Ohshima et al. [35] reported the clinicopathological significance of the expression of PD-1, which is associated with exhausted T cells. The number of PD-1 + TILs in PTLD specimens was significantly higher in patients who developed PTLD soon after transplantation [35]. In our study, in case 1, a high numbers of PD-1-positive TIL cells (average 20.8/HPF) may be related to ATL development after renal transplantation. Thus, T cell exhaustion may be associated with PTLD development. However, case 2 was not related to the high number of PD-1 + TILs in PTLD specimens. Thus, the underlying immune mechanisms may differ in each case. Based on the pathogenesis of the PD-1 + TIL mechanism in ATL cells, molecular-targeted therapy may be a therapeutic option including PD -1 Inhibitor therapy [52], in the future. Thus, immunological mechanisms or genetic mechanisms may also be related to ATL development after renal transplantation under immunosuppressive treatment. These mechanisms may assist in the early onset and rapid progression of ATL after renal transplantation.
Importantly, we reviewed the previous literatures regarding HTLV-1-related disease including ATL and HAM, after organ transplantation (Tables 1, 2, 6, 7, 8, and 9).
Previous reports on ATL after organ transplantation including renal transplantation are shown in Table 1 [3,4,5, 8,9,10,11,12,13,14,15,16,17,18,19,20]. Notably, the majority of ATL development cases after renal transplantation were derived from “recipient” positive cases (Tables 6 and 7). Furthermore, the majority of HAM development cases after renal transplantation were derived from “donor” positive cases (Tables 6 and 7). These findings may suggest that ATL is derived from HTLV-I + recipient cells and HAM is derived from HTLV-1 + donor cells, although the precise mechanism remains unknown. Thus, the origin of HTLV-1-related disease (ATL or HAM) after renal transplantation may be different. Similarly, in liver transplantation, our previous nationwide study strongly indicated that 3 out of 70 “recipient” positive cases developed ATL (HTLV-1-positive recipient-derived ATL) (Table 9). Furthermore, two out of six donor-positive and recipient-positive cases developed ATL derived from HTLV-1-positive recipients. However, none out of 6 “donor” positive cases developed HAM (Table 9). To date, 16 cases of ATL development after organ transplantation have been reported within 10 years of organ transplantation under immunosuppressive circumstances. Similarly, in an immune-deficient mouse model using a primary ATL specimen, we demonstrated and validated ATL development under immune-deficient conditions [53]. Yoshizumi et al. identified and discussed that fulminant hepatic failure during a cytokine storm in a recipient before transplantation may affect the development of ATL. I believe that the onset of ATL after transplantation is important when considering the immune environment of ATL. If we can investigate the immune environment after onset, including the differences between post-transplant ATL, post-transplant HAM, and normal ATL and HAM, we will be able to understand the pathogenesis of ATL and HAM.
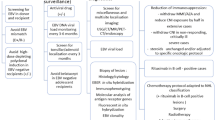
At present, although there is no established prevention and treatment for ATL or HAM after organ transplantation, based on the clinical guidelines for PTLD after organ transplantation and the JSH Practical Guideline for ATL, we diagnosed and treated ATL after HTLV-1-positive organ transplantation. We also showed the summarization and graphical representation of the strategy from diagnosis to treatment of ATL in renal transplant recipients according to the clinical guideline for PTLD after organ transplantation and the JSH Practical Guidelines for ATL (Fig. 4) [54,55,56]. Thus, our experience may be a crucial example to assist in ATL development after renal transplantation in clinical practice.
The summarization and graphical representation of the strategy from diagnosis to treatment of ATL in renal transplant recipients according to the clinical guideline for PTLD after organ transplantation and the JSH Practical Guidelines for ATL. At present, although there is no established prevention and treatment for ATL or HAM after organ transplantation, based on the clinical guideline of PTLD after organ transplantation and the JSH Practical Guidelines for ATL, we performed the diagnosis and treatment of ATL after HTLV-1-positive organ transplantation. Thus, our experiences may be one of the crucial examples to assist ATL development in patients after renal transplantation in the future. The diagnosis of ATL is closely related to blood tests (HTLV-1 clonality by Southern blot analysis), tissue biopsy (immunohistochemical analysis), and radiological imaging (ATL staging). Regarding the treatment, the strategy of treatment is (i) to eliminate the ATL and (ii) to minimize the harm to transplanted organs. According to the clinical guidelines for PTLD after organ transplantation and the JSH Practical Guidelines for ATL, the withdrawal of immunosuppressive agents, including FK or CSP, is an essential treatment approach. Therefore, chemotherapy (a CHOP-like regimen) may be a subsequent therapeutic option. According to the response and organ dysfunction in recipient, the molecular-targeted therapy (CCR4 antibody, CD30 antibody, lenalidomide) or allo-HSCT may be treatment strategy for ATL after organ transplantation. Accumulation of ATL cases after organ transplantation is essential to establish a diagnosis and strategy for ATL after organ transplantation in the future. Regarding the prevention of HTLV-1 infection in endemic areas of HTLV-1 infection, such as our institution in Miyazaki Prefecture, we considered that suggested ways to prevent the development of ATL in renal transplant recipients may be the screening of HTLV-1 antibody. We did not perform organ transplantation from HTLV-1 carrier donors to HTLV-1- negative recipients in order to prevent HTLV-1 infection. In 2014, the Japanese Society for Clinical Renal Society and the Ministry of Health, Labour, and Welfare recommended the exclusion of HTLV-1-positive donors for renal transplantation. From the negative aspects of the view, case 3 (renal transplantation from HTLV-1-positive donor to HTLV-1-negative donor in another hospital and subsequent follow-up in our hospital case) showed the need for enlightenment regarding adherence to the guidelines in Japan. Thus, in endemic areas of HTLV-1 infection, to prevent new HTLV-1 infection, organ transplantation from HTLV-1 carrier donors to HTLV-1-negative recipients should be avoided in clinical practice
In conclusion, in contrast to relatively long periods in the natural course or history of ATL (= 50–60 years), the early onset and rapid progression of ATL development with poor outcomes should be kept in mind during follow-up periods in areas endemic for HTLV-1. The HTLV-1 Positive Organ Transplant Registry by Dr. Yamauchi and Dr. Yamano was conducted in a nationwide study. Further follow-up should be essential, and the accumulation of cases should be also desired to elucidate the pathogenesis of ATL after organ transplantation in the future.
Availability of data and materials
Please contact the authors for data requests.
References
Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ. Long-term consequences of kidney donation. N Engl J Med. 2009;360(5):459–69. https://doi.org/10.1056/NEJMoa0804883.
Abbas F, El Kossi M, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29–46.
Kawano N, Shimoda K, Ishikawa F, Taketomi A, Yoshizumi T, Shimoda S, Yoshida S, Uozumi K, Suzuki S, Maehara Y, Harada M. Adult T-cell leukemia development from a human T-cell leukemia virus type I carrier after a living-donor liver transplantation. Transplantation. 2006;82(6):840–3.
Yoshizumi T, Takada Y, Shirabe K, Kaido T, Hidaka M, Honda M, Ito T, Shinoda M, Ohdan H, Kawagishi N, Sugawara Y, Ogura Y, Kasahara M, Kubo S, Taketomi A, Yamashita N, Uemoto S, Yamaue H, Miyazaki M, Takada T, Maehara Y. Impact of human T-cell leukemia virus type 1 on living donor liver transplantation: a multi-center study in Japan. J Hepatobiliary Pancreat Sci. 2016;23(6):333–41.
Kawano N, Yoshida S, Kawano S, Kuriyama T, Tahara Y, Toyofuku A, Manabe T, Doi A, Terasaka S, Yamashita K, Ueda Y, Ochiai H, Marutsuka K, Yamano Y, Shimoda K, Kikuchi I. The clinical impact of human T-lymphotrophic virus type 1 (HTLV-1) infection on the development of adult T-cell leukemia-lymphoma (ATL) or HTLV-1-associated myelopathy (HAM)/atypical HAM after allogeneic hematopoietic stem cell transplantation (allo-HSCT) and renal transplantation. J Clin Exp Hematop. 2018;58(3):107–21.
Yoshida N, Miyoshi H, Ohshima K. Clinical applications of genomic alterations in ATLL: predictive markers and therapeutic targets. Cancers. 2021;13(8):1801. https://doi.org/10.3390/cancers13081801.
Yamauchi J, Yamano Y, Yuzawa K. Risk of human T-Cell Leukemia virus type 1 infection in kidney transplantation. N Engl J Med. 2019;380(3):296–8.
Zanke BW, Rush DN, Jeffery JR, et al. HTLV-1 T cell lymphoma in a cyclosporine-treated renal transplant patient. Transplantation. 1989;48:695–7.
Tsurumi H, Tani K, Tsuruta T, et al. Adult T-cell leukemia developing during immunosuppressive treatment in a renal transplant recipient. Am J Hematol. 1992;41:292–4.
Jenks PJ, Barrett WY, Raftery MJ, et al. Development of human T-cell lymphotropic virus type I-associated adult T-cell leukemia/lymphoma during immunosuppressive treatment following renal transplantation. Clin Infect Dis. 1995;21:992–3.
Williams NP, Buchner LM, Shah DJ, et al. Adult T-cell leukemia/lymphoma in a renal transplant recipient: an opportunistic occurrence. Am J Nephrol. 1994;14:226–9.
Mori J, Kamiryo Y, Yano S, et al. Adult T-cell leukemia following ABO incompatible renal transplant patients in Japan. Renal Transplant Vasc Surg. 2000;12:137–41.
Hoshida Y, Li T, Dong Z, et al. Lymphoproliferative disorders in renal transplant patients in Japan. Int J Cancer. 2001;91:869–75.
Ichikawa Y, Iida M, Ebisui C, et al. A case study of adult t-cell lymphoma in a kidney transplant patient. Transplant Proc. 2000;32:1982–3.
Yoshizumi T, Takada Y, Shirabe K, et al. Impact of human T-cell leukemia virus type 1 on living donor liver transplantation: a multi-center study in Japan. J Hepatobiliary Pancreat Sci. 2016;23(6):333–41.
Nakamura N, Tamaru S, Ohshima K, et al. Prognosis of HTLV-I-positive renal transplant recipients. Transplant Proc. 2005;37:1779–82.
Tamaki H, Matsuoka M. Donor-derived T-cell leukemia after bone marrow transplantation. N Engl J Med. 2006;354:1758–9.
Nakamizo A, Akagi Y, Amano T, et al. Donor-derived adult T-cell leukaemia. Lancet. 2011;377:1124.
Kitamura N, Nakanishi T, Yoshida Y, et al. HTLV-I–associated posttransplant lymphoproliferative disorder following virus transmission from recipient to donor cells. Blood. 2017;130:84–6.
Glowacka I, Korn K, Potthoff SA, et al. Delayed seroconversion and rapid onset of lymphoproliferative disease after transmission of human T-cell lymphotropic virus type 1 from a multiorgan donor. Clin Infect Dis. 2013;57:1417–24.
Kawamata T, Ohno N, Sato K, et al. A case of post-transplant adult T-cell leukemia/lymphoma presenting myelopathy similar to but distinct from human T-cell leukemia virus type I (HTLV- I)-associated myelopathy. Springerplus. 2014;3:581.
Nagamine Y, Hayashi T, Kato Y, et al. Human T lymphotropic virus type-1-associated myelopathy manifesting shortly after living-donor renal transplantation. Intern Med. 2015;54:75–8.
Ramanan P, Deziel PJ, Norby SM, et al. Donor-transmitted HTLV-1-associated myelopathy in a kidney transplant recipient–case report and literature review. Am J Transplant. 2014;14:2417–21.
Nakatsuji Y, Sugai F, Watanabe S, et al. HTLV-I-associated myelopathy manifested after renal transplantation. J Neurol Sci. 2000;177:154–6.
Kuroda Y, Takashima H, Yukitake M, et al. Development of HTLV-I-associated myelopathy after blood transfusion in a patient with aplastic anemia and a recipient of a renal transplant. J Neurol Sci. 1992;109:196–9.
Inose Y, Akiyama S, Mochizuki A, et al. A case report of HTLV-1 associated myelopathy (HAM) manifested after renal transplantation. Rinsho Shinkeigaku. 2010;50:241–5.
Kuroki N, Kin M, Tateishi T. A case report of HAM development after renal transplantation. Rinsho Shinkeigaku. 2007;48:222 (in Japanese).
Toro C, Rodés B, Poveda E, et al. Rapid development of subacute myelopathy in three organ transplant recipients after transmission of human T-cell lymphotropic virus type I from a single donor. Transplantation. 2003;75:102–4.
Imirizaldu JJZ, Gomez Esteban JC, Rouco Axpe I, et al. Post-transplantation HTLV-1 myelopathy in three recipients from a single donor. J Neurol Neurosurg Psychiatry. 2003;74:1080–4.
Shintani Y, Napou Y, Fujii R, et al. One case of HAM after cadaveric renal transplantation. Ishoku. 2001;36:286.
Soyama A, Eguchi S, Takatsuki M, et al. Human T-cell leukemia virus type I–associated myelopathy following living-donor liver transplantation. Liver Transpl. 2008;14:647–50.
Ozden S, Seilhean D, Gessain A, et al. Severe demyelinating myelopathy with low human T cell lymphotropic virus type 1 expression after transfusion in an immunosuppressed patient. Clin Infect Dis. 2002;34:855–60.
Narukawa N, Shiizaki K, Kitabata Y, et al. Plasma exchange for the treatment of human T-cell lymphotropic virus type 1 associated myelopathy. Ther Apher Dial. 2001;5:491–3.
Takeuchi M, Miyoshi H, Ohshima K. Tumor microenvironment of adult T-cell leukemia/lymphoma. J Clin Exp Hematop. 2021;61(4):202–9.
Saito H, Miyoshi H, Shibayama H, Toda J, Kusakabe S, Ichii M, Fujita J, Fukushima K, Maeda T, Mizuki M, Oritani K, Seto M, Yokota T, Kanakura Y, Hosen N, Ohshima K. High numbers of programmed cell death-1-positive tumor infiltrating lymphocytes correlate with early onset of post-transplant lymphoproliferative disorder. Int J Hematol. 2021;114(1):53–64.
The Guidelines of HAM, 2019. https://www.neurology-jp.org/guidelinem/ham/ham_2019.pdf. pp 107–117, we judged the indication and contra-indication of renal transplantation in our institution.
Shiraishi Y, Sato Y, Chiba K, et al. An empirical Bayesian framework for somatic mutation detection from cancer genome sequencing data. Nucl Acids Res. 2013;41(7):e89.
Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–15. https://doi.org/10.1038/ng.3415.
Kogure Y, Kameda T, Koya J, et al. Whole-genome landscape of adult T-cell leukemia/lymphoma. Blood. 2022;139(7):967–82.
Tsukasaki K, Imaizumi Y, Tokura Y, Ohshima K, Kawai K, Utsunomiya A, Amano M, Watanabe T, Nakamura S, Iwatsuki K, Kamihira S, Yamaguchi K, Shimoyama M. Meeting report on the possible proposal of an extranodal primary cutaneous variant in the lymphoma type of adult T-cell leukemia-lymphoma. J Dermatol. 2014;41(1):26–8.
Sasaki K, Mori Y, Yoshimoto G, Sakoda T, Kato K, Inadomi K, Kamezaki K, Takenaka K, Iwasaki H, Maeda T, Miyamoto T, Akashi K. Successful treatment of Ph ALL with hematopoietic stem cell transplantation from the same HLA-haploidentical related donor of previous liver transplantation. Leuk Lymphoma. 2018;59(8):2005–7.
Takeshita M, Akamatsu M, Ohshima K, Kobari S, Kikuchi M, Suzumiya J, Uike N, Okamura T. CD30 (Ki-1) expression in adult T-cell leukaemia/lymphoma is associated with distinctive immunohistological and clinical characteristics. Histopathology. 1995;26(6):539–46.
Karube K, Kakimoto Y, Tonozuka Y, Ohshima K. The expression of CD30 and its clinico-pathologic significance in peripheral T-cell lymphomas. Expert Rev Hematol. 2021;14(8):777–87.
Cook LB, Phillips AA. How I treat adult T-cell leukemia/lymphoma. Blood. 2021;137(4):459–70.
Nakashima M, Yamochi T, Watanabe M, et al. CD30 characterizes polylobated lymphocytes and disease progression in HTLV-1-infected individuals. Clin Cancer Res. 2018;24(21):5445–57.
Baba Y, Sakai H, Kabasawa N, Harada H. Successful treatment of an aggressive adult t-cell leukemia/lymphoma with strong CD30 expression using brentuximab vedotin as combination and maintenance therapy. Intern Med. 2022. https://doi.org/10.2169/internalmedicine.9812-22.
Vase MØ, Maksten EF, Bendix K, Hamilton-Dutoit S, Andersen C, Møller MB, Sørensen SS, Jespersen B, Kampmann J, Søndergård E, Nielsen PS, D’amore F. Occurrence and prognostic relevance of CD30 expression in post-transplant lymphoproliferative disorders. Leuk Lymphoma. 2015;56(6):1677–85.
Margolskee E, Jobanputra V, Jain P, Chen J, Ganapathi K, Nahum O, Levy B, Morscio J, Murty V, Tousseyn T, Alobeid B, Mansukhani M, Bhagat G. Genetic landscape of T- and NK-cell post-transplant lymphoproliferative disorders. Oncotarget. 2016;7(25):37636–48.
Tsukasaki K, Marçais A, Nasr R, Kato K, Fukuda T, Hermine O, Bazarbachi A. Diagnostic Approaches and established treatments for adult T cell leukemia lymphoma. Front Microbiol. 2020;19(11):1207.
Leandro Venturutti L, Teater M, Zhai A, et al. TBL1XR1 mutations drive extranodal lymphoma by inducing a pro-tumorigenic memory fate. Cell. 2020;182:297–316.
Drieux F, Ruminy P, Sater V, et al. Detection of gene fusion transcripts in peripheral T-Cell lymphoma using a multiplexed targeted sequencing assay. J Mol Diagn. 2021;23(8):929–40.
Ishitsuka K, Utsunomiya A, Ishida T. PD-1 inhibitor therapy in adult T-cell leukemia-lymphoma. N Engl J Med. 2018;379(7):695. https://doi.org/10.1056/NEJMc1807852.
Kawano N, Ishikawa F, Shimoda K, Yasukawa M, Nagafuji K, Miyamoto T, Baba E, Tanaka T, Yamasaki S, Gondo H, Otsuka T, Ohshima K, Shultz LD, Akashi K, Harada M. Efficient engraftment of primary adult T-cell leukemia cells in newborn NOD/SCID/beta2-microglobulin(null) mice. Leukemia. 2005;19(8):1384–90.
Zimmermann H, Koenecke C, Dreyling MH, et al. Modified risk-stratified sequential treatment (subcutaneous rituximab with or without chemotherapy) in B-cell Post-transplant lymphoproliferative disorder (PTLD) after Solid organ transplantation (SOT): the prospective multicentre phase II PTLD-2 trial. Leukemia. 2022;36(10):2468–78.
Markouli M, Ullah F, Omar N, et al. Recent advances in adult post-transplant lymphoproliferative disorder. Cancers. 2022;14(23):5949.
Tsukasaki K, Fukushima T. JSH practical guidelines for hematological malignancies, 2018: II. Lymphoma-8. Adult t-cell leukemia-lymphoma. Int J Hematol. 2019;109(3):249–59. https://doi.org/10.1007/s12185-018-02588-5.
Acknowledgements
We thank the medical staff, including nurses, laboratory technicians, pharmacists, physical therapists, mental therapists, psychiatrists, and nutritionists, for their excellent care of the patients who were treated with renal transplantation, chemotherapy, and allo-HSCT in our institution.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NK, KY, HM, NY, and KO designed the study. NK collected the clinical data. NK wrote the paper. AT, TM, AD, and ST care for patients treated with renal transplantation. NK, SY, TK, TN, TT, and KY cared for HTLV-1 carriers and patients with ATL. KY, HM, NY, KO, FA, KN, TK, YK, YI, KM, HO, II, YM, KK, JY, YY, KK and KA supervised the study. All authors read and approved the fnal manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was conducted in compliance with good clinical practice and ethical principles of the Declaration of Helsinki. This study was approved by the ethics committees of Miyazaki Prefectural Miyazaki Hospital (19-2).
Consent for publication
We received consent from the patients and their families regarding the use of the person’s data, including the individual details and images. Furthermore, we received informed consent for publication from the patients and their families.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kawano, N., Kyohei, Y., Miyoshi, H. et al. The development of adult T cell leukemia/lymphoma in renal transplant recipients: report of two cases with literature review. Ren Replace Ther 9, 36 (2023). https://doi.org/10.1186/s41100-023-00480-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-023-00480-5