Abstract
Background
Quality of life for haemodialysis (HD) patients may be affected by symptoms during dialysis treatments, and patient groups have highlighted the need to improve post-dialysis fatigue and dialysis-related symptoms. As changes in extracellular water (ECW) may lead to cramps and other symptoms, we wished to determine whether there was an association between ECW and intra-dialytic symptoms.
Methods
We reviewed the hospital records of HD patients who completed a self-reported intra-dialytic symptom questionnaire, using a visual analogue scale, who had contemporaneous pre- and postdialysis bioimpedance ECW measurements adjusted to height (aECW).
Results
We studied dialysis sessions of 506 patients, 314 (62.1%) male, 226 (44.7%) diabetic, mean age 64.6 ± 15.7 years, weight 69.9 ± 17.4 kg, and duration of dialysis treatment 26 (9.6–60.1) months. We divided patients into three groups according to pre-dialysis aECW, and total dialysis symptom scores were greater for those in the lower tertile (25 (10–41) vs middle 18 (8.5–34) vs upper 20 (7–31), p < 0.05). Only feeling cold, dizziness, and low blood pressure were statistically different between the three pre-dialysis aECW groups, and there was no difference in post-dialysis recovery times.
We analysed the effect of the fall in aECW pre-to post-dialysis. Patients in the group with the greatest fall in aECW did not report more intra-dialytic symptoms or longer recovery times.
Conclusion
We found that patients starting dialysis with lower relative ECW were more likely to report intra-dialytic symptoms than those with greater amounts of fluid to remove, and most commonly reported symptoms were associated with intra-vascular volume depletion.
Similar content being viewed by others
Introduction
More than 2 million patients with chronic kidney disease (CKD) are treated by haemodialysis world-wide. Despite technological advances in dialysis machine and dialyzer designs [1], patient mortality remains high. Although patients are regularly monitored with laboratory tests, including dialyzer urea clearance (Kt/Vurea), haemoglobin, calcium, phosphate, parathyroid hormone, and blood pressure, interventional trials and meta-analyses have shown that these are weakly and inconsistently associated with all-cause and cardiovascular mortality and cardiovascular events [2, 3].
Haemodialysis patients experience poor health-related quality of life [4] and self-report high levels of depression [5]. Patients, along with other dialysis stakeholders, have identified symptom relief as a top priority and have called for increased symptom focus in both research and clinical practice [6,7,8]. Symptoms, such as fatigue, may be a consequence of CKD or directly attributable to the haemodialysis treatments [9], and several centres have developed haemodialysis-specific symptom questionnaires [10, 11].
Patients attending for haemodialysis with greater inter-dialytic weight gains have greater weight losses with dialysis, using faster ultra-filtration rates. These patients are reported to be at greater risk of cardiovascular mortality [12, 13]. Patients typically under-report their symptoms, and dialysis staff similarly often fail to record intra-dialytic symptoms, whereas studies which have actively questioned patients have reported that almost all patients suffered with fatigue or cramps [14]. As cramps and fatigue may be induced and exacerbated by greater and more rapid fluid removal, we wished to determine whether patients with greater extracellular volume prior to the dialysis session experienced more intra-dialytic symptoms.
Patients and methods
In keeping with UK National Health Service (NHS) guidelines to obtain patient feedback on treatment, we asked all patients attending for outpatient dialysis treatment in 3 dialysis centres under the care of the Royal Free Hospital, London, to self-report the frequency of dialysis associated symptoms, including fatigue, feeling cold, cramps, dizziness, headache, nausea, abdominal pain, back ache, pruritus, short of breath, and palpitations and time to recovery using a previously reported visual analogue scale [10, 15], when they attended for a routine out-patient mid-week dialysis session in four outlying satellite dialysis centres under the care of a university hospital in sequence in May, July, and November 2017, respectively. Psychological distress was determined using the distress thermometer score, a visual scoring system initially introduced in the management of patients with cancer [16]. Hospital-computerised medical records were reviewed to obtain their Stoke-Davies co-morbidity grades, a co-morbidity score developed in the UK and used by the UK national renal registry [17], and frailty using the Canadian geriatric frailty score, which assess functional ability, in terms of activities of daily living [18].
Pre-midweek blood samples were taken on the day of the questionnaire for standard biochemical measurement of urea, creatinine, albumin, C reactive protein (CRP) and N terminal pro-brain natriuretic peptide (NT-proBNP) (Roche Integra, Roche Diagnostics, Lewes, UK) [19] and haemoglobin along with the corresponding post-dialysis urea.
The dialysis prescription and dialysis session details were reviewed retrospectively from hospital-computerised records. Patients dialysed using either a Fresenius 4000H or 5008 dialysis machines (Fresenius MC, Bad Homburg, Germany) or BBraun DialogueR+ (BBraun, Melsungen, Germany) with a polysulphone dialyzer (Fresenius MC, Bad Homburg, Germany) [20] and anticoagulated with a bolus of tinzaparin low molecular weight heparin (Leo Laboratories, Princes Risborough, UK) [21]. All dialysates used a common concentration of bicarbonate 32 mmol/L, acetate 3.0 mmol/L, magnesium 0.5 mmol/L and glucose 5.5 mmol/L. Conductivity modules were regularly calibrated and checked [22]. We used constant ultrafiltration profiles and ultrafiltration rates (UFR) were calculated as the difference in pre- and post-dialysis weights divided by sessional time and adjusted for patient weight.
Blood pressure was measured in the sitting position immediately prior to the start of the dialysis treatment using integrated automatic oscillometric devices. Multifrequency bioimpedance (MFBIA) measurements were made with an 8 electrode multi-frequency segmental bioimpedance device (InBody 720, Seoul, South Korea) in CKD haemodialysis outpatients attending for the mid-week haemodialysis session, in a standardised protocol [23, 24]. All MFBIA measurements were made both prior to and after the dialysis session had been completed and time allowed for re-equilibration [25]. The bioimpedance devices were regularly serviced and calibrated.
In a sub-set of patients, standard two-dimensional M mode transthoracic echocardiogram (Philips IE33, Philips Medical Systems, Eindhoven, Netherlands) was performed [26].
Ethics
This audit of clinical service complied with the UK National Health Service (NHS) guidelines for clinical audit and service development and met with approval from the Health Research Authority (HRA). In keeping with UK guidelines, all patient data was anonymised prior to analysis (https://www.hra.nhs.uk).
Statistical analysis
Data is presented as mean ± standard deviation, median (interquartile range), or as percentage. Standard statistical tests were used to analyse data (D’Agostino-Pearson normality test, chi-square test, ANOVA, Kruskal-Wallis, or Spearman univariate correlation) with appropriate corrections made for multiple testing, by Tukey or Gannet-Howell post hoc testing. Statistical analysis used Prism 8.3 (Graph Pad, San Diego, USA) and Social and Political Sciences statistical package (SPSS 24.0, IBM, Armonk, NY, USA). Statistical significance was taken as p < 0.05.
Results
A total of 551 patients were asked to complete the dialysis symptom questionnaire and their dialysis centre, and 506 (92%) completed the questionnaires. Twenty-seven patients were unable to complete the questionnaires, due to their inability to read or declined to participate. An additional 18 patients were excluded due to missing bioimpedance data, or limb amputations.
We studied the dialysis sessions of the 506 patients, 314 (62.1%) male, 226 (44.7%) diabetic, mean age 64.6 ± 15.7 years, weight 69.9 ± 17.4 kg, and duration of dialysis treatment 26 (9.6–60.1) months. To compare ECW between patients, we adjusted ECW by height (aECW) [27]. We divided patients into three groups according to aECW, and there were more male patients and more diabetics in the group with the higher ECW/height tertile (Table 1), and this group had greater fat-free mass and also had higher pre-dialysis systolic blood pressures, but dialysed with a lower dialysate sodium concentration, and as such had a greater serum sodium to dialysate sodium concentration gradient. Not all patients could provide 24-h urine collections, as such we used serum β2 microglobulin as a surrogate for residual renal function, and there was no difference between tertiles. There were no differences in the prescription of anti-hypertensive medications.
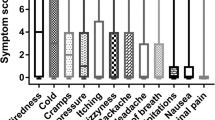
The total dialysis symptom scores were greater for those in the lower pre-dialysis aECW tertile (Fig. 1). However, when comparing individual symptom scores, then only feeling cold, dizziness and low blood pressure were statistically greater for the lower pre-dialysis aECW group compared to the middle and upper aECW groups (Table 2), and there was no difference in post-dialysis recovery times (Fig. 2), or recorded blood pressure (Table 1).
Dialysis symptom frequency scores using a visual analogue scale of 0 to 10. Patients divided according to extracellular water to height ratio into 3 groups—lower, middle, and higher tertiles. Results expressed as median, interquartile, and 90% limits. *p < 0.05, **p < 0.01, ***p < 0.001 vs those in the lower tertile
We then divided patients according to the change in ECW to height pre- to post-dialysis (ΔaECW). Patients with the greater fall in ECW adjusted for height had faster ultrafiltration rates, and greater weight loss and falls in systolic blood pressure (Table 3) but did not report more intra-dialytic symptoms (Table 4). Similarly, there were no differences in the time to recover post-dialysis (recovery < 1.0 h – ΔaECW lower tertile/middle tertile/upper tertile 41.0 vs 45.8 vs 39.6%, and 1–4 h 30 vs 26.4 vs 26%). There were no differences in the prescription of anti-hypertensive medications.
A subgroup of 424 (83.6%) had records of echocardiograms available for review, and 52.6% had left ventricular hypertrophy on standard criteria [28]. There were no differences in symptom reporting comparing those with and without left ventricular hypertrophy. However, there was a negative association between left ventricular ejection fraction and reporting shortness of breath during the dialysis session (r = − 0.139, p = 0.004).
Discussion
It is well recognised that patients with CKD treated by haemodialysis may suffer with cramps, headaches, and fatigue during dialysis sessions and take some time to fully recover after the dialysis session, which can impact on quality of life [4].
Recent workshops which have included patient representatives have highlighted the importance of fatigue and the impact this has on patient well-being [6,7,8]. However, there are two aspects of fatigue, fatigue directly related to the dialysis session and general fatigue. We wished to concentrate on symptoms directly attributable to the dialysis session.
Previous studies have observed that intra-dialytic symptoms and recovery time are increased, whether self-reported or on direct questioning, when more fluid is removed during dialysis sessions, along with faster ultra-filtration rates [14, 29, 30]. We used bioimpedance to assess volume status. There has been debate as how best to express whether patients have an increased ECW, and to adjust ECW for body size to allow comparison between patients. Comparing ECW to intracellular water (ICW) or total body water (TBW) can be confounded by changes in body composition, due to loss of cell mass (ICW) as patients get older, or differences in muscle and fat mass between genders, ethnic groups [31], and obesity [32]. As such, we chose to express ECW as a ratio of height [33].
We divided patients according to their pre-dialysis ECW to height ratio, as we anticipated that patients with greater ECW would be most likely to have more fluid removed during a dialysis session. Pre-dialysis systolic blood pressure was greater in this group, but there were no differences in NTproBNP. Whereas we had expected that the group with the greater pre-dialysis aECW would experience more intra-dialytic symptoms and have longer post-dialysis recovery times, we found that symptom reporting was highest for those in the lower aECW group, and pre-dialysis aECW had no effect on recovery times. In particular those symptoms associated with hypovolaemia, dizziness, feeling cold, and reporting low blood pressure were significantly more commonly reported in the pre-dialysis lower aECW group, despite the lower aECW starting dialysis with a higher haemoglobin concentration. This would suggest that fluid removal in patients closer to their target or dry weight leads to greater symptom reporting. As such there may be a balance to consider when prescribing dialysis treatments between achieving an ideal post-dialysis target weight and potentially increasing patient discomfort during the dialysis session.
This group had similar percentage weight loss and ultrafiltration rates compared to the other groups yet dialysed using a higher dialysate sodium and a lower gradient between the serum and dialysate sodium. Previous studies have suggested that using a higher dialysate sodium and a lower serum to dialysate gradient reduces patient symptoms [34], but our study does not support these earlier reports. However, as we used cooled dialysates, then this may have reduced any potential effect of dialysate sodium. The cardiovascular response to ultrafiltration may equally be important in whether patients report intra-dialytic symptoms. There were no differences in the prescription of antihypertensive medications. In a sub-group of patients, echocardiography data was available, and although patients with left ventricular hypertrophy did not report more symptoms, those with reduced left ventricular systolic function did report more shortness of breath, but not other symptoms. However, the echocardiograms were not recorded at the same time as bioimpedance and symptoms, and left ventricular ejection fractions may vary with intravascular volume.
Compared to earlier reports which had no objective measure of patient volume status, we measured changes in ECW, but our findings are supported by a recent study which noted that healthier patients with greater weight gains reported fewer symptoms [30]. Whereas other studies excluded patients with low intra-dialytic weight gains who may have been closer to their target weight [29] and noted the effect of faster ultrafiltration only at rates > 10 mL/kg/h, and other studies reporting an effect of ultrafiltration rates reporting rates > 15 mL/kg/h, so much higher than the rates used in our clinical practice [29, 30]. In addition, we used cooled dialysate, whereas many of the previous studies used a dialysate temperature of 37 °C, and cooling during dialysis has been shown to reduce the risk of hypotension and improve internal organ blood flows. As such, this cooling effect may have reduced symptom reporting compared to previous reports. Our study would suggest that symptoms are more likely to be reported when fluid is removed from patients starting dialysis with less ECW to be removed, which is in keeping with subjective patient interviews [14].
To determine whether a larger reduction in ECW was associated with increased intra-dialytic symptom reporting, we divided patients according to the change in aECW pre- to post-dialysis. As expected, the tertile with the greater reduction in aECW had greater percentage weight loss, ultrafiltration rate, and fall in systolic blood pressure. However, neither total dialysis symptom score nor any individual symptom score was greater in those patients with the greatest fall in aECW, and similarly, there was no effect on post-dialysis recovery time, and this is supported by a recent retrospective analysis [30]. Although one would expect that a fall in systolic blood pressure during dialysis would be associated with more intra-dialytic symptoms, studies have observed that patients starting dialysis with higher blood pressures and with more volume to be removed reported fewer symptoms [30, 34]. This may reflect that although blood pressure fell, those who were symptomatic started dialysis with lower blood pressures, so the effect of a subsequent reduction led to greater symptom reporting.
Interestingly, the group with the largest fall in aECW also dialysed with greater serum sodium to dialysate gradient. Although these patients may have been expected to suffer more cramps and other symptoms during dialysis [35], other studies which have objectively measured fluid shifts during dialysis failed to demonstrate and effect of the serum to dialysate gradient [36].
Patients with CKD treated by haemodialysis are recognised to have relatively high rates of self-reported fatigue and depression [4], and this may confound post-dialysis self-reported recovery times and fatigue [37]. We therefore used the distress thermometer, a tool more often used to determine psychological distress in cancer patients [16], to determine overall psychological wellbeing and found that distress thermometer scores were not different between pre-dialysis aECW groups, and also the change in aECW with dialysis. We used a dialysis symptom questionnaire that had been developed over 6 years [15, 16], and is similar to recently developed questionnaires [11]. The separation between distress thermometer scores and the dialysis symptom scores would suggest that the dialysis symptom questionnaire is not confounded by background health issues.
We report on the effect of changes in ECW measured using bioimpedance on patient-reported intra-dialytic symptoms. Our results may differ from previous reports due to differences in centre practices in terms of setting post-dialysis target weights, dialysate composition and temperature, patient demographics, and co-morbidity. We only measured changes in ECW and were not able to measure corresponding changes in cardiac output and vascular resistance, and changes in ECW may not reflect changes in intravascular volume [38], although patients closer to their target weight are more likely to have a reduction in cardiac output [39], so would be expected to have more symptoms.
On the other hand, we prospectively recorded intra-dialytic symptoms in a cohort of more than 500 patients with corresponding pre- and post-sessional ECW measurements.
Collaborative workshops between dialysis clinicians, health care providers, and patients have highlighted the need to improve the quality of life of dialysis patients [6,7,8]. It is therefore important to understand the causes of intra-dialytic symptoms. In this observational study of more than 500 patients, we found that by objectively measuring ECW, those patients with less ECW prior to dialysis were more likely to report intra-dialytic symptoms, particularly dizziness, feeling cold, and symptoms of low blood pressure. This highlights the need to carefully assess post-dialysis target weights, as overzealous fluid removal in patients closer to their target weight may lead to an increase in patient-reported intra-dialytic symptoms and more thought to prescribing dialysis treatments to sustain intra-vascular volume may potentially reduce patient symptoms and improve patient experiences.
Availability of data and materials
Located “R” drive audit file UCL Department of Renal Medicine, Royal Free Hospital
References
Haroon S, Davenport A. Choosing a dialyzer: what clinicians need to know. Hemodial Int. 2018;22(S2):S65–74.
Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R. Haemodialysis (HEMO) Study Group. Effect of dialysis dose and membrane flux in maintenance haemodialysis. N Engl J Med. 2002;347(25):2010–9.
Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305(11):1119–27.
Valderrábano F, Jofre R, López-Gómez JM. Quality of life in end-stage renal disease patients. AmJ KidDis. 2001;38(3):443–64.
Chilcot J, Almond MK, Guirguis A, Friedli K, Day C, Davenport A, Wellsted D, Farrington K. Self-reported depression symptoms in haemodialysis patients: Bi-factor structures of two common measures and their association with clinical factors. Gen Hosp Psychiatry. 2018;54:31–6.
Evangelidis N, Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, Crowe S, Harris T, Van Biesen W, Winkelmayer WC, Sautenet B, O'Donoghue D, Tam-Tham H, Youssouf S, Mandayam S, Ju A, Hawley C, Pollock C, Harris DC, Johnson DW, Rifkin DE, Tentori F, Agar J, Polkinghorne KR, Gallagher M, Kerr PG, SP MD, Howard K, Howell M, Craig JC. Standardized Outcomes in Nephrology–Hemodialysis (SONG-HD). Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am J Kidney Dis. 2017;70(4):464–75.
Tong A, Winkelmayer WC, Wheeler DC, van Biesen W, Tugwell P, Manns B, Hemmelgarn B, Harris T, Crowe S, Ju A, O'Lone E, Evangelidis N. Craig JC; SONG-HD. Nephrologists’ perspectives on defining and applying patient-centered outcomes in haemodialysis. Clin J Am Soc Nephrol. 2017;12(3):454–66.
Tong A, Manns B, Hemmelgarn B, Wheeler DC, Evangelidis N, Tugwell P, Crowe S, Van Biesen W, Winkelmayer WC, O'Donoghue D, Tam-Tham H, Shen JI, Pinter J, Larkins N, Youssouf S, Mandayam S, Ju A, Craig JC. SONG-HD Investigators. Establishing core outcome domains in haemodialysis: report of the Standardized Outcomes in Nephrology-Haemodialysis (SONG-HD) Consensus Workshop. Am J Kidney Dis. 2017;69(1):97–107.
Chilcot J, Guirguis A, Friedli K, Almond M, Davenport A, Day C, Wellsted D, Farrington K. Measuring fatigue using the Multidimensional Fatigue Inventory-20: a questionable factor structure in haemodialysis patients. Nephron. 2017;136(2):121–6.
Caplin B, Kumar S, Davenport A. Patients’ perspective of haemodialysis-associated symptoms. Nephrol Dial Transplant. 2011;26(8):2656–63.
Flythe JE, Dorough A, Narendra JH, Wingard RL, Dalrymple LS, DeWalt DA. Development and content validity of a hemodialysis symptom patient-reported outcome measure. Qual Life Res. 2019;28(1):253–65.
Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, Savoldi S, Fischer MS, Londrino F, Cancarini G. Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant. 2007;22(12):3547–52.
Assimon MM, Wenger JB, Wang L, Flythe JE. Ultrafiltration rate and mortality in maintenance haemodialysis patients. Am J Kidney Dis. 2016;68(6):911–22.
Cox KJ, Parshall MB, Hernandez SH, Parvez SZ, Unruh ML. Symptoms among patients receiving in-center hemodialysis: a qualitative study. Hemodial Int. 2017;21(4):524–33.
Caplin B, Alston H, Davenport A. Does online haemodiafiltration reduce intra-dialytic patient symptoms ? Nephron Clin Pract. 2013;124(3-4):184–90.
Alston H, Burns A, Davenport A. Loss of appendicular muscle mass in haemodialysis patients is associated with increased self-reported depression, anxiety and lower general health scores. Nephrology (Carlton). 2018;23(6):546–51.
Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17(6):1085–92.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95.
Booth J, Pinney J, Davenport A. N-terminal proBNP--marker of cardiac dysfunction, fluid overload, or malnutrition in haemodialysis patients? Clin J Am Soc Nephrol. 2010;5(6):1036–40.
Tangvoraphonkchai K, Riddell A, Davenport A. Platelet activation and clotting cascade activation by dialyzers designed for high volume online haemo-diafiltration. Hemodial Int. 2018;22(2):192–200.
Davenport A. Low-molecular-weight heparin as an alternative anticoagulant to unfractionated heparin for routine outpatient haemodialysis treatments. Nephrology (Carlton). 2009;14(5):455–61.
Sandhu E, Crawford C, Davenport A. Weight gains and increased blood pressure in outpatient haemodialysis patients due to change in acid dialysate concentrate supplier. Int J Artif Organs. 2012;35(9):642–7.
Fürstenberg A, Davenport A. Comparison of multifrequency bioelectrical impedance analysis and dual-energy X-ray absorptiometry assessments in outpatient haemodialysis patients. Am J Kidney Dis. 2010;57(1):123–9.
Papakrivopoulou E, Booth J, Pinney J, Davenport A. Comparison of volume status in asymptomatic haemodialysis and peritoneal dialysis outpatients. Nephron Extra. 2012;2(1):48–54.
Tangvoraphonkchai K, Davenport A. Changes in body composition following haemodialysis as assessed by bioimpedance spectroscopy. Eur J Clin Nutr. 2017;71(2):169–72.
Papakrivopoulou E, Lillywhite S, Davenport A. s N-terminal probrain-type natriuretic peptide a clinically useful biomarker of volume overload in peritoneal dialysis patients? Nephrol Dial Transplant. 2012;27(1):396–401.
van de Kerkhof J, Hermans M, Beerenhout C, Konings C, van der Sande FM, Kooman JP. Reference values for multifrequency bioimpedance analysis in dialysis patients. Blood Purif. 2004;22(3):301–6.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8.
Hussein WF, Arramreddy R, Sun SJ, Reiterman M, Schiller B. Higher ultrafiltration rate is associated with longer recovery time in patients undergoing conventional haemodialysis. Am J Nephrol. 2017;46:3–10.
Correa S, Pena-Esparragoza JK, Scovner KM, McCausland FR. Predictors of intradialytic symptoms: an analysis of data from the hemodialysis study. AmJKidDis. 2020;76:331–9.
Davenport A, Hussain Sayed R, Fan S. The effect of racial origin on total body water volume in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2011;6(10):2492–8.
Davenport A. Differences in prescribed Kt/V and delivered haemodialysis dose--why obesity makes a difference to survival for haemodialysis patients when using a ‘one size fits all’ Kt/V target. Nephrol Dial Transplant. 2013;28(Suppl 4):iv219–23.
Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int. 2014;86(3):489–96.
Yoowannakul S, Vongsanim S, Tangvoraphonkchai K, et al. Falls in systolic blood pressure during dialysis which require no nursing intervention are associated with increased patient intra-dialytic symptom self-reporting and prolonged post-dialysis recovery times. Ren Replace Ther. 2020;6:2. https://doi.org/10.1186/s41100-019-0249-0.
Basile C, Pisano A, Lisi P, Rossi L, Lomonte C, Bolignano D. High versus low dialysate sodium concentration in chronic haemodialysis patients: a systematic review of 23 studies. Nephrol Dial Transplant. 2016;31(4):548–63.
Kumar S, Khosravi M, Massart A, Potluri M, Davenport A. Are serum to dialysate sodium gradient and segmental bioimpedance volumes associated with the fall in blood pressure with haemodialysis? Int J Artif Organs. 2014;37(1):21–8.
Davenport A, Guirguis A, Almond M, Day C, Chilcot J, Da Silva GM, Fineberg N, Friedl K, Spencer B, Wellsted D, Farrington K. Post-dialysis recovery time is extended in patients with greater self-reported depression screening questionnaire scores. Hemodial Int. 2018;22(3):369–76.
Kotecha T, Martinez-Naharro A, Yoowannakul S, et al. Acute changes in cardiac structural and tissue characterisation parameters following haemodialysis measured using cardiovascular magnetic resonance. Sci Rep. 2019;9(1):1388.
Yoowannakul S, Vongsanim S, Kotecha T, et al. Haemodialysis patients with less extracellular water overload and smaller cardiac atrial chamber sizes are at greater risk of a fall in blood pressure during dialysis. Ther Apher Dial. 2020. https://doi.org/10.1111/1744-9987.13490 PMID: 32216122.
Acknowledgements
Drs Suree Yoowannakul, Kamonwan Tangvoraphonkchai, Surachet Vongsanim, and Ahmed Mohamed were in receipt of International Society of Nephrology and Kindey Research UK training scholarships.
Code availability
Not applicable
Funding
None
Author information
Authors and Affiliations
Contributions
Andrew Davenport registered the audit; Suree Yoowannakul, Kamonwan Tangvoraphonkchai, Surachet Vongsanim, and Ahmed Mohamed handed out and collected the questionnaires. Data analysis was by Andrew Davenport. All authors contributed to the first draft, and all authors approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Retrospective audit complies with NHS HRA guidelines. Consent to participate is not applicable.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yoowannakul, S., Vongsanim, S., Tangvoraphonkchai, K. et al. Patient-reported symptoms during dialysis: the effect of pre-dialysis extracellular water and change in extracellular water post-dialysis. Ren Replace Ther 7, 4 (2021). https://doi.org/10.1186/s41100-021-00321-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-021-00321-3