Abstract
Purpose
This study aimed to evaluate cytokine levels in plasma samples over time from living-donor renal transplant recipients with no evidence of pathological and clinical rejection at least 1 year post-procedure.
Methods
We examined plasma cytokine levels in 15 living-donor renal transplant recipients who were treated at our hospital from 2015 to 2018 and who presented with no evidence of pathological or clinical rejection for 1 year or longer. We collected blood samples before renal transplantation and at 1 week and 1 year post-procedure. We evaluated levels of 40 cytokines in plasma using Bio-Plex Pro™ Human Chemokine Assay kit.
Results
We detected no increase in plasma cytokine levels at either the 1 week or the 1 year time points. Plasma levels of 22 cytokines remained stable throughout and levels of 18 cytokine decreased after transplantation.
Conclusion
Plasma cytokine levels remained unchanged or were decreased in our patient cohort that included stable cases of living-donor renal transplantation. Our results suggest that renal transplantation may promote amelioration of chronic inflammation associated with end-stage renal failure and dialysis.
Similar content being viewed by others
Background
Approximately 38,000 patients begin renal dialysis each year in Japan, while the number of renal transplantations remains low, at 1800 a year. The 5-year rejection rate after living-donor renal transplantation is 7% and the 10-year rate is 15% according to the Factbook 2018; these numbers indicate that there is significant loss of transplant function over a relatively brief period of time. The most frequent reason for loss of transplant function is chronic rejection. Early diagnosis of chronic rejection is critical; once symptoms appear, including proteinuria and decreased renal function, treatment options are limited and unfavorable outcomes are likely. At the present time, chronic rejection is diagnosed by renal biopsy; there are no minimally invasive methods available for real-time diagnosis. Toward this end, we focused on plasma cytokines, as levels of these immune/inflammatory mediators may vary in response to surgical invasion and immunosuppressant therapy.
Previous reports have focused on an association between acute rejection within 3 months of the renal transplantation procedure and levels of serum cytokines [1,2,3,4]; others have focused on the association of cytokine levels with progression to end-stage renal failure, uremia, and modulations in response to dialysis [5]. However, to the best of our knowledge, there are no reports that have examined the changes in cytokine levels over time in a comprehensive manner for a period of 1 year or longer among patients with successful renal transplantation. Here, we evaluated levels of 40 distinct cytokines in 15 living-donor recipients both prior to and at time points after successful renal transplantation.
Materials and methods
This was a retrospective cohort study. Of the 30 living-donor renal transplantations carried out at the Department of Urology, Iwate Medical University, from 2015 to August 2018, we identified 15 cases that could be tracked for 1 year or longer who presented with no signs or symptoms of clinical or pathological rejection. One case is excluded because of acute rejection within 1 week after transplantation.
One out of 30 living-donor renal transplantations carried out at the Department of Urology, Iwate Medical University, from 2015 to August 2018 had acute rejection and 3 had chronic rejection. The rate of rejection of transplant cases at our hospital was almost the same as the reports so far.
We collected blood samples (10 ml in tubes with sodium ethylenediamine tetra-acetic acid (EDTA) anticoagulant) before renal transplantation, and at 1 week and 1 year after transplantation; samples were stored at 4 °C until initial processing. To evaluate plasma cytokine levels, blood cells were pelleted by centrifugation at 1500 rpm for 5 min; supernatants were separated and dispensed into micro Spitz tubes, and stored it at − 80 °C. The 40 cytokines evaluated using the Bio-Plex Pro™ Human Chemokine Assay (Bio-Rad, Hercules, CA, USA) include IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-16, CCL1, CCL2, CCL3, CCL7, CCL8, CCL11, CCL13, CCL15, CCL17, CCL19, CCL20, CCL21, CCL22, CCL23, CCL24, CCL25, CCL26, CCL27, CXCL1, CXCL2, CXCL5, CXCL6, CXCL9, CXCL10, CXCL11, CXCL12, CXCL13, CXCL16, CX3CL1, IFN-γ, MIF, GM-CSF, and TNF-α. We used statistical analysis software JMP11.0 (SAS Institute Japan Co.) and analyzed cytokine concentrations from blood plasma collected prior to transplantation, and at 1 week and 1 year after transplantation, by the paired t tests with p value < 0.05 as statistically significant.
Results
The average age of the patients in our study cohort was 32 years (range, 13 to 64 years); 11 of the transplant recipients were male and four were female. With respect to ABO blood typing, nine of the living-donor transplants were matched to the recipient, four were non-matched, and two were incompatible. With respect to HLA matching, 12 of the living-donor transplants had three mismatches, one case had four mismatches, and three cases had five mismatches. The mean period of dialysis prior to transplant was 12 months (range 0 to 72 months). Primary diagnoses included diabetic nephropathy (four cases), IgA nephropathy (three cases), hypoplastic kidney (two cases), antinuclear cytoplasmic antibody (ANCA)-associated glomerulonephritis (two cases), Alport syndrome (one case), and unknown etiology (three cases). The average age of the donors was 55 (range 24 to 70). The relationship of the donor to the recipient included father (four cases), mother (five cases), husband (one case), or wife (five cases; Table 1). The immunosuppressant regimens included basiliximab (BXM), methylprednisolone (mPSL), tacrolimus (FK), and mycophenolate mofetil (MMF). Clinical parameters including estimated glomerular filtration rate (eGFR), serum C-reactive protein (CRP), and white blood cell count (WBC), including cell differential (neutrophils (Neut)), were examined (Table 2).
There is no statistically significant difference in eGFR between at 1 week and 1 year after transplantation, and there are no clinically suggestive findings of transplant rejection (p = 0.622: paired t tests with p value < 0.05 as statistically significant). WBC and Neut increased significantly at 1 week after transplantation due to the effect of surgical invasion (p = 0.02, p = 0.0004), but 1 year after transplantation, there was no statistically significant difference and no findings suggestive of chronic inflammation or rejection were found. CRP showed no significant changes between at 1 week and 1 year after transplantation (p = 0.26, p = 0.19).
No statistically significant differences over time period including pre-procedure to 1 week or 1 year after transplantation were detected for plasma levels of IL-1β, IL-2, IL-6, IL-8, IL-10, IL-16, CCL2, CCL15, CCL17, CCL19, CCL20, CCL24, CXCL2, CXCL5, CXCL6, CXCL10, CXCL11, CXCL12, CXCL13, IFN-γ, MIF, and GM-CSF. Figure 1 includes a typical response.
Plasma cytokine levels that undergo significant decreases at 1 week but not at 1 year after transplantation include CCL11, CCL21, CCL26, CXCL1, and IL-4 (Fig. 2).
Plasma cytokines that undergo significant decreases both at 1 week and at 1 year after transplantation include CCL1, CCL3, CCL7, CCL8, CCL13, CCL22, CCL25, CCL27, TNF-α, CXCL9, and CX3CL1 (Fig. 3).
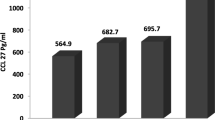
Plasma cytokines that undergo significant decreases at 1 year but not at 1 week after transplantation include CXCL16 and CCL23 (Fig. 4). Of particular note, we detected no significant increases in any of the plasma cytokines when comparing pre-procedure levels to those detected at either 1 week or 1 year after transplantation (Table 3).
Discussion
Numerous cytokines and chemokines are implicated in the pathogenesis of renal dysfunction [6,7,8,9]. Chemokines are cytokines with common structural features that function mainly to promote migration and infiltration of immune cells. Chemokines are further classified into CC chemokines, CXC chemokines, and CX3C chemokines based on the spacing of their characteristic cysteine residues; CC chemokines are primarily involved in promoting lymphocyte and macrophage infiltration while CXC chemokines primarily target polynuclear leukocytes [8]. However, much is still unknown regarding their roles in the pathogenesis of renal failure and/or modulating outcomes associated with renal transplantation. Several reports have documented an association between specific cytokines and acute rejection occurring within three months of a transplantation procedure [1,2,3]; another report documented an association between circulating cytokine levels and end-stage renal failure, uremia, and dialysis [5]. There are no similar reports that include a comprehensive assessment of the changes in circulating cytokine levels a period of 1 year or longer in patients with successful renal transplants. In this study, we focused on the changes in plasma cytokine concentrations over time in patient who underwent a successful live-donor renal transplantation.
We were also able to classify the 15 patient cases based on the cytokines responses to include group 1 in which no changes were identified throughout and group 2 in which cytokine levels decreased after the transplantation procedure.
Cytokines in group 1 are cytokine levels that did not change between before transplantation and 1 year after transplantation. Cytokines in group 2 are cytokine levels that decreased 1 year after transplantation compared to before transplantation (Fig. 5).
Furthermore, although we did not include these cases in our examination due to the small sample size, our preliminary examination of three cases that presented with chronic rejection revealed increased levels of six specific plasma cytokines, including IL-2, IL-6, CXCL5, CXCL6, CXCL16, CCL24, and GM-CSF, at 1 year after renal transplantation (Supplementary Fig. 1). These results suggest that we may be able to detect chronic rejection at an early stage by monitoring one or more of these cytokines on a more or less regular basis after the transplant.
We were also interested in exploring the findings in group 2, which includes patients in which specific cytokines underwent a significant decrease after transplantation. Previous reports have suggested that patients with end-stage renal failure or uremia, and/or who were undergoing dialysis before renal transplantation, may have elevated levels of plasma cytokines due to the chronic activation of monocytes and macrophages [5]. Activation of macrophages results in the synthesis and release of inflammatory cytokines including TNFα and secondary increases in levels of CCL1 and CX3CL1 [10, 11]. Activated macrophages also release CCL3, CCL7, CCL8, CCL13, and CXCL9 [12]. As such, baseline levels of TNFα, CCL1, CX3CL1, CCL3, CCL7, CCL8, and CCL13 may be comparatively high immediately prior to the renal transplantation procedure; levels of these cytokines will then undergo a gradual and significant decline and will remain at this lower level in the absence of transplant rejection.
Ischemia-reperfusion-related issues occurring in response to renal transplantation may have an impact on the values identified immediately post-procedure. Macrophages have been reported as involved in ischemia-reperfusion disorder [13] in which they synthesize and secrete IL-1β, IL-6, and TNFα; elevated levels of IL-1β result in increased levels of CCL23 [14]. Moreover, as CXCL16 may promote arteriosclerosis associated with end-stage renal failure [15], an examination of the role of renal transplantation and the progress of arteriosclerosis may be worthwhile.
Cytokines have multiple receptors that are detected on numerous cell types. Given the complexities associated with cytokine—cell receptor interactions that may serve to augment or to neutralize one other, we are unable to provide a full interpretation of our findings. We still do not know much about the detailed functions of each cytokine or chemokine in all given situations and microenvironments; this is a critical issue that requires further elucidation.
Among the limitations of this study, we recognize that this is a retrospective study. However, we have obtained complete and detailed information for each patient from our multi-year database. We also recognize that the results are based on findings from patients in a single-center study and that the number of cases is small. Further research will permit us to explore its generalized applicability.
Conclusions
In this study, we examined plasma cytokine levels over time in 15 patients who underwent living-donor renal transplantation at our hospital and who did not present with pathological or clinical rejection for a period of 1 year or longer. In this stable cohort, we identified no significant increase in levels of 40 distinct cytokines when comparing levels at 1 week and 1 year after transplantation to those evaluated pre-procedure; most plasma levels were stable throughout, and some decreased.
As such, we conclude that renal transplantation does not promote increases in cytokine levels that would be indicative of an ongoing inflammatory response. Based on these findings, we hope to explore the possibility that early stage chronic rejection might be monitored via changes in specific plasma cytokines over time.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ANCA:
-
Antinuclear cytoplasmic antibody
- BMX:
-
Basiliximab
- mPSL:
-
Methylprednisolone
- FK:
-
Tacrolimus
- MMF:
-
Mycophenolate mofetil
- eGFR:
-
Estimated glomerular filtration rate
- CRP:
-
Serum C-reactive protein
- WBC:
-
White blood cell
- Neut:
-
Neutrophils
References
Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int. 2005;67:1216–33.
Tefik T, Ciftci HS, Karadeniz MS, et al. Predictive value of interleukin 2 and interleukin 8 on early rejection in living related kidney transplant recipients. Transplant Proc. 2019;51:1078–81.
Lo DJ, Weaver TA, Kleiner DE, et al. Chemokines and their receptors in human renal allotransplantation. Transplantation. 2011;91:70–7.
Rotondi M, Rosati A, Buonamano A, et al. High pretransplant serum levels of CXCL10/IP-10 are related to increased risk of renal allograft failure. Am J Transplant. 2004;4:1466–74.
Akalin E, Dikman S, Murphy B, et al. Glomerular infiltration by CXCR3+ ICOS+ activated T cells in chronic allograft nephropathy with transplant glomerulopathy. Am J Transplant. 2003;3:1116–20.
Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–701.
Turner JE, Paust HJ, Steinmetz OM, Panzer U. The Th17 immune response in renal inflammation. Kidney Int. 2010;77:1070–5.
Stasikowska O, Wagrowska-Danilewicz M. Chemokines and chemokine receptors in glomerulonephritis and renal allograft rejection. Med Sci Monit. 2007;13:Ra31–6.
Wada T, Yokoyama H, Furuichi K, et al. Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor (MCAF/MCP-1). Faseb j. 1996;10:1418–25.
Gombert M, Dieu-Nosjean MC, Winterberg F, et al. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol. 2005;174:5082–91.
Nishimura M, Umehara H, Nakayama T, et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168:6173–80.
Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–81.
Jo SK, Sung SA, Cho WY, et al. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant. 2006;21:1231–9.
Forssmann U, Delgado MB, Uguccioni M, et al. CKbeta8, a novel CC chemokine that predominantly acts on monocytes. FEBS Lett. 1997;408:211–6.
Hu ZB, Chen Y, Gong YX, et al. Activation of the CXCL16/CXCR6 Pathway by Inflammation Contributes to Atherosclerosis in Patients with End-stage Renal Disease. Int J Med Sci. 2016;13:858–67.
Acknowledgements
We thank members of the Department of Urology, Iwate Medical University, for their support. The authors would like to thank Enago (www.enago.jp ) for the English language review.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
AI constructed the figures and tables and performed the statistical analyses. JS contributed to the preparation of the manuscript and all aspects of data collection and analysis. TM, TA, and WO assisted with clinical data. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Research Committee, Iwate Medical University. All procedures were performed in accordance with the ethical standards of Iwate Medical University and with the Declaration of Helsinki. An alternative of informed consent (approved by the Institutional Review Board of Iwate Medical University) was obtained from all patients included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ito, A., Sugimura, J., Matsuura, T. et al. Plasma cytokine levels before and 1 year after successful living-donor renal transplantation. Ren Replace Ther 6, 48 (2020). https://doi.org/10.1186/s41100-020-00298-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-020-00298-5