Abstract
Objective
Abdominal aortic calcification (AAC) is an important marker of subclinical atherosclerosis and a predictor of cardiovascular disease. This study aims to explore the association between carotenoid intakes and AAC.
Methods
We included 2889 participants from the National Health and Nutrition Examination Survey (NHANES). Dietary carotenoid intakes were obtained through 24-h dietary recall interviews. Severe AAC was defined as a Kauppila score > 5. The main analysis utilizes logistic and restricted cubic spline models.
Result
Severe AAC was detected in 378 (13.08%) participants. In fully adjusted models, the odds ratios (OR) with 95% confidence intervals (CI) of α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein with zeaxanthin and total carotenoid intakes for individuals with severe AAC were 0.53 (0.23–0.77), 0.39 (0.19–0.80), 0.18 (0.05–0.62), 0.40 (0.20–0.78), 0.53 (0.32–0.88) and 0.38 (0.18–0.77) in the highest versus lowest quartile intake, respectively. Dose–response analyses revealed that all of the carotenoids were associated with decreased risk of severe AAC in a nonlinear trend. Total carotenoid intakes of at least 100ug/kg/day were associated with decreased odds for severe AAC.
Conclusion
α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein with zeaxanthin and total carotenoids were inversely associated with the risk of severe AAC in adults.
Similar content being viewed by others
Introduction
Atherosclerosis, a chronic inflammatory disease of large arteries, is a leading cause of cardiovascular diseases [1, 2]. Atherosclerosis has become an epidemic due to population growth and aging, and increased prevalence of comorbidities such as obesity and diabetes among others [3,4,5]. The abdominal aorta is among the earliest sites to develop atherosclerosis and calcification, which is a good marker of subclinical atherosclerosis and a predictor of cardiovascular disease [6, 7]. The absence of AAC is highly effective at ruling out coronary artery disease [8, 9]. The prevalence of AAC increases with age, present in 22.4% of males and 16.4% of females age under 45 years and 100% of both males and females at 75 years and older [10]. Therefore, early detection of AAC is of great significance in the primary prevention of cardiovascular disease.
Unhealthy lifestyle and diet are important contributors to atherosclerosis. More intake of fruits, vegetables and seaweed reduces the risk of atherosclerosis [11,12,13]. Carotenoids are fat-soluble pigments with antioxidant properties that are naturally present in fruits, vegetables and seaweed [14]. The relationship between carotenoid intakes and hypertension [15], depression [16] and cancer [17] has been reported in epidemiological studies. Li demonstrated that at least 100 ug/kg per day intakes of carotenoids was associated with lower risk of hypertension [15]. Ge found a U-shaped dose–response relationships between both β-carotene and lutein with zeaxanthin intakes and the risk of depressive symptoms [16]. Previous studies have reported that high dosage of lycopene is associated with lower risk of prostate cancer [17] and AAC [18]. However, the association between carotenoid intakes and AAC has not been fully investigated.
In this study, using data from National Health and Nutrition Examination Survey (NHANES), we aimed to explore the relationship between carotenoid intakes and AAC.
Methods
Study population
The data on carotenoid intakes and AAC were obtained from NHANES 2013–2014 cohort. NHANES is a nationally representative survey, designed to assess the health and nutritional status of the civilian noninstitutionalized population in the 50 US states and the District of Columbia. The relevant information from the survey is publicly available at https://www.cdc.gov/nchs/nhanes/ [19].
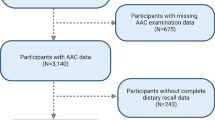
The NHANES 2013–2014 cohort included 10,175 participants. Participants with incomplete information on both carotenoid intakes (n = 7035) and AAC (n = 243) were excluded. Furthermore, we excluded participants with extreme dietary energy intake (male: < 500 or > 8000 kcal/day, female: < 500 or > 5000 kcal/day). The final analytical samples included 2889 individuals. Details are provided in Fig. 1.
Dietary carotenoid intake assessment
NHANES database provides information on dietary carotenoids directly. The dietary carotenoid intakes of all included participants were obtained through two 24-h dietary recall interviews. The first diet recall interview was conducted in person at the Mobile Examination Center, and the second was conducted by telephone 3–10 days later. Each participant reports the type and amount of all food and beverages consumed 24 h prior to the interview. Multiple measurement guides (such as glasses, bowls, mugs, drink boxes, bottles, etc.) were used to estimate the amounts of food. Dietary data in NHANES were quantified by the Nutrition Methodology Working Group. In our study, the intake of α-carotene, β-carotene, β-cryptoxanthin, lycopene and lutein with zeaxanthin was calculated by averaging over the two recall periods. Total carotenoids were defined as the sum of the aforementioned five dietary carotenoids.
AAC evaluation
AAC was assessed from lumbar lateral spine dual-energy X-ray absorptiometry scans (DXA, Densitometer Discovery A, Hologic, Marlborough, MA, USA). The Kauppila score was used to quantify the presence and severity of AAC. In the Kauppila score system, both anterior and posterior aortic walls were divided into four segments, corresponding to the region in front of the lumbar vertebrate L1–L4. The score 0 represents no calcification, 1 (≤ 1/3 of the aortic wall), 2 (> 1/3 to ≤ 2/3 of the aortic wall) or 3 (> 2/3 of the aortic wall) for these four segment. The Kauppila AAC score is the sum of calcification score, resulting in a range from “0” to “6” for each segment and “0” to “24” for the total score. According to previous studies, a higher AAC score corresponded to a more serious calcification condition of the abdominal aorta and severe AAC is defined as an AAC score > 5 [20].
Covariates
Standard questionnaires were used to collect age, gender, race, family income, smoking status, physical activity and alcoholic intake. Race was classified as Hispanic, non-Hispanic white, non-Hispanic black or other race. Family monthly poverty level index were categorized as ≤ 1.30, 1.31 to 3.50 and > 3.50 (richest) [21]. Smoking status were categorized as current smoker, former smoker and never smoker based on whether they were currently smoking and smoked at least 100 cigarettes in his/her lifetime [22]. Alcohol drinking status were classified as none, moderate drinking (0–4 in males, 0–3 drinks/day in females) and heavy drinking (≥ 5 in males, ≥ 4 drinks/day in females) [23]. Metabolic equivalent (MET) was used to quantify physical activity. In NHANES, the MET for vigorous work or leisure-time activity was 8.0, moderate work or leisure-time activity 4.0, and walking or bicycling transportation 4.0, respectively. Participants were classified into inactive (< 600 MET-minutes/week)) and active (≥ 600 MET-minutes/week according to the physical activity guidelines for adults [24]. Energy intake data were obtained from two 24-h dietary recall and calculated as an average of 2-day energy intake. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Hypertension was defined as if blood pressure ≥ 130/80 mmHg or taking antihypertensive medication [25]. Diabetes was defined as any of the following: taking insulin or diabetes pills, hemoglobin A1c ≥ 6.5% or fasting plasma glucose ≥ 126 mg/dl [26]. Dyslipidemia was defined as any of the following: total cholesterol ≥ 240 mg/dl, low-density lipoprotein cholesterol ≥ 160 mg/dl, high-density lipoprotein cholesterol < 40 mg/dl or currently taking cholesterol-lowering medication [27].
Statistical analysis
Characteristics are described as means (SD) for continuous variables and frequency (percentages) for categorical variables. Differences between groups were tested using Student’s t-tests for continuous variables and Chi-squared tests for categorical variables. Carotenoid intakes were analyzed as a categorical variable (quartile). Logistic regression models were performed to estimate the association between carotenoid intakes and severe AAC. Model 1 included adjustment for age and gender. Model 2 included the variables in model 1 and race, family income, energy intake, BMI, smoking, alcohol drinking, physical activity, hypertension, diabetes and dyslipidemia. The lowest quartile (Q1) of carotenoid intakes was defined as the reference group. The dose–response analysis was conducted by restricted cubic spline model. In sensitivity analyses, ACC was evaluated as continuous variable (AAC score 0–24). Linear regression models were used to evaluate the association between dietary carotenoid intakes and ACC score. Interaction and stratified analyses were conducted according to age (< 60 or ≥ 60 years), gender (male or female), race (Hispanic white, non-Hispanic white, non-Hispanic black or other race), hypertension (yes or no), diabetes (yes or no) and dyslipidemia (yes or no). A p value < 0.05 was considered statistically significant. All tests were two-sided. All analyses were performed using SPSS 26.0 (IBM Inc., Chicago, IL, USA) and R 3.3.0 software.
Results
The baseline characteristics of included population are presented in Table 1. The prevalence of severe AAC was observed in 13.08% participants. Overall, participants with severe AAC were more likely to be older, non-Hispanic White, and have lower BMI and lower energy intake. They were also more likely to be smokers and drinker and suffered from hypertension, diabetes and dyslipidemia.
Table 2 presents the odds ratios (ORs) with 95% CIs of severe AAC based on quartiles of five carotenoids and total of them. The relationships between carotenoid intakes and severe AAC are shown in Table 2. The crude ORs with 95% CI of severe AAC in the highest versus lowest quartiles were 0.25 (0.17–0.35) for α-carotene, 0.31 (0.22–0.43) for β-carotene, 0.37 (0.26–0.54) for β-cryptoxanthin, 0.27 (0.19–0.38) for lycopene, 0.53 (0.39–0.71) for lutein with zeaxanthin and 0.18 (0.13–0.27) for total carotenoid. After adjustment for age and gender in Model 1, the results remained stable and statistically significant. In Model 2, the ORs with 95% CI of severe AAC were 0.53 (0.23–0.77) for α-carotene, 0.39 (0.19–0.80) for β-carotene, 0.18 (0.05–0.62) for β-cryptoxanthin, 0.40 (0.20–0.78) for lycopene, 0.53 (0.32–0.88) for lutein with zeaxanthin and 0.38 (0.18–0.77) for total carotenoid.
Dose–response relationship between carotenoid intakes and severe AAC is shown in Additional file 1: Fig. S1. The results showed that higher carotenoids were associated with decreased risk of severe AAC in a nonlinear trend (p for nonlinearity < 0.05). Sensitivity analyses showed that higher carotenoid intakes were significantly associated with AAC score in fully adjusted models (Additional file 1: Table S1). There was no evidence of effect modification by age, gender, race, hypertension diabetes and dyslipidemia for carotenoid intakes and severe AAC associations (all p interaction > 0.05).
Discussion
In this study, using a representative national sample of US population aged 40 years and over, we found that dietary carotenoid intakes (including α-carotene, β-carotene, β-cryptoxanthin, lutein with zeaxanthin, lycopene, dietary carotenoid and total carotenoid) were inversely associated with severe AAC. The associations remained significant after adjusting for confounding variables. This suggested that carotenoid intakes play a protective role in the development of AAC. Subgroup analysis found that this association was independent of age, gender, race, hypertension, diabetes and dyslipidemia indicating that the associations were consistent in different population settings.
Previous studies have suggested that fruit and vegetable consumption have a great impact on calcification [28,29,30]. The CAIFOS (Calcium Intake Fracture Outcome Study) found that each 50-gram increase in apple intake per day results in 24% lower odds of developing severe AAC [(odd ratio OR): 0.76 (0.62 to 0.93), p = 0.009)]. In a previous NHANES study, every one standard deviation increase (9.4 g/day) in dietary fiber intake was associated with 28% lower risk of severe AAC [OR 0.72 (95% CI, 0.57 to 0.90), p = 0.004] [12]. Other studies have reported that the Mediterranean diet, which is rich in vegetables and fruits, negatively correlated with the risk of AAC [31, 32]. However, carotenoid has not been fully studied in previous research.
As carotenoids are widely found in fruits, vegetables and seaweed species, these studies indirectly suggest that carotenoid intakes reduce AAC risk. A population-based retrospective cohort study of the 6,095 chronic kidney disease participants demonstrated that higher intake of carotenoids associated with lower all-cause mortality. [α-carotene, (HR = 0.77, 95% CI 0.65 to 0.92, P = 0.002), β-cryptoxanthin (HR = 0.83, 95% CI 0.70 to 0.98, P = 0.019), lycopene (HR = 0.77, 95% CI 0.65 to 0.91, P = 0.002) and lutein + zeaxanthin (HR = 0.82, 95% CI 0.70 to 0.96, P = 0.002)] [33]. Our study showed that carotenoid intakes are inversely associated with severe AAC, and carotenoid intakes have a nonlinear negative dose–response relationship with severe AAC. Total carotenoid intakes of at least 100ug/kg/day were associated with decreased odds for severe AAC. The results of the present study are consistent with previous studies on fruit and vegetable consumption and AAC, suggested that carotenoid intakes may have a significant favorable impact on cardiovascular health.
The mechanisms behind the association between carotenoid intakes and AAC remain unclear. Several possible mechanisms which might explain this association have been proposed. First, it has been reported that inflammation caused by oxidative stress through reactive oxygen species (ROS) attack may play an important role in the pathophysiological process of vascular calcification [34,35,36]. Carotenoids are diet-derived antioxidants that can lower ROS activity, effectively protect endothelial cells from oxidation and cellular damages and consequently reduce the risk of developing AAC. Second, dietary carotenoid intakes were beneficial to lower blood pressure, which is an important risk factor for AAC [37]. Third, previous studies have indicated that IL-6 and TNF-α can promote vascular smooth muscle cell (VSMC) calcification and β-carotene can reduce the mRNA levels of IL-6 and TNF-α [38].
Interventions focused on reinstating nutrient levels in populations at nutritional risk may yield greater effectiveness compared to interventions concentrating on populations with sufficient nutrient levels and supplementing beyond adequacy [39]. This is particularly notable in light of the contradictory findings regarding the association of antioxidant intake or blood status, such as carotenoids, with chronic diseases or their risk factors, as well as the limited effects observed in antioxidant supplementary trials.
Limitations of the study
This study had some limitations. First, the cross-sectional study cannot infer a causal relationship between carotenoid intakes and AAC. In addition, due to the cross-sectional design, reverse causation cannot be excluded. Second, although we adjusted for several potential confounders, there may still be some unknown confounding factors that cannot be ruled out. Third, the generalizability of our result may be limited because all participants were US residents aged 40 years and older in NHANES 2013–2014 due to the database limitations. Fourth, the complicated mechanism between carotenoid intakes and AAC warrants further investigation. Finally, carotenoid intake data were obtained from dietary recall interviews, which might have risk of recall bias.
Conclusion
In conclusion, our study has found a negative and nonlinear association between carotenoid intakes and AAC in adults. This may provide a new strategy for preventing AAC, thereby reducing the risk of cardiovascular diseases. Further prospective studies are needed to confirm our results and elucidate the mechanisms involved.
Availability of data and materials
The data generated and analyzed during the current study are obtained from the NHANES 2013–2014 cohort. NHANES is a program conducted by the National Center for Health Statistics (NCHS), and the relevant information from the survey is publicly available at https://www.cdc.gov/nchs/nhanes/.
Abbreviations
- AAC:
-
Abdominal aortic calcification
- NHANES:
-
National Health and Nutrition Examination Survey
- OR:
-
Odds ratios
- CI:
-
Confidence intervals
- NCHS:
-
National Center for Health Statistics
- MET:
-
Metabolic equivalent
- BMI:
-
Body mass index
- ROS:
-
Reactive oxygen species
- VSMC:
-
Vascular smooth muscle cell
References
Zhong C, Yang X, Feng Y, Yu J. Trained immunity: an underlying driver of inflammatory atherosclerosis. Front Immunol. 2020;11:284.
Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9:73–102.
Lee SJ, Kim H, Oh BK, et al. Association of inter-arm systolic blood pressure differences with arteriosclerosis and atherosclerosis: a cohort study of 117,407 people. Atherosclerosis. 2022;342:19–24.
Henning RJ. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: a review of the pathophysiology and treatment of obesity. Am J Cardiovasc Dis. 2021;11(4):504–29.
Chevli PA, Freedman BI, Hsu FC, et al. Plasma metabolomic profiling in subclinical atherosclerosis: the Diabetes Heart Study. Cardiovasc Diabetol. 2021;20(1):231.
Szulc P. Abdominal aortic calcification: a reappraisal of epidemiological and pathophysiological data. Bone. 2016;84:25–37.
Golestani R, Tio R, Zeebregts CJ, et al. Abdominal aortic calcification detected by dual X-ray absorptiometry: A strong predictor for cardiovascular events. Ann Med. 2010;42(7):539–45.
Allam AHA, Thompson RC, Eskander MA, et al. Is coronary calcium scoring too late? Total body arterial calcium burden in patients without known CAD and normal MPI. J Nucl Cardiol. 2018;25(6):1990–8.
Strong JP, Malcom GT, McMahan CA, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281(8):727–35.
Chuang ML, Massaro JM, Levitzky YS, et al. Prevalence and distribution of abdominal aortic calcium by gender and age group in a community-based cohort (from the Framingham Heart Study). Am J Cardiol. 2012;110(6):891–6.
Sun Y, Zhang H, Tian W. Dietary fiber and prevalence of abdominal aortic calcification in the United States (from the national health and nutrition examination survey data [2013-2014]). Nutr J. 2022;21(1):25.
Bondonno NP, Lewis JR, Prince RL, et al. Fruit intake and abdominal aortic calcification in elderly women: a prospective cohort study. Nutrients. 2016;8(3):159.
Qin Z, Chang K, Liao R, Jiang L, Yang Q, Su B. Greater dietary inflammatory potential is associated with higher likelihood of abdominal aortic calcification. Front Cardiovasc Med. 2021;8:720834.
Chuyen HV, Eun JB. Marine carotenoids: bioactivities and potential benefits to human health. Crit Rev Food Sci Nutr. 2017;57(12):2600–10.
Li Z, Chen J, Zhang D. Association between dietary carotenoid intakes and hypertension in adults: National Health and Nutrition Examination Survey 2007–2014. J Hypertens. 2019;37(12):2371–9.
Ge H, Yang T, Sun J, Zhang D. Associations between dietary carotenoid intakes and the risk of depressive symptoms. Food Nutr Res. 2020. https://doi.org/10.29219/fnr.v64.3920.
Van Hoang D, Pham NM, Lee AH, Tran DN, Binns CW. Dietary carotenoid intakes and prostate cancer risk: a case-control study from Vietnam. Nutrients. 2018;10(1):70.
Hu L, Liu Q, Ou Y, et al. Dietary lycopene is negatively associated with abdominal aortic calcification in US adults: a cross-sectional study. Ann Med. 2023;55(1):2195205.
Liu H, Wang L, Chen C, Dong Z, Yu S. Association between dietary niacin intake and migraine among American Adults: National Health and Nutrition Examination Survey. Nutrients. 2022;14(15):3052.
Chen Y, Chang Z, Zhao Y, et al. Association between the triglyceride-glucose index and abdominal aortic calcification in adults: a cross-sectional study. Nutr Metab Cardiovasc Dis. 2021;31(7):2068–76.
Vilar-Gomez E, Nephew LD, Vuppalanchi R, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. 2022;75(6):1491–506.
Kahende JW, Adhikari B, Maurice E, Rock V, Malarcher A. Disparities in health care utilization by smoking status–NHANES 1999–2004. Int J Environ Res Public Health. 2009;6(3):1095–106.
Phillips JA. Dietary guidelines for Americans, 2020–2025. Workplace Health Saf. 2021;69(8):395.
Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–8.
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):2199–269.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9.
Lu Y, Zhang H, Lu J, et al. Prevalence of dyslipidemia and availability of lipid-lowering medications among primary health care settings in China. JAMA Netw Open. 2021;4(9):e2127573.
Machado AD, Gómez LM, Marchioni DML, et al. Association between dietary intake and coronary artery calcification in non-dialysis chronic kidney disease: The PROGREDIR Study. Nutrients. 2018;10(3):372.
Hartiala O, Kajander S, Knuuti J, et al. Life-course risk factor levels and coronary artery calcification. The Cardiovascular Risk in Young Finns Study. Int J Cardiol. 2016;225:23–9.
Nicoll R, Howard JM, Henein MY. A review of the effect of diet on cardiovascular calcification. Int J Mol Sci. 2015;16(4):8861–83.
Bergia RE 3rd, Biskup I, Giacco R, et al. The MEDGICarb-Study: design of a multi-center randomized controlled trial to determine the differential health-promoting effects of low- and high-glycemic index Mediterranean-style eating patterns. Contemp Clin Trials Commun. 2020;19:100640.
Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128(3):229–38.
Hu Y, Cai X, Zhang N, et al. Relation between dietary carotenoid intake, serum concentration, and mortality risk of CKD patients among US adults: National Health and Nutrition Examination Survey 2001–2014. Front Med (Lausanne). 2022;9:871767.
Qiao Y. Reactive oxygen species in cardiovascular calcification: role of medicinal plants. Front Pharmacol. 2022;13:858160.
Tóth A, Balogh E, Jeney V. Regulation of vascular calcification by reactive oxygen species. Antioxidants (Basel). 2020;9(10):963.
Chen NX, O’Neill KD, Dominguez JM 2nd, Moe SM. Regulation of reactive oxygen species in the pathogenesis of matrix vesicles induced calcification of recipient vascular smooth muscle cells. Vasc Med. 2021;26(6):585–94.
Xavier AA, Pérez-Gálvez A. Carotenoids as a source of antioxidants in the diet. Subcell Biochem. 2016;79:359–75.
Zhou X, Gan T, Fang G, Wang S, Mao Y, Ying C. Zeaxanthin improved diabetes-induced anxiety and depression through inhibiting inflammation in hippocampus. Metab Brain Dis. 2018;33(3):705–11.
Duffield-Lillico AJ, Begg CB. Reflections on the landmark studies of beta-carotene supplementation. J Natl Cancer Inst. 2004;96(23):1729–31.
Acknowledgements
Not applicable
Funding
This work was supported by the Guangzhou University of Chinese Medicine Discipline High-Quality Enhancement Project - Functional Assessment and Risk Warning of Traditional Chinese Medicine for Disease Prevention, the State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, the Guangdong Provincial Key Laboratory of Clinical Research on Traditional Chinese Medicine(No. SZ2021ZZ32), the Science and Technology Planning Project of Guangdong Province(No. 2023B1212060063), and the Self-Funded Science and Technology Innovation Project in Foshan City, The Sixth Affiliated Hospital of South China University of Technology (No. 232001007374).
Author information
Authors and Affiliations
Contributions
WC and YL analyzed, interpreted data and drafted the manuscript. ML and HL interpreted the data, revised the manuscript for intellectual content. CC and YL designed and conceptualized study, interpreted the data and drafted the manuscript for intellectual content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
NHANES was approved by National Center for Health Statistics Research Ethic Review Board. All subjects signed the informed consent during the recruitment period.
Competing interests
There are no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Fig S1.
The dose-response relationship between dietary carotenoid intakes and severe AAC. Table S1. Associations and Midlife Cognitive Function dietary carotenoid intakes and abdominal aortic calcification score.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, W., Li, Y., Li, M. et al. Association between dietary carotenoid intakes and abdominal aortic calcification in adults: National Health and Nutrition Examination Survey 2013–2014. J Health Popul Nutr 43, 20 (2024). https://doi.org/10.1186/s41043-024-00511-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41043-024-00511-9