Abstract
Background
Acute pericarditis causes acute inflammation of the pericardium. Although most cases of pericarditis are idiopathic with no identifiable cause, its etiology can be infectious, such as viral, bacterial, mycotic, and tuberculous infections, or non-infectious, including post-pericardiotomy, metastatic malignant tumor, connective tissue disease, or uremia. However, there has been no report of pericarditis caused by adenoviral infection in patients undergoing peritoneal dialysis (PD). Herein, we report a case of pericarditis and pericardial effusion caused by adenoviral infection in a patient undergoing PD.
Case presentation
A 59-year-old man who had been undergoing PD in our department for 3 years had a bout of acute enteritis. He was later admitted to the emergency department of our hospital because of malaise and loss of consciousness due to pericardial effusion. Testing after admission revealed elevated adenovirus antibody titers. Pericardial effusion improved although no changes in his PD prescription were made. The patient was hospitalized and admitted to maintain hemodynamics and prevent hypotension. Since insufficient dialysis volume was ruled out by peritoneal equilibrium tests and dialysis volume assessment, the patient was kept under observation, and no changes were made regarding the method of dialysis. Pericardial effusion and the C-reactive protein level both gradually declined, and the patient’s weight remained steady. The adenovirus antibody titer alone increased to 1:64 at approximately 2 weeks after hospitalization. The final diagnosis was acute pericarditis due to adenoviral infection rather than uremia or dialysis-associated pericarditis.
Conclusions
We treated a patient with a rare case of pericardial effusion caused by viral (adenoviral) pericarditis in a patient undergoing PD. In addition to testing for the usual causes, uremic and dialysis-associated pericarditis must always be ruled out in patients receiving dialysis. In cases of pericarditis with a viral origin, diagnosis and treatment must be comprehensive.
Similar content being viewed by others
Background
Acute pericarditis is a disease that causes acute inflammation of the pericardium, and its epidemiology has not been adequately studied. An autopsy study reported an incidence of pericarditis of approximately 1% [1]. It has also been reported that pericarditis occurs in approximately 5% of patients with non-ischemic chest pain [2]. Most cases of pericarditis are idiopathic, as they have no identifiable cause. However, it can arise from infectious causes, such as viral, bacterial, mycotic, and tuberculous infections, or non-infectious causes, including post-pericardiotomy, metastatic malignant tumor, connective tissue disease, or uremia. Imazio et al. found that 83.2% of cases were idiopathic, with identifiable causes being malignant tumors in 5.1% of cases, tuberculous in 3.8%, autoimmunity in 7.3%, and pyogenic disorders in 0.7% [3]. Viral pericarditis is usually caused by Coxsackie B virus or echovirus. In patients receiving dialysis, uremic pericarditis must also be included in the differential diagnosis. However, there has been no report of pericarditis caused by an adenovirus in a patient undergoing peritoneal dialysis (PD). Herein, we report a case of pericarditis and pericardial effusion caused by adenoviral infection in a patient undergoing PD.
Case presentation
A 59-year-old Japanese man developed oliguria and ascites when he was 11 years old and was diagnosed with nephrotic syndrome, although a renal biopsy was not performed. After approximately 3 years of repeated hospitalization and discharge, he continued to undergo outpatient treatment with prednisolone and diuretics but stopped attending hospital appointments for about 40 years. In March 2009, he developed proteinuria and renal dysfunction and was referred to our department. He started PD in August 2013 and has been managed by our department since then. In late June 2016, he developed acute enteritis. In early July, he began to feel dizzy and ill. In late July, he lost consciousness at work and was brought to our hospital. The patient’s father also had end-stage kidney disease, and had undergone hemodialysis (details, including the underlying condition, are unknown).
PD before hospitalization included three cycles of 2000 mL of DIANEAL® N PD-4 1.5 PD solution (Baxter, Deerfield, IL; 7 h) and 1500 mL of EXTRANEAL® PD solution (Baxter; 12-h dwell period). Further, peritoneal equilibration tests in 2016 revealed a dialysate to plasma ratio of solute concentration of creatinine (Cr) of 0.64 (low average) and the dialysate glucose at 4 h versus the dialysate glucose at time zero of 0.36 (low average). Previously, dialysis efficiency and volume, as measured in 2015, were a total Kt/V of 2.5 (peritoneal Kt/V, 1.73 + renal Kt/V, 0.76) and a total weekly creatinine clearance (CCr) of 89.3 L/week/1.73 m2 (peritoneal CCr, 48.7 L/week/1.73 m2 + renal CCr, 40.7 L/week/1.73 m2).
Physical examination findings on arrival were as follows: height, 162.8 cm; weight, 58.8 kg; temperature, 36.5 °C; blood pressure, 104/79 mmHg; heart rate 97 bpm; respiratory rate, 22 breaths/min; and percutaneous arterial oxygen saturation, 98% (on room air). Mild pallor of the palpebral conjunctiva was observed with no jaundice of the bulbar conjunctiva. Respiratory sounds were clear, but pericardial rub and a systolic murmur (Levine II/IV) that were loudest at the apex were present. The abdomen was mildly distended and soft; there were no areas of tenderness, and bowel sounds were normal. The PD catheter exit site was normal, and there was no pedal edema.
Electrocardiography (ECG) revealed a low voltage compared to those of previous tests (Fig. 1). A chest radiograph showed an increased cardiothoracic ratio (Fig. 2a), and pericardial effusion was visible on computed tomography (Fig. 2b). Echocardiography showed no abnormal movement of the left ventricular wall; the ejection fraction (EF) was 70%, left ventricular end diastolic diameter/left ventricular end systolic diameter was 39/22 (mm), interventricular septum/posterior wall thickness was 14/10 (mm), aorta/left atrium diameter was 34/33 (mm), early diastolic wave/atrial systolic wave was 100/116, deceleration time was 201 ms, early filling velocity on transmitral Doppler (E)/early relaxation velocity on tissue Doppler (E′) (E/E′) was 12.6, and the inferior vena cava diameter was 22 mm (no respiratory variation). No pulmonary hypertension, tricuspid valve regurgitation, aortic regurgitation, or mitral valve stenosis or regurgitation were observed, except a mild aortic stenosis; however, pericardial effusion was present (8 mm anterior to the right ventricle and 13.5 mm posterior to the left ventricle). Peritoneal dialysate analysis, urinalysis, and blood biochemistry tests did not reveal any significant infection, such as PD-associated peritonitis, urinary tract infection, or pneumonia (Tables 1 and 2). Viral antibody measurement patterns were consistent with those of previous cytomegalovirus and Epstein-Barr viral infections, and antibody titers to adenovirus type-2, Coxsackie virus type A2, and echovirus type 9 were elevated.
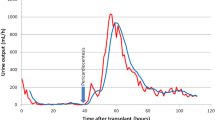
Figure 3 shows the patient’s clinical course. As the patient had recently developed acute enteritis and there was a possibility of a previous viral infection, the provisional diagnosis was viral pericarditis. Pericardiocentesis was not due to lack of storage; therefore, the properties of pericardial fluid could not be confirmed. Although the diameter of the inferior vena cava was enlarged, there was no tricuspid valve regurgitation, and intravenous saline was administered for several days after the patient was admitted to maintain hemodynamics and prevent hypotension due to the reduced cardiac EF resulting from pericardial effusion. Compared to past data, there were no signs of fluid overload and no major changes in blood urea nitrogen or serum creatinine levels were observed. Since insufficient dialysis volume was ruled out by peritoneal equilibrium tests and dialysis volume assessment, the patient was kept under observation and no changes were made regarding the method of dialysis. Pericardial effusion and the C-reactive protein (CRP) level both gradually declined, and there was almost no weight change during hospitalization, as the patient’s weight remained steady at approximately 59 kg. The adenovirus type-2 antibody titer was later found to be elevated at 1:32 after hospitalization. Although Coxsackie virus and echovirus antibody titers were increased, the adenovirus type-2 antibody titer alone increased further to 1:64 at approximately 2 weeks after hospitalization. The final diagnosis was acute pericarditis due to adenoviral infection. The patient’s subsequent course was uneventful, and he was discharged in early August. At an outpatient appointment in late August, both the cardiothoracic ratio and pericardial effusion had improved, and the adenovirus type-2 antibody titer decreased to 1:16.
Discussion and conclusions
In the present case, a patient receiving PD developed pericardial effusion due to viral pericarditis. Acute pericarditis is a condition in which fluid accumulates in the pericardial space as a result of pericardial inflammation. Its cause may be idiopathic, infectious, or non-infectious, post-myocardial infarction (Dressler syndrome), metastatic malignant tumors, trauma, and amyloidosis. According to Imazio et al., the idiopathic type generally accounts for most cases [4, 5].
Diagnosis is based on clinical signs and investigation findings, including ECG and echocardiography. Clinical symptoms include chest pain, fever, and dyspnea. Pericarditis can be differentiated from myocardial infarction as the chest pain is worsened by lying flat and taking a deep breath and is relieved by sitting or leaning forward. In idiopathic or viral infection cases, precursor symptoms, such as upper respiratory and gastrointestinal symptoms, and general malaise may be evident 1–2 weeks before the onset of pericarditis. The tests performed include ECG, echocardiography, chest radiography, and blood tests. Characteristic ECG findings are convex upward ST elevation on all leads other than aVR and inversions that do not coincide with the regions supplied by the coronary artery. These features change over time, with ST elevation normalizing after a few days, and only a negative T-wave remains. The trace is completely normalized after 1–2 months. Severe pericardial effusion causes QRS waves to decay to a constant potential. ECG reveals an echo-free space in the pericardial cavity. Chest radiography reveals enlargement of the heart due to pericardial effusion. Blood test results often include elevated CRP levels and white blood cell count (WBC count), which are suggestive of an inflammatory process. If myocarditis is also present, cardiac enzymes are mildly elevated.
The course and prognosis of pericarditis vary depending on the etiology. Idiopathic and viral cases generally have good prognoses, as patients recover spontaneously within 2–6 weeks. However, its recurrence rate is 18.3%, cardiac tamponade occurs in 3.1% of cases, and 1.5% of patients progress to constrictive pericarditis [3].
If there is a clear cause for treatment, the cause is treated. Treatment of idiopathic or viral pericarditis is mainly symptomatic, using non-steroidal anti-inflammatory drugs. Combination therapy or monotherapy with corticosteroids is effective in first-onset cases and in preventing recurrence. Steroids are used if the cause is a connective tissue disease or an autoimmune condition. Uremic pericarditis is treated by commencing dialysis or improving its efficiency. However, if there is no improvement, steroid use should be considered. If cardiac tamponade is present, pericardial drainage is performed. Pericardial fenestration is performed in the event of repeated pleural effusion leading to the development of cardiac tamponade or heart failure.
In the present case, some form of infection was suggested by the elevated WBC count, elevated CRP level, and mildly elevated procalcitonin levels. However, there were no signs suggestive of serious bacterial infection, and mycotic and tuberculous infections were ruled out by negative βD-glucan elevation and interferon-gamma release assay. Connective tissue disease-related autoantibody tests were negative; there was no myocardial infarction, and there were no positive signs that were suggestive of malignant tumors on imaging or tumor markers. The patient had no history of trauma. Amyloidosis was not seriously considered because of the short duration of dialysis, and M proteins were not detected. Therefore, the most likely differential diagnoses were either an infection, particularly a viral infection, or uremic or dialysis-related pericarditis.
Pericarditis in dialysis patients may be uremic or dialysis-related [6]. Pericarditis occurs in patients with end-stage kidney disease, either before or immediately after commencing dialysis. Although the details of its pathogenesis are unknown, it may involve factors including increased vascular permeability due to the accumulation of uremic toxins, immune system dysfunction, and fluid overload. Waker et al. reported that 41% of patients with acute and chronic renal failure were diagnosed with uremic pericarditis on autopsy, which is a very high rate [7]. However, Hakim et al. reported that 7% of patients developed pericarditis within 6 months of commencing dialysis [8]. It is conceivable that advances in dialysis therapy and the introduction of erythropoietin may have influenced this shift. Diabetes-associated pericarditis develops in patients with diabetes undergoing dialysis and is usually caused by insufficient dialysis or inadequate fluid removal (fluid overload). Therefore, it is necessary to optimize dialysis therapy. Furthermore, Tseng et al. found that 85.1% of patients with diabetes and 82.9% of those without diabetes showed improvement after hemodialysis was intensified [9]. Conversely, Connors et al. reported that the improvement rate was as low as 57% after the commencement of dialysis [10]. A study on pericarditis in patients receiving PD found that 4.3% of these patients developed pericarditis more than 4 months after starting dialysis, which is comparable to the rate among patients receiving hemodialysis [11]. As our patient had been undergoing PD for more than 3 years, dialysis-associated pericarditis was considered as a differential diagnosis. However, this was ultimately ruled out because the results of the dialysis efficiency and dialysis volume tests from the previous year did not reveal any major problems. There was also no subcutaneous edema, the pericarditis resolved with no change in the PD prescription, and there was almost no change in body weight when the patient was hospitalized.
Adenoviral infections occur throughout the year in all age groups. Although approximately half are subclinical infections, they account for 5–10% of pediatric respiratory diseases, and may also cause acute gastroenteritis and conjunctivitis. There have also been recent cases of fatal infections, including hepatitis, pneumonia, and meningitis, in immunocompromised individuals, such as organ transplant recipients and patients with acquired immune deficiency syndrome. With regards to the relationship between adenovirus and pericarditis, Ivanova et al. reported that among 235 patients with myocarditis or pericarditis, 63 were negative for Coxsackie type B virus immunoglobulin M (IgM) antibodies, and in the above-mentioned 63 patients, six were positive for adenovirus IgM antibodies, including four patients with pericarditis [12]. Prodromal symptoms such as upper respiratory and gastrointestinal symptoms and general malaise are often present before the onset of pericarditis. Since the patient had pericardial effusion with inflammatory response and was preceded by enteritis, viral pericarditis was suspected. Viral tests were performed for Coxsackie virus and other viruses that cause pericarditis, as well as adenovirus, which causes enteritis, and a diagnosis of adenovirus-induced pericarditis was made based on elevated adenovirus antibodies.
There have been few reports on viral pericarditis in patients receiving dialysis [13, 14] (Table 3). However, all reported cases have been in patients undergoing hemodialysis. The causative viruses in those reports were influenza type A and Coxsackie type B viruses. There have been no previously reported cases of adenoviral infection leading to pericarditis in patients receiving dialysis. The main treatment was continuation of hemodialysis and pericardial pericardiectomy, but some patients improved after hemodialysis was changed to PD [13]. There have been no reports of pericarditis and pericardial effusion caused by adenovirus in patients receiving PD. Hence, this is an extremely valuable case report, although it has some limitations, including the fact that we were unable to perform pericardiocentesis to test the pericardial fluid.
We treated a patient receiving PD who had pericardial effusion caused by adenoviral pericarditis. In addition to testing for the usual causes, uremic and dialysis-associated pericarditis must always be excluded in patients receiving dialysis. Furthermore, in patients receiving PD, pericarditis due to viral infections should be kept in mind.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Abbreviations
- PD:
-
Peritoneal dialysis
- CCr:
-
Creatinine clearance
- ECG:
-
Electrocardiography
- EF:
-
Ejection fraction
- E:
-
Early filling velocity on transmitral Doppler
- E′:
-
Early relaxation velocity on tissue Doppler
- CRP:
-
C-reactive protein
- WBC:
-
White blood cell
- IgM:
-
Immunoglobulin M
References
Friman G, Fohlman J. The epidemiology of viral heart disease. Scand J Infect Dis Suppl. 1993;88:7–10.
Launbjerg J, Fruergaard P, Hesse B, Jørgensen F, Elsborg L, Petri A. Long-term risk of death, cardiac events and recurrent chest pain in patients with acute chest pain of different origin. Cardiology. 1996;87:60–6.
Imazio M, Cecchi E, Demichelis B, Ierna S, Demarie D, Ghisio A, et al. Indicators of poor prognosis of acute pericarditis. Circulation. 2007;115:2739–44.
Permanyer-Miralda G, Sagristá-Sauleda J, Soler-Soler J. Primary acute pericardial disease: a prospective series of 231 consecutive patients. Am J Cardiol. 1985;56:623–30.
Zayas R, Anguita M, Torres F, Giménez D, Bergillos F, Ruiz M, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol. 1995;75:378–82.
Naobumi M, Epicarditis TS. In: Tokuichiro S, Kazuhiro, editors. Cardiovascular bedside manual of dialysis patients. Tokyo: Academic Press; 2004. p. 117–23 (in Japanese)
Wacker W, Merrill JP. Uremic pericarditis in acute and chronic renal failure. J Am Med Assoc. 1954;156:764–5.
Hakim JG, George A, Siziya S. Echocardiographic assessment of left ventricular hypertrophy diastolic dysfunction and pericardial disease in patients on maintenance haemodialysis. East Afr Med J. 1996;73:505–8.
Tseng JR, Lee MJ, Yen KC, Weng CH, Liang CC, Wang IK, et al. Course and outcome of dialysis pericarditis in diabetic patients treated with maintenance hemodialysis. Kidney Blood Press Res. 2009;32:17–23.
Connors JP, Kleiger RE, Shaw RC, Voiles JD, Clark RE, Harter H, et al. The indications for pericardiectomy in the uremic pericardial effusion. Surgery. 1976;80:689–94.
Silverberg S, Oreopoulos DG, Wise DJ, Uden DE, Meidok H, Jones M, et al. Pericarditis in patients undergoing long-term hemodialysis and peritoneal dialysis. Incidence, complications and management. Am J Med. 1977;63:874–9.
Ivanova SK, Angelova SG, Stoyanova AP, Georgieva IL, Nikolaeva-Glomb LK, Mihneva ZG, et al. Serological and molecular biological studies of parvovirus B19, Coxsackie B viruses, and adenoviruses as potential cardiotropic viruses in Bulgaria. Folia Med (Plovdiv). 2016;58:250–6.
Cohen GF, Burgess JH, Kaye M. Peritoneal dialysis for the treatment of pericarditis in patients on chronic hemodialysis. Can Med Assoc J. 1970;102:1365–8.
Osanloo E, Shalhoub RJ, Cioffi RF, Parker RH. Viral pericarditis in patients receiving hemodialysis. Arch Intern Med. 1979;139:301–3.
Acknowledgments
The authors are grateful to the medical staff of Shimane University Hospital, Izumo, Japan.
Funding
The authors received no funding for this case report.
Author information
Authors and Affiliations
Contributions
ME was responsible for this manuscript. SF, KT, RY, AY, KY, KT, HS, and TI provided information on the discussion and treatment of the patient. ME collected the data and drafted the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient. For case reports, a formal approval from a local ethics committee was not required.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Egawa, M., Fujii, S., Takase, K. et al. Pericardial effusion caused by viral pericarditis in a patient receiving peritoneal dialysis. Ren Replace Ther 8, 17 (2022). https://doi.org/10.1186/s41100-022-00406-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-022-00406-7