Abstract
Background
Currently, it is unclear whether the progression of chronic kidney disease (CKD) could be an independent predictor of antibody response after administration of a COVID-19 vaccine. This study aimed to investigate the immune response to COVID-19 vaccination in patients with CKD stage G4 to G5 without renal replacement therapy and G5D using the recommended dose and schedule.
Methods
This retrospective single-center cohort study evaluated immunogenicity regarding antibody response after COVID-19 vaccination in our hospital for late-stage CKD patients aged ≥ 60 years. We evaluated antibody responses in 48 patients with CKD G4, 35 patients with CKD G5, and 70 patients undergoing hemodialysis (HD; CKD G5D).
Results
After the second vaccination, anti-SARS-CoV-2-S (Spike) IgG levels were found to be positive (> 0.8 U/mL) in all CKD G4 and G5 patients (100%), and 69 of 70 HD patients (98.5%). The median (interquartile range [IQR] S-IgG level (Ab titers) was 358 [130.2–639.2], 218 [117–377], and 185.5 [95.1–323.5] U/mL in the CKD G4, G5, and HD groups, respectively. The median S-IgG levels were significantly lower in the HD group than in the CKD G4 group (p < 0.01). However, there was no significant difference in the antibody titers between the CKD G4 and G5 groups. To further analyze the decline in S-IgG levels after 6 months, we additionally assessed and compared antibody titers at 1 month and 6 months after the second vaccination in the HD group. Compared with the median S-IgG levels of 185.5 [95.1–323.5] U/mL 1 month after the second dose, the median S-IgG level 6 months thereafter was significantly decreased at 97.4 [62.5–205.5] U/mL (p < 0.05).
Conclusions
We highlight two major factors of variability in the vaccine response. First, in elderly patients with late-stage CKD, antibody titers tended to be lower in the G5D group than in the G4 and G5 groups despite the shorter time since vaccination; therefore, CKD stage progression might cause a decline in antibody titers. Second, waning immune responses were observed 6 months after second dose administration in HD patients advocating a potential need for a third booster dose vaccine after 6 months.
Similar content being viewed by others
Background
The high-risk groups for coronavirus disease 2019 (COVID-19)-mediated critical illness include those with obesity, diabetes mellitus, older age and chronic kidney disease (CKD) [1,2,3], with CKD emerging as the most prevalent of all [3]. Thus, patients with CKD, with their elevated risk of mortality from COVID-19, require urgent action for preventing severe outcomes.
Presently, studies have shown that responses to COVID-19 mRNA vaccines are likely to be lower in patients with CKD than in the general population [4,5,6]. Therefore, immunization against COVID-19 with effective vaccines is an important component of health-maintenance strategies for these patients [4].
Currently, it is unclear whether the progression of CKD could be an independent predictor of antibody response after administration of a COVID-19 vaccine. This study aimed to investigate the immune response to COVID-19 vaccination in patients with CKD stage G4 to G5 without renal replacement therapy (RRT) and G5D using the recommended dose and schedule.
Methods
Study design and participants
This retrospective single-center cohort study was performed in Kameda Medical Center to evaluate immunogenicity in terms of antibody response after COVID-19 mRNA vaccination in patients with late-stage CKD aged 60 years or older.
Due to Japan’s vaccine delivery systems, group vaccination was conducted mostly with two doses of Comirnaty COVID-19 vaccine (BioNTech-Pfizer BNT162b2).
We evaluated antibody responses in 48 patients with CKD G4, 35 patients with CKD G5, and 70 patients undergoing hemodialysis (HD) (CKD G5D). We excluded patients aged below 60 years, receiving treatment for immunosuppression, and with malignancy or hematologic disorders.
As we reported earlier [7], severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific antibodies in HD groups were evaluated and compared to that of control group; thus, in this study, we evaluated the antibody responses among late-stage CKD patients based on a residual renal function. The details of the control group, which is important for this study, are shown in Table 1 because these data were not presented in our previous report. The control group was composed of 35 participants (a population expected to have optimal antibody response) who were volunteers that met the criteria of over 60 years of age with no evidence of kidney failure, active cancer or ongoing treatment for immunosuppression. They were selected over a four-month period by consecutive sampling from patients visiting our gastroenterology outpatient clinic.
All participants had received the first and second dose of COVID-19 mRNA vaccines (following the recommended interval of 21 days for the BNT162b2 vaccine) between May 12, 2021 and October 6, 2021. Sample collection on antibody titers follow-up was continued until November 24, 2021.
Humoral response assessment
Serum samples were tested for SARS-CoV-2 antibodies (immunoglobulin G [IgG] levels) using the Elecsys® Anti-SARS-CoV-2 S RUO (Roche Diagnostics, Basel, Switzerland) test system. Antibody titers > 0.8 U/mL were considered as positive immune response to vaccination.
Outcomes
The primary outcomes evaluated in this study included quantitative humoral responses to the second dose of COVID-19 mRNA vaccine. Anti-SARS-CoV-2-S (Spike) IgG levels were evaluated among the CKD G4, G5, and G5D groups. In the G5D group, a comparison between 1 and 6 months after the second vaccination was also performed.
Statistical analysis
The data were analyzed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA). To compare the three groups (non-normally distributed samples), the data were analyzed using the non-parametric Kruskal–Wallis and post hoc Dunn’s tests. Mann–Whitney U test was used to compare non-normally distributed data between two groups. *p < 0.05 and **p < 0.01 are depicted.
Ethical considerations and disclosures
All patients provided written informed consent to participate in this study, which was approved by the institute’s committee on human research (Approval Number 21–025).
Results
Table 1 lists and compares demographic and laboratory data of the study groups. CKD G4 consisted of 34 males (70.8%), with a mean age of 77.1 years, CKD G5 consisted of 23 males (65.7%), with a mean age of 75.3 years, and HD group consisted of 47 males (67.1%), with a mean age of 73.1 years. The median time on dialysis was 5.5 years in the HD patients. The percentage of male patients and the incidence of diabetes mellitus were almost the same in the G4, G5, and G5D groups.
After the second vaccination, anti-SARS-CoV-2-S (Spike) IgG levels were found to be positive (> 0.8 U/mL) in all CKD G4 and G5 patients (100%), and 69 of 70 HD patients (98.5%). The median (interquartile range [IQR]) S-IgG level (Ab titers) was 358 [130.2–639.2], 218 [117–377], and 185.5 [95.1–323.5] U/mL in the CKD G4, G5, and HD groups, respectively.
The median S-IgG levels were significantly lower in the HD group than in the CKD G4 group (p < 0.01) (Fig. 1). However, there was no significant difference in the antibody titers between the CKD G4 and G5 groups (Fig. 1). No life-threatening allergic reaction or side effect was observed post-vaccination.
Antibody response following vaccination with the second dose of coronavirus disease 2019 (COVID-19) vaccine. The median antibody titers after second vaccination were significantly lower in the HD group than in the CKD G4 group (p < 0.01). There was no significant difference in the antibody titers between the CKD G4 and G5 groups
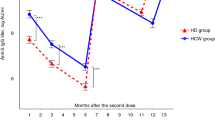
To further analyze the decline in S-IgG levels after 6 months, we additionally assessed and compared the antibody titers at 1 month and 6 months after the second vaccination in the HD group. Compared with the median S-IgG level 1 month after the second dose (185.5 [95.1–323.5] U/mL), the median S-IgG level 6 months thereafter was significantly decreased (97.4 [62.5–205.5] U/mL) (p < 0.05) (Fig. 2).
Antibody response following vaccination with the second dose of coronavirus disease 2019 (COVID-19) vaccine in hemodialysis patients. The median antibody titer decreased from 185.5 [95.1–323.5] U/mL 1 month after the second vaccination to 97.4 [62.5–205.5] U/mL 6 months after the second vaccination (p < 0.05)
Discussion
We retrospectively evaluated the antibody responses of patients with CKD stages G4, G5, and G5D after the second-dose administration of the COVID-19 mRNA vaccine. It has been reported that obesity, diabetes mellitus, older age, male sex, and CKD affect response to COVID-19 vaccine [1,2,3]; however, in our study, the percentage of male patients and the incidence of diabetes mellitus were almost the same among the G4, G5, and G5D groups; thus, we evaluated the antibody responses based on a gradual loss of kidney function.
There is growing evidence for the protective efficacy of the COVID-19 vaccine [8,9,10]. However, due to their impaired immune system, patients with CKD and those undergoing RRT produce a suboptimal response to the vaccine [7]. Furthermore, there have been no clear data on the COVID-19 vaccination of patients with CKD stages G4 to G5.
In our study, median antibody titers in patients with GFR 15–29, < 15, and < 5 mL/min were 358, 218, and 185.5 U/mL, respectively. Thus, the degree of renal impairment may influence the titers of anti-SARS-CoV-2-S IgG. In later stages of CKD, especially in the hemodialysis stage, vaccination was more likely to induce a poor antibody response [11]. Therefore, kidney function appears to be an independent predictor of IgG levels.
Regarding the antibody titers 6 months after the second dose of COVID-19 vaccine, waning immune responses was observed in this study. These findings are consistent with previous preliminary reports [8, 12,13,14]. Moreover, these studies indicate that immune responses to vaccines are considerably reduced in patients undergoing dialysis, for whom a vaccination strategy including three doses of vaccine has been recommended [13], and administration of a third dose of the BNT162b2 vaccine to solid-organ transplant recipients significantly improved the immunogenicity of the vaccine [12]. Therefore, our results confirm the potential need for booster doses, especially in those with lower antibody titers, after administering the second dose of COVID-19 vaccine.
With regard to the third-dose vaccination strategy in patients with late-stage CKD and low immune responses, we have to consider the effective dose and schedule of the vaccine [13,14,15]. To increase effectiveness in CKD patients, higher vaccine doses or an increased number of vaccine injections may be necessary for booster vaccination, similar to influenza and hepatitis B vaccinations [16].
We encourage further studies to assess whether a stronger antibody response could be obtained with administration of higher doses of vaccine booster against COVID-19 in late-stage CKD.
The study limitations include variation in sampling date among patients with CKD stages G4–5 and the lack of cellular immune response data. In the G4 and G5 groups, differences in the intervals and frequency of outpatient visits were observed, rendering it difficult to compare the aforementioned variables at the same time-point and resulting in a significant variation in the sampling date. In contrast, the G5D group had less variation because they visited the hospital regularly for hemodialysis. Moreover, regarding the measurement of follow up antibody titers 6 months after the second vaccination, since CKD stage progression was observed in some patients in the G4 and G5 groups, we could not compare antibody titers. However, antibody titers tended to be lower in the G5D group than in the G4 and G5 groups despite the shorter time since vaccination, and we observed a decrease in antibody titers after 6 months.
Conclusions
We highlight two major factors of variability in vaccine response. First, in elderly patients with late-stage CKD, antibody titers tended to be lower in the G5D group than in the G4 and G5 groups despite the shorter time since vaccination; therefore, CKD stage progression might cause a decline in antibody titers. Second, waning immune responses were observed 6 months after administering the second dose in HD patients advocating a potential need for a third-dose booster vaccine after 6 months.
Although no threshold has been established for protective immunity, a third-dose booster COVID-19 vaccine after 6 months may be necessary to sustain a protective immunity, particularly in patients with late-stage CKD with a low immune response.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CKD:
-
Chronic kidney disease
- RRT:
-
Renal replacement therapy
References
Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, Gan H, Sun YL, Fu W, Li W, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–55.
Jordan RE, Adab P, Cheng KK: Covid-19: risk factors for severe disease and death. BMJ (Clinical research ed) 2020, 368:m1198.
Gansevoort RT, Hilbrands LB. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol. 2020;16(12):705–6.
Broseta JJ, Rodríguez-Espinosa D, Rodríguez N, Mosquera MDM, Marcos M, Egri N, Pascal M, Soruco E, Bedini JL, Bayés B, et al. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78(4):571–81.
Windpessl M, Bruchfeld A, Anders H-J, Kramer H, Waldman M, Renia L, Ng LFP, Xing Z, Kronbichler A. COVID-19 vaccines and kidney disease. Nat Rev Nephrol. 2021;17(5):291–3.
Carr EJ, Kronbichler A, Graham-Brown M, Abra G, Argyropoulos C, Harper L, Lerma EV, Suri RS, Topf J, Willicombe M, et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney international reports. 2021;6(9):2292–304.
Matsunami M, Suzuki T, Terao T, Kuji H, Matsue K. Immune response to SARS-CoV-2 vaccination among renal replacement therapy patients with CKD: a single-center study. Clin Exp Nephrol. 2022;26(3):305–7.
Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet (London, England). 2021;398(10309):1407–16.
Hou YC, Lu KC, Kuo KL: The efficacy of COVID-19 vaccines in chronic kidney disease and kidney transplantation patients: a narrative review. Vaccines 2021, 9(8).
Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW: SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science (New York, NY) 2021:eabm0620.
DaRoza G, Loewen A, Djurdjev O, Love J, Kempston C, Burnett S, Kiaii M, Taylor PA, Levin A. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis. 2003;42(6):1184–92.
Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–2.
Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100(3):702–4.
Davidovic T, Schimpf J, Abbassi-Nik A, Stockinger R, Sprenger-Mähr H, Lhotta K, Zitt E. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: time for a boost. Kidney Int. 2021;100(6):1334–5.
Frantzen L, Thibeaut S, Moussi-Frances J, Indreies M, Kiener C, Saingra Y, Santini J, Stroumza P, El-Haik Y, Cavaillé G. COVID-19 vaccination in haemodialysis patients: good things come in threes. Nephrol Dial Transpl. 2021;36(10):1947–9.
Khan SF, Bowman BT. Vaccinating the Patient with ESKD. Clin J Am Soc Nephrol. 2019;14(10):1525–7.
Acknowledgements
The authors would like to thank Dr. So Nakaji for recruiting healthy volunteers from Kameda Medical Center’s Gastroenterology Unit.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
MM designed and wrote the manuscript. TS and KM contributed to the protocol design. JF, TT, KU, SS, TT, KN, MN, MO, JY and HK contributed to patient follow up and data management. MM, TS, and KM analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Kameda Medical Center (Approval Number 21–025) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent was obtained from all the patients to publish the data obtained in this study.
Competing interests
The authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Matsunami, M., Suzuki, T., Fukuda, J. et al. Comparison of antibody response following the second dose of SARS-CoV-2 mRNA vaccine in elderly patients with late-stage chronic kidney disease. Ren Replace Ther 8, 13 (2022). https://doi.org/10.1186/s41100-022-00402-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-022-00402-x