Abstract
Background
A core collection is a subset of an entire collection that represents as much of the genetic diversity of the entire collection as possible. The establishment of a core collection for crops is practical for efficient management and use of germplasm. However, the establishment of a core collection of mushrooms is still in its infancy, and no established core collection of the economically important species Flammulina velutipes has been reported.
Results
We established the first core collection of F. velutipes, containing 32 strains based on 81 genetically different F. veltuipes strains. The allele retention proportion of the core collection for the entire collection was 100%. Moreover, the genetic diversity parameters (the effective number of alleles, Nei’s expected heterozygosity, the number of observed heterozygosity, and Shannon’s information index) of the core collection showed no significant differences from the entire collection (p > 0.01). Thus, the core collection is representative of the genetic diversity of the entire collection. Genetic structure analyses of the core collection revealed that the 32 strains could be clustered into 6 groups, among which groups 1 to 3 were cultivars and groups 4 to 6 were wild strains. The wild strains from different locations harbor their own specific alleles, and were clustered stringently in accordance with their geographic origins. Genetic diversity analyses of the core collection revealed that the wild strains possessed greater genetic diversity than the cultivars.
Conclusion
We established the first core collection of F. velutipes in China, which is an important platform for efficient breeding of this mushroom in the future. In addition, the wild strains in the core collection possess favorable agronomic characters and produce unique bioactive compounds, adding value to the platform. More attention should be paid to wild strains in further strain breeding.
Similar content being viewed by others
Background
A core collection is a subset of accessions that presents the maximum possible genetic diversity contained in an entire collection with minimum redundancy [1, 2]. The establishment of a core collection for crops is practical for efficient management of germplasm. Core collections of most major food crops, such as Oryza sativa, Zea mays, Glycine max and Triticum aestivum, have already been established [3,4,5,6,7].
A core collection is traditionally constructed based on morphological and agronomic characters using different strategies, such as the constant allocation (C) strategy, the logarithm (L) strategy, the proportional allocation (P) strategy, and the random sampling (R) strategy [2, 8,9,10]. However, most morphological and agronomic characters are quantitative traits that can be easily affected by environmental variation [11,12,13]. Therefore, phenotypic data cannot directly reflect the genetic diversity of germplasm resources [11].
Conversely, molecular markers can directly reflect a germplasm’s genetic diversity at the DNA sequence level. Compared with other molecular markers, simple sequence repeats (SSRs) are randomly repeated DNA sequences, generally 1 to 6 base pairs in length per unit. SSRs can spread extensively throughout a genome. They are typically co-dominant, highly polymorphic, reproducible and easy to score [14,15,16]. Based on molecular marker data, Kim et al. [17] developed software named PowerCore by applying the advanced maximization (M) strategy with heuristic searching to establish a core collection (allele mining collection); it allows all alleles to be captured in a minimum number of accessions [17]. It has been successfully used with many economically important crops, such as Oryza sativa, Glycine max, Olea europaea, Vigna radiata, and Sesamum indicum and has been proven to be most suitable for establishing a core collection based on molecular data [18,19,20,21,22].
However, the development of core collections of edible mushrooms is still at an early stage, and core collections have been established only in Pleurotus ostreatus and Lentinula edodes [23,24,25]. Flammulina velutipes is cultivated on a large scale in East Asia [26,27,28]. China is currently the largest producer of F. velutipes, with an annual production of 2.4 million tons [29]. In our previous study, we obtained 124 strains (110 cultivars from the spawn market of China and 14 wild strains from Yunnan, Sichuan, and Hunan provinces), and excluded cultivars labeled with confusing names, then screened out 81 strains that are genetically different [30]. In order to efficiently manage and utilize of these genetically different strains, a smaller representative core collection without redundant strains is urgently needed.
In this study, we aimed to (i) establish the core collection of F. velutipes; (ii) evaluate the genetic diversity of the core collection and the entire collection; and (iii) analyze the core collection’s genetic structure.
Methods
Strain materials and DNA extraction
We used 81 strains of F. velutipes in this study, including 67 cultivars and 14 wild strains (Additional file 1: Table S1). Genomic DNA was extracted for each strain with the CTAB-based method [31]. In each case, fresh mycelium was harvested from potato dextrose agar medium after inoculation for 10 days at 23 °C. The DNA concentration and purity were measured with a NanoDrop2000 spectrophotometer. The DNA solution of each sample was diluted to 100 ng/μl.
SSR genotyping
The 25 polymorphic SSR markers used in this study were developed by our previous study [30]. The forward primer of each SSR was labeled with fluorescent dye (FAM) at the 5′ end (TSINGKE, Kunming). Polymerase chain reactions (PCR) were carried out in a total volume of 25 μl, containing 1 μl template DNA, 1 μl bovine serum albumin, 2.5 μl reaction buffer, 0.5 μl deoxynucleoside triphosphate, 1 μl for each primer, 0.3 μl Taq DNA polymerase, and 17.7 μl ddH2O. PCR was conducted on an ABI 2720 Thermal Cycler (Applied Biosystems, Foster City, CA) or an Eppendorf Master Cycler (Netheler-Hinz, Hamburg, Germany) under the following parameters: 94 °C for 4 min, then 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, followed by a final extension step of 72 °C for 8 min. The PCR products were run in an ABI 3730 Genetic Analyzer using GeneScan 500 Rox as a size standard (Applied Biosystems); after a denaturation step at 98 °C for 5 min and shock chilling on ice, alleles of each locus were scored in base pairs with the GeneMapper v3.2 software package (Applied Biosystems), the size of the PCR products for each SSR was recorded in an Excel spreadsheet.
Development of a core collection for F. velutipes
The core collection was established based on genotyping data using PowerCore software [17]. The heuristic algorithm that finds the optimum path from the initial to the final stages for sample selection was used (http://genebank.rda).
Data analysis
The genetic diversity parameters (the effective number of alleles Ae, Nei’s expected heterozygosity H, the number of observed heterozygosity Ho, and Shannon’s information index I) and allele frequency of the core collection and of the entire collection were established with PopGene v1.31 [32]. A dendrogram of the genetic relationships among the core collection strains was constructed based on the simple matching (SM) coefficient by applying the unweighted pair group method with arithmetic mean (UPGMA) using the NTSYSpc v2.10e [33]. The genetic structure of the core collection was analyzed with STRUCTURE v2.3.4 based on an admixture model. Models were tested for K-values ranging from 2 to 10, with 10 independent runs per K value. For each run, the initial burn-in period was set to 100,000 with 100,000 MCMC iterations. To determine the most probable value of K, the deltaK method was used and implemented in Structure Harvester [34, 35].
Results
Core collection construction
A total of 153 alleles were amplified by 25 SSRs in the 81 strains [30]. In this study, based on PowerCore calculation, the 153 alleles could be represented using a minimum of 32 strains, including 19 cultivars and 13 wild strains (Table 1). This finding suggests that the 32 strains could be a core collection of the 81 strains. The core collection sampling proportion is about 39.5%: for cultivars about 27.9%, and for wild strains about 92.9%.
Genetic diversity of the core collection
Statistics to describe the genetic diversity of the core collection and the entire collection for 25 SSR markers are summarized in Table 2. The t-tests of the mean genetic diversity parameters (Ae, H, Ho, I) of the core collection and the entire collection were non-significant (p > 0.01) (Table 2), which reveals that the genetic diversity of the core collection has no significant difference from that of the entire collection. In addition, based on an UPGMA dendrogram of the core collection and the entire collection (Fig. 1), the strains in different groups of the entire collection were uniformly selected for the core collection. Thus, the core collection could represent the genetic diversity of the entire collection. However, the distributions of allele frequency differ between the entire collection and the core collection (Additional file 2: Table S2).
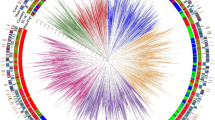
UPGMA dendrogram of core collection (32 accessions) and entire collection (81 accessions) of F. velutipes constructed based on genetic similarity coefficients of SSR data in the core collection and entire collection, respectively. Strains connected with dashed lines show placement of core collection strains in entire collection
We further investigated the genetic diversity parameters (Ae, H, Ho, I) of the cultivars and wild strains in core collection, which demonstrated that the wild strains possess greater genetic diversity than the cultivars (Table 3).
Among the 153 alleles in the core collection, 72 (47%) were specific for each group and could differentiate the six groups from each other (Fig. 2). Group 6 had the highest number of specific alleles (30), followed by group 4 (13), group 5 (11), group 1 (8), group 2 (7), and group 3 (3) (Fig. 2). Nearly 53% of the alleles (81 of 153) were common to all the groups and can thus be categorized as conserved alleles (Fig. 2).
Genetic structure of the core collection
The admixture model-based clustering method was used in the STRUCTURE program to infer the genetic structure of the core collection. The optimum number of K was analyzed using delta K (ΔK). A strong peak of ΔK is six, which indicated that there were six groups in the core collection (Fig. 3). The cultivars were assigned to groups 1 to 3, and the wild strains were assigned to groups 4 to 6 (Fig. 4).
A similar result was also shown in the dendrogram constructed with the UPGMA method (Fig. 1). For the cultivars, white strains were assigned to groups 2 and 3, and yellow strains were distributed throughout groups 1 to 3. The wild strains were clustered in groups 4 to 6, and each group was stringently in accordance with its geographic origins. The strains in group 4 were collected from Hunan Province, with the exception of strain F77, which was purchased from Spawn Company in Jilin Province in northeastern China. This strain shares similar alleles with the strains collected from Hunan Province, indicating that it may have been isolated from Hunan Province. The strains in groups 5 and 6 were collected from Sichuan and Yunnan Provinces, respectively.
Discussion
Representation of the core collection
The successful formation of a core collection depends on maximum allelic representation efficiency and elimination of redundancy from the entire collection [2]. In this study, we successfully developed a core collection of F. velutipes with 100% allelic representation under 39.5% of the sampling proportion (28.4% for cultivars and 92.9% for wild strains) based on 25 SSR markers. The genetic diversity parameters of the core collection could represent of the entire collection. And the strains selected in the core collection can represent the different allele components of each group in the entire collection (Fig. 1). Our results proved that the advanced M strategy is powerful in capturing 100% allelic diversity in a core collection [17, 22]. The differences of the allele frequency between the entire collection and the core collection may be due to the redundant alleles including some homozygous loci that were excluded during the core collection construction.
Further strain improvement of F. velutipes based on the core collection
Most crops inevitably undergo a drastic loss of genetic diversity during cultivation, and F. velutipes is no exception [30, 36,37,38]. Lower genetic diversity among cultivars may lead to inbreeding depression [39]. Thus, the core collection established in this study could effectively protect the cultivars’ heterogeneous germplasm resources and help avoid inbreeding depression for further strain improvement. Furthermore, the specific alleles harbored in different groups of cultivars may indicate different agronomic characters in each group. For example, in our previous experiment, the mycelium growth rate at 23 °C of a yellow strain F1 (6.38 ± 0.1 mm/d) in group 1 was significantly greater than that of the industrialized white strain F3 (6.35 ± 0.07 mm/d) (p < 0.01) (unpublished data). Therefore, F1 could be used to crossbreed with the industrialized white strains to facilitate mycelium growth and shorten the production time.
The wild strains harbor higher genetic diversity than the cultivars in the core collection. Meanwhile, several economically important agronomic traits, such as tolerance to high temperature and rich contents of sesquiterpenes, can also be found in wild strains [30, 40]. In the cultivation of F. velutipes, temperature is usually an important limiting factor. The cultivars’ fruiting temperature needed for stringent control is less than 15 °C, which will result in high energy costs [30]. However, we have gathered several wild strains of F. velutipes from subtropical regions of China in summer, despite most of the wild strains of this species mainly forming fruiting bodies in winter. Thus, those wild strains are ideal samples to domesticate for tolerance to high temperatures. In fact, we did find a wild strain (F98), collected from Longling, Yunnan, that can grow more vigorously than cultivars at a higher temperature (18 °C) [30]. Further analyses on chemical components showed that this strain contains 15 new sesquiterpenes with various skeletons, some of which showed moderate antidiabetes and antitumor bioactivity [40]. Thus, it is quite essential to keep as many wild strains as possible in constructing a core collection for F. velutipes.
Conclusions
In conclusion, we have established the first core collection of F. velutipes in China, which is an important platform for efficient breeding of this mushroom in the future. The core collection is representative of the entire collection. In addition, the wild strains in the core collection possess favorable agronomic characters and produce unique bioactive compounds, adding value to the platform. More attention should be paid to wild strains in further strain breeding.
References
Frankel OH, AHD B. Current plant genetic resources-a critical appraisal. In genetics: new frontiers, vol. 4. New Delhi: Oxford & IBH Publishing Co; 1984. p. 1–11.
Brown AHD. Core collections: a practical approach to genetic resources management. Genome. 1989;31:818–24.
Li Y, Shi Y, Cao Y, Wang T. Establishment of a core collection for maize germplasm preserved in Chinese National Genebank using geographic distribution and characterization data. Genet Resour Crop Evol. 2005;51:845–52.
Hao CY, Zhang XY, Wang LF, Dong XW, Shang XW, Jia JZ. Genetic diversity and core collection evaluations in common wheat germplasm from the northwestern spring wheat region in China. Mol Breeding. 2006;17:69–77.
Balfourier F, Roussel V, Strelchenko P, Exbrayat-Vinson F, Sourdille P, Boutet G, et al. A worldwide bread wheat core collection arrayed in a 384-well plate. Theor Appl Genet. 2007;114:1265–75.
Oliveira MF, Nelson RL, Geraldi IO, Cruz CD, de Toledo JFF. Establishing a soybean germplasm core collection. Field Crops Res. 2010;119:277–89.
Zhao W, Cho GT, Ma KH, Chung JW, Gwag JG, Park YJ. Development of an allele-mining set in rice using a heuristic algorithm and SSR genotype data with least redundancy for the post-genomic era. Mol breeding. 2010;26:639–51.
Holbrook CC, Anderson WF, Pittman RN. Selection of a core collection from the US germplasm collection of peanut. Crop Sci. 1993;33:859–61.
Hintum TJ, Bothmer R, Visser DL. Sampling strategies for composing a core collection of cultivated barley (Hordeum vulgare s. Lat.) collected in China. Hereditas. 1995;122:7–17.
Ortiz R, Ruiz-Tapia EN, Mujica-Sanchez A. Sampling strategy for a core collection of Peruvian quinoa germplasm. Theor Appl Genet. 1998;96:475–83.
Hu J, Zhu J, Xu HM. Methods of constructing core collections by stepwise clustering with three sampling strategies based on the genotypic values of crops. Theor Appl Genet. 2000;101:264–8.
Xiao Y, Liu W, Dai Y, Fu C, Bian Y. Using SSR markers to evaluate the genetic diversity of Lentinula edodes’ natural germplasm in China. World J Microbiol Biotechnol. 2010;26:527–36.
Zhang RY, Hu DD, Gu JG, Hu QX, Zuo XM, Wang HX. Development of SSR markers for typing cultivars in the mushroom Auricularia auricula-judae. Mycol Prog. 2012;11:587–92.
Zane L, Bargelloni L, Patarnello T. Strategies for microsatellite isolation: a review. Mol Ecol. 2002;11:1–16.
Freeland JR. Molecular Ecology. In: Molecular markers in ecology. Thomson Press: Chichester; 2005. p. 43–57.
Selkoe KA, Toonen RJ. Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecol Lett. 2006;9:615–29.
Kim KW, Chung HK, Cho GT, Ma KH, Chandrabalan D, Gwag JG, et al. PowerCore: a program applying the advanced M strategy with a heuristic search for establishing core sets. Bioinformatics. 2007;23:2155–62.
Zhang Y, Zhang X, Hua W, Wang L, Zhuo C. Analysis of genetic diversity among indigenous landraces from sesame (Sesamum indicum L.) core collection in China as revealed by SRAP and SSR markers. Genes Genom. 2010;32:207–15.
Belaj A, del Dominguez-García MDC, Atienza SG, Urdíroz NM, Rosa RD, Satovic Z, et al. Developing a core collection of olive (Olea europaea L.) based on molecular markers (DArTs, SSRs, SNPs) and agronomic traits. Tree Genet Genomes. 2012;8:365–78.
Kaga A, Shimizu T, Watanabe S, Tsubokura Y, Katayose Y, Harada K, et al. Evaluation of soybean germplasm conserved in NIAS genebank and development of mini core collections. Breed Sci. 2012;61:566–92.
Moe KT, Gwag JG, Park YJ. Efficiency of PowerCore in core set development using amplified fragment length polymorphic markers in mungbean. Plant Breed. 2012;131:110–7.
Tiwari KK, Singh A, Pattnaik S, Sandhu M, Kaur S, Jain S, et al. Identification of a diverse mini-core panel of Indian rice germplasm based on genotyping using microsatellite markers. Plant Breed. 2015;134:164–71.
Li H, Chen Q, Huang CY, Xie BG, Zhang A. A method for establishing core collection of Pleurotus ostreatus cultivated in China based on SSR markers. Acta Hortic Sin. 2012;39:2023–32.
Zhang XM, Yao Q, Gong ZY, Gao XX, Li J, Liu X, et al. Development of EST-SSR markers in Pleurotus ostreatus and construction of primary core collection. Mycosystema. 2013;32:64–80.
Liu J, Wang Z-R, Li C, Bian Y-B, Xiao Y. Evaluating genetic diversity and constructing core collections of Chinese Lentinula edodes cultivars using ISSR and SRAP markers. J Basic Microbiol. 2015;55:749–60.
Mizuno R, Ichinose H, Honda M, Takabatake K, Sotome I, Takai T. Use of whole crop sorghums as a raw material in consolidated bioprocessing bioethanol production using Flammulina velutipes. Biosci Biotechnol Biochem. 2009;73:1671–3.
Van Peer AF, Park SY, Shin PG, Jang KY, Yoo YB, Park YJ, et al. Comparative genomics of the mating-type loci of the mushroom Flammulina velutipes reveals widespread synteny and recent inversions. PLoS One. 2011;6:e22249.
Shi M, Yang Y, Guan D, Zhang Y, Zhang Z. Bioactivity of the crude polysaccharides from fermented soybean curd residue by Flammulina velutipes. Carbohydr Polym. 2012;89:1268–76.
Li X, Li Y. Quality comparison and analysis on white Flammulina velutipes grown with bottle lines in China. Edible Fungi China. 2014;33:20–4.
Liu XB, Feng B, Li J, Yan C, Yang ZL. Genetic diversity and breeding history of winter mushroom (Flammulina velutipes) in China uncovered by genomic SSR markers. Gene. 2016;591:227–35.
Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–5.
Yeh FC, Yang RC, Boyle T. POPGENE, version 1.31. Microsoft window-based freeware for population genetic analysis. Alberta: University of Alberta; 1999. p. 1–28.
Rohlf FJ. Phylogenetic models and reticulations. J Classif. 2000;17:185–9.
Evanno G, Regnaut S. Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–20.
Earl DA. vonHoldt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour. 2012;4:359–61.
Reif JC, Hamrit S, Heckenberger M, Schipprack W, Maurer HP, Bohn M, et al. Trends in genetic diversity among European maize cultivars and their parental components during the past 50 years. Theor Appl Genet. 2005;111:838–45.
Doebley JF, Gau BS, Smith BD. The molecular genetics of crop domestication. Cell. 2006;127:1309–21.
Hyten DL, Song Q, Zhu Y, Choi IY, Nelson RL, Costa JM, et al. Impacts of genetic bottlenecks on soybean genome diversity. Proc Natl Acad Sci U S A. 2006;103:16666–71.
Xu J. Analysis of inbreeding depression in Agaricus bisporus. Genet Soc Am. 1995;141:137–45.
Tao Q, Ma K, Yang Y, Wang K, Chen B, Huang Y, et al. Bioactive Sesquiterpenes from the edible mushroom Flammulina velutipes and their biosynthetic pathway confirmed by genome analysis and chemical evidence. J Org Chem. 2016;81:9867–77.
Acknowledgements
The authors are grateful to Dr. Min Zhang (Liaoning Academy of Agricultural Sciences), Dr. Yong-Chang Zhao (Yunnan Academy of Agricultural Sciences), Dr. Jin-Xia Zhang (Chinese Academy of Agricultural Sciences), Dr. Bao-Gui Xie (Fujian Agriculture and Forestry University), Dr. Ping Zhang (Hunan Normal University) and Mr. Pan-Meng Wang (Kunming Institute of Botany, Chinese Academy of Sciences) for providing strains for this study. Prof. Dr. Won-Sik Kong (Mushroom Research Division, National Institute of Horticultural and Herbal Science, Rural Development Administration, Eumsung, Republic of Korea) is acknowledged for generously sharing the genome sequencing data of the strain KACC42780 for us. We also thank Dr. Bang Feng and Ms. Yang-Yang Cui (Kunming Institute of Botany, Chinese Academy of Sciences) for improving the manuscript. The anonymous reviewers are also gratefully acknowledged for their comments and suggestions. Dr. Heather Hallen-Adams (Food Science and Technology, University of Nebraska-Lincoln) is acknowledged for having polished the English.
Funding
This study was supported by the National Basic Research Program of China (973 Program, No. 2014CB138305).
Availability of data and materials
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
ZLY and XBL conceived and designed the experiments. XBL and JL collected the strains. XBL generated the DNA sequences and analyzed the data. All authors wrote the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
“Not applicable”.
Consent for publication
“Not applicable”.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Strains used in this study. (DOC 82 kb)
Additional file 2:
Allele frequence distributions between entire collection and core collection. (XLS 46 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, X.B., Li, J. & Yang, Z.L. Genetic diversity and structure of core collection of winter mushroom (Flammulina velutipes) developed by genomic SSR markers. Hereditas 155, 3 (2018). https://doi.org/10.1186/s41065-017-0038-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41065-017-0038-0