Abstract
Background
Despite advances in decompressive craniectomy (DC) for the treatment of traumatic brain injury (TBI), these patients are at risk of having a poor long-term prognosis. The aim of this study was to predict 1-year mortality in TBI patients undergoing DC using logistic regression and random tree models.
Methods
This was a retrospective analysis of TBI patients undergoing DC from January 1, 2015, to April 25, 2019. Patient demographic characteristics, biochemical tests, and intraoperative factors were collected. One-year mortality prognostic models were developed using multivariate logistic regression and random tree algorithms. The overall accuracy, sensitivity, specificity, and area under the receiver operating characteristic curves (AUCs) were used to evaluate model performance.
Results
Of the 230 patients, 70 (30.4%) died within 1 year. Older age (OR, 1.066; 95% CI, 1.045–1.087; P < 0.001), higher Glasgow Coma Score (GCS) (OR, 0.737; 95% CI, 0.660–0.824; P < 0.001), higher d-dimer (OR, 1.005; 95% CI, 1.001–1.009; P = 0.015), coagulopathy (OR, 2.965; 95% CI, 1.808–4.864; P < 0.001), hypotension (OR, 3.862; 95% CI, 2.176–6.855; P < 0.001), and completely effaced basal cisterns (OR, 3.766; 95% CI, 2.255–6.290; P < 0.001) were independent predictors of 1-year mortality. Random forest demonstrated better performance for 1-year mortality prediction, which achieved an overall accuracy of 0.810, sensitivity of 0.833, specificity of 0.800, and AUC of 0.830 on the testing data compared to the logistic regression model.
Conclusions
The random forest model showed relatively good predictive performance for 1-year mortality in TBI patients undergoing DC. Further external tests are required to verify our prognostic model.
Similar content being viewed by others
Background
TBI is a common cause of death and disability worldwide affecting all age groups [1]. Decompressive craniectomy (DC), surgically removing a component of the skull, has been performed in TBI patients for many years [2, 3], especially those with high intracranial pressure (ICP) [4], which can effectively increase cerebral perfusion pressure. Two randomized clinical trials have been conducted, the DECRA [5] and RESCUEicp [6] trials, which focused on the prognosis of TBI patients after DC. DECRA demonstrated that early bifronto-temporo-parietal DC decreased the length of stay in the ICU but was associated with unfavorable outcomes; RESCUEicp reported that DC in patients with TBI and refractory intracranial hypertension led to lower mortality.
In previous research, short-term outcome predictive scoring models, discharge status [7] and 30-day mortality [8], were well established in TBI patients with DC. However, a long-term mortality prediction model is a crucial issue that has not received sufficient attention. Despite several studies on long-term outcomes, such as predictors of 1-year mortality in older brain-injured patients [9] and functional outcomes from 3 to 24 months following severe brain injury [10], the study population was not specifically focused on patients after DC. It is necessary to identify the predictors of long-term mortality in TBI patients after DC to gain a better understanding of the progression of the disease, contributing to better daily care and improvements in the quality of life of patients.
Machine learning has been widely used in disease diagnosis and prognosis prediction [11, 12]. For example, machine learning was applied to predict in-hospital morbidity and mortality after TBI, which demonstrated relatively good predictive performance [13]. To date, the use of machine learning techniques to predict the long-term prognosis of TBI patients after DC has rarely been explored. Thus, the purpose of this study was to develop prognostic models to predict the 1-year mortality of TBI patients undergoing DC by using logistic regression and random tree models.
Methods
Patient population
This retrospective study was approved by the Ethics Committee of Tangdu Hospital, Fourth Military Medical University. We reviewed 947 consecutive TBI patients treated at Tangdu Hospital from January 1, 2015, to April 25, 2019. Our main inclusion criterion covered patients who underwent DC with a history of TBI. The main indications and approach were described in previous studies [14, 15]. The exclusion criteria were as follows: (1) an interval from injury to admission of more than 24 h; (2) death in the hospital; (3) other severe systemic diseases, such as malignant tumors, cirrhosis, and uremia; and (4) loss to follow-up.
Variables and data collection
The following data were extracted from the registry database by five study nurses: patient demographic characteristics; Glasgow Coma Score (GCS) score in admission; biochemical tests including aPTT, INR, platelet counts, D-dimer, fibrinogen, glucose, red blood cell, and neutrophil/lymphocyte ratio (NLR); initial CT scan characteristics including contusion volume, subarachnoid hemorrhage (Fisher scales), midline shift, and basal cistern status; perioperative bleeding; and worsening neurologic condition, including mechanical ventilation, tracheotomy, deep venous thrombosis, and hypotension that needed noradrenaline to correct. INR, aPTT, and platelet counts were used to define traumatic coagulopathy, according to a previous study [16], aimed at simplifying the prognostic model. Coagulopathy was defined as an aPTT > 36 s and/or a PT in INR > 1.2 and/or a platelet count < 100 × 109 per liter.
Prognostic model
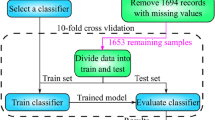
Of the 230 patients, 172 patients (75%) were randomly selected for training, and the remaining 58 patients (25%) were selected for testing. The random seed was set as 66,511. The ratio of non-survivors to survivors was 1:2, so synthetic minority oversampling technique (SMOTE) was used to balance the training data. We conducted a performance comparison of the logistic regression and random tree models. The 10-fold cross-validation, repeated three times, was performed by using the original 75% of the data treated as training data. Features were selected by using the univariate logistic regression method. Hyperparameter optimization was achieved by the grid search method. Three parameters were determined for the random tree model: “Gini” impurity criterion, mtry = 4, and tree = 100. We determined the number of mtry by grid optimization algorithm. An open-source programming language R 3.6.1 and an efficient machine learning tool GraphLab Create were used for machine learning coding.
We compared the predictive performance of the logistic regression and random tree models according to accuracy, sensitivity, specificity, and AUC. The AUCs of the two models on the testing data are the results of back-substituting the training set to the models. The definitions of accuracy, sensitivity, and specificity were described in a previous study [17].
Statistical analysis
Categorical variables are expressed as frequencies (percentages), and continuous variables with skewed distributions are presented as medians and interquartile ranges (IQRs). Univariate logistic regression was used to identify significant predictive variables (P < 0.05), which were entered into the multivariate regression by using the forward LR method to determine the independent risk factors for 1-year mortality. A nomogram model was developed to predict the probability of 1-year mortality. All data were analyzed with the statistical software SPSS 20.0 (IBM, New York, NY).
Results
Patient population
Of the 947 patients treated in this research center during the study period, 306 patients who underwent DC were identified. According to our inclusion and exclusion criteria, 230 patients were enrolled. By February 26, 2020, 89 (38.7%) patients had died, and 141 (61.3%) had survived. The survival analysis showed that the 3-month survival rate was 0.826, the 6-month survival rate was 0.774, the 1-year survival rate was 0.695, and the 3-year survival rate was 0.623 (Fig. 1).
The patient demographic and clinical characteristics are summarized in Table 1. Seventy patients (30.4%) died within 1 year of undergoing DC. The median age was 59 years (IQR, 50–65), and 16 (22.86%) patients were female. In this study, the most frequent mechanism of injury was motor vehicle accidents (114 patients; 49.57%); falling was also common (82 patients; 36.65%). The median GCS score at admission was 4.5 (IQR, 3–7). The total biochemical tests were as follows: Fibrinogen was 1.86 g/L (IQR, 1.39–2.27). D-dimer was 42.56 mg/mL (IQR, 18.47–98.10). Fifty-one (22.17%) patients had coagulopathy disorders. Abnormal glucose (>8.33 mmol/L) and red blood cells (man >5.5×1012/L or <4.0×1012/L, woman >5.0×1012/L or <3.5×1012/L) were shown in 159 (69.13%) patients and 56 (24.56%) patients, respectively. The N/L ratio was 14.55 (IQR, 8.29–24.57). In the initial CT scan characteristics, the contusion volume was 15.99 cm3 (IQR, 0–40.82), and subarachnoid hemorrhage (Fisher scale) was 2 (0–3) in total. Midline shift and completely effaced basal cisterns were noted in 97 (42.17%) and 40 (17.39%) patients, respectively. Perioperative bleeding was more than 750 mL shown in 178 (77.39%) patients. Worsening neurologic conditions are listed as follows: mechanical ventilation, tracheotomy, deep venous thrombosis, and hypotension requiring noradrenaline were noted in 84 (36.52%), 102 (44.35%), 22 (9.57%), and 39 (16.96%) patients, respectively.
Prognostic factors predicting 1-year mortality in the univariate and multivariate analyses
Univariate analyses of the relationship between clinical variables and 1-year mortality are shown in Table 1. Age (P < 0.001), lower GCS (P < 0.001), higher D-dimer (P < 0.001), coagulopathy (P= 0.027), hypotension (P= 0.021), completely effaced basal cisterns (P= 0.004), and perioperative bleeding > 750 mL (P = 0.049) were associated with 1-year mortality. Table 2 lists the results of the multivariate regression analysis to predict 1-year mortality. Older age (P < 0.001), lower GCS (P < 0.001), higher D-dimer (P = 0.015), coagulopathy (P < 0.001), hypotension (P < 0.001), and completely effaced basal cisterns (P= 0.004) were independent predictors of 1-year mortality.
Next, we incorporated all independent predictors identified in multivariate regression analysis to create nomograms, as shown in Fig. 2. The nomogram was constructed by setting a score to each parameter with a point ranging from 0 to 100. Summing the points arranged for each predictor yields the total score, which is ultimately converted into an individual probability of 1-year mortality (from 1 to 99%). Based on the condition of the patients, this nomogram can predict 1-year mortality both simply and intuitively.
Prediction performance of logistic regression and random tree models
To identify the importance of each predictor for 1-year mortality, we chose the feature selection method by applying the random forest algorithm. Three parameters were identified as the most important for predicting 1-year mortality: age, GCS, and D-dimer (Fig. 3). Interestingly, perioperative bleeding was identified as the last associated factor for 1-year mortality.
Their ROC and AUC were calculated to evaluate their discriminative ability (Fig. 4). To evaluate the prediction performance of the logistic regression and random tree models, 10-fold cross-validation was performed on the training data (Table 3). Before SMOTE was used to balance the training data, we developed the logistic regression model. On the training data, it achieved an overall accuracy of 0.750, sensitivity of 0.731, specificity of 0.758, and AUC of 0.770 at the optimal cutoff point (0.307); on the testing data, it achieved an overall accuracy of 0.672, sensitivity of 1.000, specificity of 0.525, and AUC of 0.765 when at optimal cutoff point (0.195). Next, we balanced the training data by using SMOTE. The logistic regression model achieved an overall accuracy of 0.756, sensitivity of 0.615, specificity of 0.817, and AUC of 0.760 on the training data and an overall accuracy of 0.741, sensitivity of 0.889, specificity of 0.675, and AUC of 0.843 on the testing data at the optimal cutoff point. The random forest model achieved an overall accuracy of 0.983, sensitivity of 1.000, specificity of 0.975, and AUC of 0.998 on the training data and an overall accuracy of 0.810, sensitivity of 0.833, specificity of 0.800, and AUC of 0.830 on the testing data at the optimal cutoff point. The accuracy, sensitivity, specificity, and AUC at the cutoff point (0.5) of the three prognostic models are shown in Table 3.
Discussion
In our study, we found that older age, lower GCS, higher D-dimer, coagulopathy, hypotension, and completely effaced basal cisterns were independent predictors of 1-year mortality in patients with TBI after DC. Compared to the logistic regression model, the random tree model presented a better performance on the training data with respect to accuracy, sensitivity, specificity, and AUC (regardless of whether the cutoff point was 0.5 or the optimal point). So did the random tree model in regard to accuracy, sensitivity, and specificity on the testing data when the cutoff point was 0.5. Although the sensitivity of the random tree model was inferior to that of the logistic regression model on the testing data at the optimal cutoff point, the accuracy and specificity of the random tree model were superior. The AUCs of the two models are similar on the testing data. Overall, this finding suggests that the random tree is a valuable and accurate model to predict 1-year mortality in TBI patients after DC. Additionally, our study chose the time of 1-year mortality based on the survival analysis of TBI patients undergoing DC, which showed that the mortality rate within 1 year after discharge was very high. Since we turned the spotlight on the long-term outcomes of TBI patients, patients who died in the hospital were excluded. The predictors of inpatient death and postdischarge mortality were disparate, as shown by a previous study [9]. Thus, our study on 1-year mortality, which excluded patients who died in the hospital, could show better predictive performance to some extent.
Age and GCS, which were already found to be important predictors of TBI, were also confirmed in our study [13, 18]. Tian et al. [7] identified that age was one of the independent risk factors for discharge status after DC, and Tang et al. [8] also observed that age was a risk factor for 30-day mortality after DC. Combined with our research, older age is considered a risk factor for both short-term and long-term outcomes of TBI patients after DC. Older people tend to suffer from more basic diseases than young people, and the rehabilitation of the body is poorer after TBI. GCS, which was similar to age, was also a powerful predictor for the outcomes of TBI after DC [8, 19]. D-dimer, a degradation product of fibrinogen, reflects the level of fibrinolysis in the body. Many studies found that higher d-dimer at admission was associated with a higher risk of progressive hemorrhagic injury [20, 21], while a meta-analysis of the prognostic role of d-dimer level on admission in TBI patients found no significant relationship between d-dimer and the risk of poor functional outcome at 3 months [22]. In our study, higher D-dimer was one of the predictors for 1-year mortality after DC. In our opinion, the prognostic role of d-dimer may be related to the study population and specific outcomes. Secondary coagulopathy after TBI is considered an important factor for unfavorable outcomes [23, 24], and our results also confirmed this finding. TBI-induced coagulopathy is very common, ranging from 7 to 54% [25, 26]. Coagulopathy generated by TBI is a systemic manifestation of local injury [27]. Procoagulant vesicles (including tissue factors, cardiolipin, vWF) from damaged brain tissue are released into the systemic circulation [28,29,30], disrupting the balance between coagulation and anticoagulation. This distinct pathogenetic pathway has attracted increasing attention, and how to intervene in this process is crucial for the prognosis of TBI patients. Hypotension was another risk factor for 1-year mortality. The prognostic role of hypotension in TBI is poorly elaborated. Tang et al. [8] found that intraoperative hypotension was associated with 30-day mortality in TBI patients after DC. In our study, we recorded the incidence of hypotension throughout the course of the disease. Additionally, noradrenaline was injected to maintain the vital signs in patients with hypotension. Our study suggests that hypotension is a crucial predictor of long-term prognosis that should not be ignored in TBI patients after DC. However, some more specific questions between hypotension and the outcome of TBI need to be addressed. For example, it is unclear whether the course of hypotension in patients with TBI is associated with patient outcomes. The risk factors underlying hypotension in TBI need to be explored further. Completely effaced basal cistern status, which represents severely elevated ICP, was found to be an important predictor of outcome in a previous study [8, 31]. Basal cistern effacement is closely associated with pupillary reactivity midline shift. Thus, it can represent a uniquely useful neuroimaging characteristic to guide intervention in TBI [32]. Our study focused on developing prognostic models of predicting the 1-year mortality of TBI patients undergoing DC. The TBI patients who underwent DC or not were different in some characteristics. And further studies are needed to explore these differences.
Many studies on TBI have been conducted using modern machine learning algorithms owing to their good prediction performance. Matsuo et al. [13] demonstrated that random forest showed good performance for poor outcome prediction at discharge and ridge regression for in-hospital mortality prediction in TBI, both of which achieved an accuracy of almost 0.9. Based on the feature selection method, age and GCS appeared to be the most important predictors for both poor outcome and mortality in their study, which was consistent with our findings. A total of 232 patients with TBI were included and separated into training data and test data, which was comparable to our samples of 230 patients. The prediction of mortality was better than our results, which were 0.886 accuracy and 0.875 AUC on the testing data. The difference in performance is mainly due to the prediction of death at different times, and there is no doubt that long-term mortality is harder to predict than in-hospital mortality. Rughani et al. [33] used an artificial neural network to predict the in-hospital survival of TBI patients, which achieved an accuracy of 0.878 and an AUC of 0.860. They included 11 variables in the model: age, sex, total GCS score, individual components of the GCS score at the scene of injury and emergency department, and first systolic blood pressure. Nonetheless, some vital parameters, such as biochemical tests, CT scan characteristics, and neurologic worsening conditions, were absent from their model. Although one study predicted 18-month mortality in severe TBI after DC using the IMPACT prognostic model [19], whose AUC was 0.77, our random tree prediction model achieved AUCs of 0.998 and 0.830 on the testing and training data, respectively. This suggests that machine learning models perform better in outcome prediction than traditional logistic regression models. At present, machine learning algorithms have been increasingly used in the prognosis of TBI [13, 34, 35], and they enable us to optimize the treatment strategy and provide better daily care.
Limitations
There are several limitations to our study. First, it was a single-center, retrospective, and nonrandomized study, so selection bias may exist. Second, we did not include ICP data in this study because ICP monitoring was not performed on every patient. Thus, we could not include this vital variable to avoid apparent selection bias. Another limitation is that some information about the patient after discharge, such as rehabilitation therapy, was absent, but this provides an opportunity for prospective research to analyze this variable. Finally, our prognostic model was mainly targeted at TBI patients undergoing DC, so the performance of our model may decrease when it is applied to all TBI patients.
Conclusions
Our findings confirm that older age, lower GCS, higher D-dimer, coagulopathy, hypotension, and completely effaced basal cisterns were associated with 1-year mortality. The random forest model showed relatively good predictive performance for 1-year mortality, which achieved an overall accuracy of 0.810, sensitivity of 0.833, specificity of 0.800, and AUC of 0.830 on the testing data. Our results indicated that machine learning achieved good performance for TBI outcomes. By virtue of machine learning with more accurate prediction performance, we can provide TBI patients with better precision medicine and care directions.
Availability of data and materials
Please contact the authors for data requests.
Abbreviations
- DC:
-
Decompressive craniectomy
- TBI:
-
Traumatic brain injury
- AUCs:
-
Area under the receiver operating characteristic curves
- GCS:
-
Glasgow Coma Score
- ICP:
-
Intracranial pressure
References
Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104(2):213–38. https://doi.org/10.1016/j.mcna.2019.11.001.
Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90(2):187–96. https://doi.org/10.3171/jns.1999.90.2.0187.
Kwan K, Schneider J, Ullman JS. Chapter 12: Decompressive craniectomy: long term outcome and ethical considerations. Front Neurol. 2019;10:876. https://doi.org/10.3389/fneur.2019.00876.
Bor-Seng-Shu E, Figueiredo EG, Amorim RL, et al. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J Neurosurg. 2012;117(3):589–96. https://doi.org/10.3171/2012.6.Jns101400.
Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Eng J Med. 2011;364(16):1493–502. https://doi.org/10.1056/NEJMoa1102077.
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Eng J Med. 2016;375(12):1119–30. https://doi.org/10.1056/NEJMoa1605215.
Tian R, Liu W, Dong J, Zhang J, Xu L, Zhang B, et al. Prognostic predictors of early outcomes and discharge status of patients undergoing decompressive craniectomy after severe traumatic brain injury. World Neurosurg. 2019;126:e101–8. https://doi.org/10.1016/j.wneu.2019.01.246.
Tang Z, Yang K, Zhong M, Yang R, Zhang J, Jiang Q, et al. Predictors of 30-day mortality in traumatic brain-injured patients after primary decompressive craniectomy. World Neurosurg. 2020;134:e298–305. https://doi.org/10.1016/j.wneu.2019.10.053.
Peck KA, Calvo RY, Sise CB, Johnson J, Yen JW, Sise MJ, et al. Death after discharge: predictors of mortality in older brain-injured patients. J Trauma Acute Care Surg. 2014;77(6):978–83. https://doi.org/10.1097/ta.0000000000000356.
Wilkins TE, Beers SR, Borrasso AJ, Brooks J, Mesley M, Puffer R, et al. Favorable functional recovery in severe traumatic brain injury survivors beyond six months. Journal of Neurotrauma. 2019;36(22):3158–63. https://doi.org/10.1089/neu.2018.6153.
Chen JH, Asch SM. Machine learning and prediction in medicine - beyond the peak of inflated expectations. The New England Journal of Medicine. 2017;376(26):2507–9. https://doi.org/10.1056/NEJMp1702071.
Doupe P, Faghmous J, Basu S. Machine learning for health services researchers. Value in Health. J Int Soc Pharmacoeconomics Outcomes Res. 2019;22(7):808–15. https://doi.org/10.1016/j.jval.2019.02.012.
Matsuo K, Aihara H, Nakai T, Morishita A, Tohma Y, Kohmura E. Machine learning to predict in-hospital morbidity and mortality after traumatic brain injury. Journal of Neurotrauma. 2020;37(1):202–10. https://doi.org/10.1089/neu.2018.6276.
Kolias AG, Kirkpatrick PJ, Hutchinson PJ. Decompressive craniectomy: past, present and future. Nature Reviews Neurology. 2013;9(7):405–15. https://doi.org/10.1038/nrneurol.2013.106.
Mao X, Miao G, Hao S, et al. Decompressive craniectomy for severe traumatic brain injury patients with fixed dilated pupils. Therapeutics Clin Risk Manag. 2015;11:1627–33. https://doi.org/10.2147/tcrm.S89820.
Lustenberger T, Talving P, Kobayashi L, Barmparas G, Inaba K, Lam L, et al. Early coagulopathy after isolated severe traumatic brain injury: relationship with hypoperfusion challenged. The Journal of Trauma. 2010;69(6):1410–4. https://doi.org/10.1097/TA.0b013e3181cdae81.
Liu J, Xiong Y, Zhong M, Yang Y, Guo X, Tan X, et al. Predicting long-term outcomes after poor-grade aneurysmal subarachnoid hemorrhage using decision tree modeling. Neurosurgery. 2020;87(3):523–9. https://doi.org/10.1093/neuros/nyaa052.
Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329–37. https://doi.org/10.1089/neu.2006.0035.
Honeybul S, Ho KM. Predicting long-term neurological outcomes after severe traumatic brain injury requiring decompressive craniectomy: a comparison of the CRASH and IMPACT prognostic models. Injury. 2016;47(9):1886–92. https://doi.org/10.1016/j.injury.2016.04.017.
Yuan F, Ding J, Chen H, Guo Y, Wang G, Gao WW, et al. Predicting progressive hemorrhagic injury after traumatic brain injury: derivation and validation of a risk score based on admission characteristics. Journal of Neurotrauma. 2012;29(12):2137–42. https://doi.org/10.1089/neu.2011.2233.
Tong WS, Zheng P, Zeng JS, Guo YJ, Yang WJ, Li GY, et al. Prognosis analysis and risk factors related to progressive intracranial haemorrhage in patients with acute traumatic brain injury. Brain Inj. 2012;26(9):1136–42. https://doi.org/10.3109/02699052.2012.666437.
Zhang J, He M, Song Y, Xu J. Prognostic role of D-dimer level upon admission in patients with traumatic brain injury. Medicine (Baltimore). 2018;97(31):e11774. https://doi.org/10.1097/md.0000000000011774.
Sun Y, Wang J, Wu X, XI C, Gai Y, Liu H, et al. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury--analysis of 242 cases. British Journal of Neurosurgery. 2011;25(3):363–8. https://doi.org/10.3109/02688697.2011.552650.
Franschman G, Boer C, Andriessen TM, et al. Multicenter evaluation of the course of coagulopathy in patients with isolated traumatic brain injury: relation to CT characteristics and outcome. J Neurotrauma. 2012;29(1):128–36. https://doi.org/10.1089/neu.2011.2044.
Chhabra G, Rangarajan K, Subramanian A, Agrawal D, Sharma S, Mukhopadhayay AK. Hypofibrinogenemia in isolated traumatic brain injury in Indian patients. Neurol India. 2010;58(5):756–7. https://doi.org/10.4103/0028-3886.72175.
Greuters S, van den Berg A, Franschman G, et al. Acute and delayed mild coagulopathy are related to outcome in patients with isolated traumatic brain injury. Crit Care (London, England). 2011;15(1):R2. https://doi.org/10.1186/cc93e99.
J Z, F Z, Blood DJJ. Coagulopathy induced by traumatic brain injury: systemic manifestation of a localized injury. 2018;131(18): 2001-2006. doi: https://doi.org/10.1182/blood-2017-11-784108.
Zhao Z, Wang M, Tian Y, Hilton T, Salsbery B, Zhou EZ, et al. Cardiolipin-mediated procoagulant activity of mitochondria contributes to traumatic brain injury-associated coagulopathy in mice. Blood. 2016;127(22):2763–72. https://doi.org/10.1182/blood-2015-12-688838.
Wu Y, Liu W, Zhou Y, Hilton T, Zhao Z, Liu W, et al. von Willebrand factor enhances microvesicle-induced vascular leakage and coagulopathy in mice with traumatic brain injury. Blood. 2018;132(10):1075–84. https://doi.org/10.1182/blood-2018-03-841932.
Tian Y, Salsbery B, Wang M, Yuan H, Yang J, Zhao Z, et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 2015;125(13):2151–9. https://doi.org/10.1182/blood-2014-09-598805.
Kolias AG, Adams H, Timofeev I, Czosnyka M, Corteen EA, Pickard JD, et al. Decompressive craniectomy following traumatic brain injury: developing the evidence base. British Journal of Neurosurgery. 2016;30(2):246–50. https://doi.org/10.3109/02688697.2016.1159655.
Nourallah B, Menon DK, Zeiler FA. Midline shift is unrelated to subjective pupillary reactivity assessment on admission in moderate and severe traumatic brain injury. Neurocritical Care. 2018;29(2):203–13. https://doi.org/10.1007/s12028-018-0526-8.
Rughani AI, Dumont TM, Lu Z, Bongard J, Horgan MA, Penar PL, et al. Use of an artificial neural network to predict head injury outcome. Journal of Neurosurgery. 2010;113(3):585–90. https://doi.org/10.3171/2009.11.Jns09857.
Folweiler KA, Sandsmark DK, Diaz-Arrastia R, Cohen AS, Masino AJ. Unsupervised machine learning reveals novel traumatic brain injury patient phenotypes with distinct acute injury profiles and long-term outcomes. Journal of Neurotrauma. 2020;37(12):1431–44. https://doi.org/10.1089/neu.2019.6705.
Hernandes Rocha TA, Elahi C, Cristina da Silva N, et al. A traumatic brain injury prognostic model to support in-hospital triage in a low-income country: a machine learning-based approach. J Neurosurg. 2019:1–9. https://doi.org/10.3171/2019.2.Jns182098.
Acknowledgements
We thank Ping Wang and Yaning Cai for their help with the development and maintenance of the neurotrauma database.
Funding
This work was supported by grants from the Chang Jiang Scholar Program of China and the National Natural Science Foundation of China (81630027, 81571215).
Author information
Authors and Affiliations
Contributions
WC, GS, and SY contributed equally to this work. All authors met the ICMJE criteria for authorship. YQ, WC, GS, and SY contributed to the conception and design. XW, JL, and HL performed the statistical analysis. GZ, HG, and DF helped to draft the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethical committee of Tangdu Hospital, Fourth Military Medical University (K201907-06), and the consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, W., Ge, S., Shi, Y. et al. Death after discharge: prognostic model of 1-year mortality in traumatic brain injury patients undergoing decompressive craniectomy. Chin Neurosurg Jl 7, 24 (2021). https://doi.org/10.1186/s41016-021-00242-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41016-021-00242-4