Abstract
Introduction
Recently, modified thoracoabdominal nerve block through perichondrial approach (M-TAPA) has been introduced as a novel trunk block. To date, studies comparing its clinical advantages with those of existing local anesthetic techniques are scarce. We aimed to compare the analgesic efficacy of M-TAPA to that of wound infiltration analgesia (WIA) in patients who underwent gynecological laparoscopic surgeries.
Methods
We studied medical records from January 2020 to July 2021 at Hokkaido University Hospital. The primary outcome was the number of analgesic requirements in the first 24 h postoperatively. Secondary outcomes were the time until the first analgesic requirement and adverse events regarding local anesthetic techniques. To address confounding, a regression model was used.
Results
Data from 90 of 231 patients were analyzed (M-TAPA group, n = 40; WIA group, n = 50). For the primary outcome, means and 95% confidence intervals for each group and between-group differences were as follows: 2.25 (1.74, 2.76), 2.28 (1.81, 2.75), and −0.03 (−0.72, 0.66), respectively. Adjusted mean difference was 0.39 (−0.32, 1.11). There were no significant differences in means between groups, with or without adjustment for covariates (p = 0.93, 0.28). Furthermore, no significant difference was detected in the time until the first analgesic requirement and adverse events related to local anesthesia.
Conclusion
Our results demonstrate that M-TAPA did not reduce postoperative analgesic requirements compared to WIA. In a future clinical trial, sufficient visceral pain control may be required to evaluate the effectiveness of M-TAPA over WIA in patients undergoing laparoscopic gynecological surgery.
Similar content being viewed by others
Background
Laparoscopic surgery for gynecological disease is widely performed due to the advantage in cosmesis, postoperative pain, and morbidity [1]. Although it is less invasive than open laparotomy, patients undergoing laparoscopic surgery often experience significant pain [2]. Uncontrolled acute postoperative pain is associated with impaired physical function and prolonged opioid use, which consequently retards early recovery [3]. To provide effective analgesia and minimize opioid use, multimodal analgesia with non-opioid analgesics, such as local anesthesia, is recommended [4]. Transversus abdominis plane block (TAPB) [5], rectus sheath block (RSB) [6], and wound infiltration anesthesia (WIA) [7] are commonly used in various abdominal surgeries as components of multimodal analgesia.
Recently, modified thoracoabdominal nerve block through perichondrial approach (M-TAPA) [8] has been introduced as a novel technique of trunk block. Some reports have demonstrated that M-TAPA could be beneficial for lower laparotomy [9], sleeve gastrectomy [10], and laparoscopic cholecystectomy [11, 12]. We previously reported that this technique could provide a broad range of analgesia in the thoracoabdominal wall, with significant inter-individual variation [13]. Although a cumulative number of successful cases has been reported, few studies have evaluated the clinical advantage of M-TAPA over existing local anesthetic techniques [12]. Some previous studies indicate that trunk block provides longer analgesia than WIA [14]. Thus, in this retrospective, exploratory study, we hypothesized that M-TAPA could reduce the postoperative analgesic requirements compared to WIA in patients undergoing gynecological laparoscopic surgery.
Methods
Patient selection
This single-center retrospective study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Review Committee of Hokkaido University Hospital (IRB No. 021-0077; Nov 26, 2021). We aimed to compare the analgesic effectiveness of M-TAPA to that of WIA in patients who underwent gynecological laparoscopic surgeries. Written informed consent was not obtained owing to the retrospective design of this study. Consent was obtained through an opt-out method, and the opportunity to refuse was provided through the Hokkaido University Hospital website (https://www.huhp.hokudai.ac.jp/date/rinsho-johokokai/approval/2021-11/).
We studied the medical records of patients who underwent gynecological laparoscopic surgery between January 2020 and July 2021 at Hokkaido University Hospital. Exclusion criteria were as follows: emergency surgery, age < 20 or ≥ 70 years, surgery duration ≥ 6 h, no use of local anesthetic, or provision of peripheral nerve blocks other than M-TAPA (i.e., TAPB, RSB). Notably, we excluded patients who received both flurbiprofen and acetaminophen or neither of them intraoperatively, as this could influence postoperative analgesic requirements.
As “gynecological laparoscopic surgery” includes different procedures, and this might influence the results; we classified the types of surgical procedures into four groups: myomectomy with or without ovarian surgery, total vaginal hysterectomy with or without ovarian surgery, cauterization of endometriosis, and ovarian surgery only, according to previous studies [15, 16].
Management of general anesthesia
Anesthetic and perioperative management were performed according to our institutional practices. Of note, although we usually select total intravenous anesthesia (TIVA) using propofol in this population, the anesthetic method differed because of the unstable supply of propofol occasioned by the coronavirus disease pandemic during the study period. Preoperative sedatives or analgesics were not administered. In the operating room, all patients were monitored for noninvasive blood pressure, electrocardiography, and peripheral oxygen saturation. A venous line was secured prior to induction. The anesthetic method was determined by the anesthesiologist in charge. TIVA was maintained with propofol (2.5–4 μg/mL) and remifentanil (0.1–0.5 μg/kg/min), targeting a bispectral index (BIS) value of 40–60. Inhalational anesthesia was maintained with 0.7–1 minimum alveolar concentration of end-tidal sevoflurane or desflurane and remifentanil. Intraoperative fentanyl administration was determined by an assigned anesthesiologist. All patients were intubated after the administration of rocuronium (0.6 mg/kg) and maintained with end-tidal carbon dioxide at 35–45 mmHg. Additionally, either of acetaminophen or flurbiprofen was given for postoperative pain control.
Surgical procedures and local anesthetic technique
In our institution, gynecological laparoscopic surgeries are performed through the 4-trocar procedure combining 12 mm-, 10 mm-, 5 mm-thick ports. The port sites are umbilicus, suprapubic midline, and right and left lower abdomen. Generally, 10-mm- or 12-mm-thick ports, mainly associated with postoperative somatic pain, are inserted in the umbilicus and right lower quadratus.
M-TAPA was performed by a single anesthesiologist (KA) after induction of general anesthesia as previously described [8, 13]. Briefly, a high-frequency linear probe (EDGE, Sonosite, Tokyo, Japan) was positioned across the costal arch, and the four key structures were visualized: external oblique muscle, internal oblique muscle, transversus abdominis muscle, and costal cartilage. An 18-gauge Touhy needle was then inserted from the caudal to cranial direction using the in-plane technique until the needle tip was positioned between the posterior aspect of the costal cartilage and the transversus abdominis muscle. Twenty-five milliliters of 0.25% ropivacaine was injected on each side (Fig. 1a, b). A picture of the body surface during the procedure is shown as Fig. 2. We note that this group included 30 patients who were involved in our prior prospective study evaluating sensory coverage [13].
Ultrasound image of M-TAPA. a An appropriate image for M-TAPA. b Image obtained after injecting the local anesthetic solution. The arrows denote the Touhy needle. M-TAPA modified thoracoabdominal nerve block through perichondrial approach, EOM external oblique muscle, IOM internal oblique muscle, TAM transversus abdominis muscle, CC costal cartilage, LA local anesthetic
In the WIA group, local anesthesia was administered by the surgeons at the time of wound closure. There were differences in the types and volumes of local anesthetic solutions. However, in most cases, 10–20 mL of 0.25% levobupivacaine or 0.375% ropivacaine was injected around the port incision site.
Postoperative pain management in the ward
After emergence from anesthesia and confirmation of a modified Aldrete score of ≥ 9, the patients were discharged to the gynecological ward. As the ward nurses were not informed of which local anesthetic technique was utilized, this study was considered to be observer-blind. In our institution, to avoid postoperative nausea and vomiting, intravenous opioid infusion was not used for patients who underwent laparoscopic gynecological surgery. Instead, intravenous acetaminophen, flurbiprofen, or intramuscular pentazocine (relatively rare) was administered at the patient’s request. Usually, the nurses administered acetaminophen or flurbiprofen first and then chose either analgesic to ensure an interval of at least 6 h between the same drugs. In addition to these rescue analgesics, 60 mg of scheduled oral loxoprofen was initiated from the next day of operation.
Outcome measures and statistical analysis
The primary outcome was the number of postoperative analgesic requirements other than the scheduled oral loxoprofen in the first 24 h. Secondary outcomes were the time until the first analgesic requirement and adverse events associated with the local anesthetic technique in the perioperative period.
Due to the retrospective design of this study, the sample size was determined based on the number of patients enrolled during the study period. Assuming that M-TAPA reduced the postoperative analgesic requirements by 1 compared to WIA with a standard deviation of 2, with a two-sided significance level of 0.05%, the target sample size was 50 patients per group for a power of 70%. During the study period, more than 200 gynecological laparoscopic surgeries were expected to be performed. Even if 50% of the patients met the exclusion criteria, we anticipated to have a sufficient sample size to assess the difference in postoperative analgesic requirements between M-TAPA and WIA. Patient characteristics are presented as mean ± standard deviation. For the primary outcome, means for each group and between-group differences were estimated.
To adjust for confounding, a regression model with types of surgical procedures, patient body mass index, administered intraoperative non-opioid analgesics (acetaminophen or flurbiprofen), and anesthetic method (TIVA or inhalational anesthesia) as covariates was used to estimate the least-squares mean for between-group differences. All the estimates are shown with their 95% confidence interval (CI). Two-sided p-values for the between-group comparison were also calculated. The time until the first analgesic requirement was compared using Kaplan–Meier method and log-rank test. All statistical calculations were performed using JMP Pro 16 software (SAS Institute, Japan). Data were compared between groups using a two-tailed Student’s t-test. The categorical data were analyzed using the chi-square test. A p-value of < 0.05 was considered significant.
Results
Eligibility for analysis
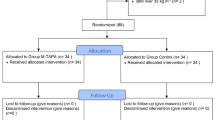
A total of 231 gynecological laparoscopic surgeries were performed during the study period. We excluded the following: emergency surgery (n = 9), age < 20 or ≥ 70 years (n = 10), surgery duration ≥ 6 h (n = 30), use of no local anesthesia or peripheral nerve block other than M-TAPA (n = 86), and administration of both intravenous flurbiprofen and acetaminophen or neither of them (n = 6). Of the remaining 90 patients, 40 were in the M-TAPA group, and 50 were in the WIA group (Fig. 3).
Patient characteristics and statistical analysis
Patient characteristics are listed in Table 1. There were no significant differences except for patient body weight, body mass index, and anesthetic method.
Table 2 shows the number of postoperative analgesic requirements in the first 24 h. For the primary outcome, means and 95% CIs for each group and between-group differences were 2.25 (1.74, 2.76), 2.28 (1.81, 2.75), and −0.03 (−0.71, 0.65), respectively. The least-squares mean (with the regression model) for between-group difference was 0.39 (−0.32, 1.11). No significant differences were observed between groups, with or without adjustment for covariates (p = 0.93, 0.28). For the secondary outcomes, there was no significant difference in the time until the first analgesic requirement (p = 0.52, Fig. 4). Furthermore, no perioperative adverse events associated with local anesthetic techniques, such as toxicity or hematoma, were observed.
Discussion
This retrospective study examined the analgesic efficacy of M-TAPA versus WIA in patients who underwent gynecological laparoscopic surgery by comparing the number of postoperative analgesic requirements and the time until the first analgesic requirement. The main finding of this study was that there was no difference in postoperative analgesic requirements between M-TAPA and WIA in the patients undergoing gynecological laparoscopic surgery. Furthermore, no difference in the time until the first analgesic requirement was observed.
M-TAPA is a novel trunk block, first reported by Tulgar et al. in 2019 [8]. To date, there is little research investigating its analgesic efficacy compared to existing local anesthetic techniques. Recently, Bilge et al. [11] reported the first randomized controlled trial indicating that M-TAPA decreased postoperative pain scores and tramadol consumption in the first 24 h and improved the quality of recovery-40 (QoR-40) scores after laparoscopic cholecystectomy compared with their standard postoperative analgesic regimen, without WIA. Subsequently, Güngör et al. [12] reported concordant results, utilizing WIA in the control group. These recent reports suggest that M-TAPA could be beneficial for laparoscopic cholecystectomy.
To the best of our knowledge, this is the first study investigating the analgesic efficiency of M-TAPA for laparoscopic gynecological surgery, which is often involved in studies regarding trunk peripheral nerve blocks. The discrepancy between the studies targeting laparoscopic cholecystectomy could be partially explained by the pain origin of the surgery performed. The postoperative pain after laparoscopic cholecystectomy mainly derives from the port incision site [17], which can be well controlled by M-TAPA. In contrast, pain after laparoscopic gynecological surgery mainly originates from visceral pain [18]. Kane et al. [16] reported that TAP block did not improve the postoperative quality of recovery-40 scores and pain scores or decrease postoperative opioid consumption in laparoscopic hysterectomy, which may support our results. Notably, half of the patients in both groups used the first rescue analgesic within 1 h, when the local anesthesia would be still effective [13]. Actually, they complained of “menstrual-like pain” at our postoperative rounds. Thus, our results were strongly influenced by visceral pain and suggest that sufficient visceral pain control is required in a future clinical trial to evaluate the effectiveness of M-TAPA over WIA in patients undergoing laparoscopic gynecological surgery.
When we evaluate the advantages of the novel nerve block technique, an adequate alternative local anesthetic technique should be provided to the control group to avoid overrating its clinical advantage [19]. Although some studies reported that trunk blocks provide longer analgesia than WIA [14], robust evidence that trunk blocks are clinically superior to WIA has not been shown. Of note, WIA has significant advantages over peripheral nerve blocks, as it does not require an anesthesiologist’s skill or ultrasound devices and is time-saving. Therefore, it is commonly used for multimodal analgesia, and we suppose it could be an appropriate control to evaluate the clinical efficacy of M-TAPA. Although we performed M-TAPA before surgery, postoperative application could be an option in a future study, as it will provide longer analgesia for the incision site [12].
In the present study, several types of surgical procedures were performed in accordance with the clinical settings. Hysterectomy has been suggested to induce more severe postoperative pain than other procedures, such as ovarian surgeries [18]. Therefore, some studies included only hysterectomy to ensure uniformity of patient characteristics [16, 20]. Thus, we utilized a regression model to address this confounding, and the mean difference of the analgesic requirement between M-TAPA vs WIA changed from −0.03 to 0.39. This may reflect that the WIA group included more patients who underwent total hysterectomy.
Theoretically, M-TAPA performed before incision ameliorates somatic pain and reduces intraoperative opioid use. However, no significant differences were observed between the two groups. As this was a retrospective study, there were no specific criteria regarding intraoperative opioid administration. The assigned anesthesiologists might use a sufficient dose of remifentanil throughout the operation to control adrenergic reactions due to pneumoperitoneum or visceral pain. To investigate the effect of intraoperative analgesic reduction, a prospective study with appropriate anesthetic protocol may be required.
Our study has some limitations. This was a single-center, retrospective study that did not unify the anesthetic protocol or postoperative analgesic regimen. The sample size of the M-TAPA group was 10 participants smaller than our expectation. Thus, we cannot rule out the possibility of under-powering to detect the difference in primary outcome. The choice of the local anesthetic technique was not random, which may have resulted in a selection bias of patient characteristics. Furthermore, our study lacked chronological postoperative pain assessment using a scale such as VAS or NRS, due to the unavailability of medical records. This also meant that the objective criteria to use rescue analgesia (i.e., NRS ≥ 4) were absent. We utilized a regression model including the types of surgical procedures as one of the covariates to adjust for confounding; however, future prospective studies on patients receiving the same surgical procedure are needed for a more appropriate comparison.
Conclusions
Our study demonstrated no difference between M-TAPA and WIA in the postoperative analgesic requirements in the first 24 h and the time until the first analgesic requirement in patients who underwent gynecological laparoscopic surgery. A future clinical trial with sufficient visceral pain control may be required to evaluate the effectiveness of M-TAPA over WIA in patients undergoing laparoscopic gynecological surgery.
Availability of data and materials
The datasets are available from the corresponding author on reasonable request.
Abbreviations
- M-TAPA:
-
Modified thoracoabdominal nerve block through perichondrial approach
- WIA:
-
Wound infiltration analgesia
- TAPB:
-
Transversus abdominis plane block
- RSB:
-
Rectus sheath block
- TIVA:
-
Total intravenous anesthesia
- BIS:
-
Bispectral index
- CI:
-
Confidence interval
References
Nieboer TE, Hendriks JC, Bongers MY, Vierhout ME, Kluivers KB. Quality of life after laparoscopic and abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2012;119:85–91.
Wu CL, Cohen SR, Richman JM, Rowlingson AJ, Courpas GE, Cheung K, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103:1079–88; quiz 109-10.
Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–98.
Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152:691–7.
McDonnell JG, O’Donnell B, Curley G, Heffernan A, Power C, Laffey JG. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: a prospective randomized controlled trial. Anesth Analg. 2007;104:193–7.
Hamid HKS, Ahmed AY, Alhamo MA, Davis GN. Efficacy and safety profile of rectus sheath block in adult laparoscopic surgery: a meta-analysis. J Surg Res. 2021;261:10–7.
Bisgaard T, Klarskov B, Kristiansen VB, Callesen T, Schulze S, Kehlet H, et al. Multi-regional local anesthetic infiltration during laparoscopic cholecystectomy in patients receiving prophylactic multi-modal analgesia: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 1999;89:1017–24.
Tulgar S, Selvi O, Thomas DT, Deveci U, Özer Z. Modified thoracoabdominal nerves block through perichondrial approach (M-TAPA) provides effective analgesia in abdominal surgery and is a choice for opioid sparing anesthesia. J Clin Anesth. 2019;55:109.
Altıparmak B, Toker MK, Uysal A, Turan M, Demirbilek SG. Reply to Tulgar et al.: Perichondral approach for blockage of thoracoabdominal nerves: anatomical basis and clinical experience in three cases. J Clin Anesth. 2019;54:150–51.
Aikawa K, Tanaka N, Morimoto Y. Modified thoracoabdominal nerves block through perichondrial approach (M-TAPA) provides a sufficient postoperative analgesia for laparoscopic sleeve gastrectomy. J Clin Anesth. 2019;59:44–5.
Bilge A, Başaran B, Et T, Korkusuz M, Yarımoğlu R, Toprak H, et al. Ultrasound-guided bilateral modified-thoracoabdominal nerve block through a perichondrial approach (M-TAPA) in patients undergoing laparoscopic cholecystectomy: a randomized double-blind controlled trial. BMC Anesthesiol. 2022;22:329.
Güngör H, Ciftci B, Alver S, Gölboyu BE, Ozdenkaya Y, Tulgar S. Modified thoracoabdominal nerve block through perichondrial approach (M-TAPA) vs local infiltration for pain management after laparoscopic cholecystectomy surgery: a randomized study. J Anesth. 2022. https://doi.org/10.1007/s00540-022-03158-0.
Aikawa K, Yokota I, Maeda Y, Morimoto Y. Evaluation of sensory loss obtained by modified-thoracoabdominal nerves block through perichondrial approach in patients undergoing gynecological laparoscopic surgery: a prospective observational study. Reg Anesth Pain Med. 2022;47:134–5.
Yu N, Long X, Lujan-Hernandez JR, Succar J, Xin X, Wang X. Transversus abdominis-plane block versus local anesthetic wound infiltration in lower abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 2014;14:121.
El Hachem L, Small E, Chung P, Moshier EL, Friedman K, Fenske SS, et al. Randomized controlled double-blind trial of transversus abdominis plane block versus trocar site infiltration in gynecologic laparoscopy. Am J Obstet Gynecol. 2015;212(182):e1-9.
Kane SM, Garcia-Tomas V, Alejandro-Rodriguez M, Astley B, Pollard RR. Randomized trial of transversus abdominis plane block at total laparoscopic hysterectomy: effect of regional analgesia on quality of recovery. Am J Obstet Gynecol. 2012;207(419):e1-5.
Mitra S, Khandelwal P, Roberts K, Kumar S, Vadivelu N. Pain relief in laparoscopic cholecystectomy–a review of the current options. Pain Pract. 2012;12:485–96.
Choi JB, Kang K, Song MK, Seok S, Kim YH, Kim JE. Pain characteristics after total laparoscopic hysterectomy. Int J Med Sci. 2016;13:562–8.
Tran Q, Bravo D, Leurcharusmee P, Neal JM. Transversus abdominis plane block: a narrative review. Anesthesiology. 2019;131:1166–90.
Calle GA, López CC, Sánchez E, De Los Ríos JF, Vásquez EM, Serna E, et al. Transversus abdominis plane block after ambulatory total laparoscopic hysterectomy: randomized controlled trial. Acta Obstet Gynecol Scand. 2014;93:345–50.
Acknowledgements
We would like to thank Anna Shimamura for supporting data collection and Editage (www.editage.com) for English language editing.
Funding
The authors have no sources of funding to declare.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. CA collected the data, prepared the first draft, and analyzed the data. KA and YM were involved in data analysis and critical revision of the manuscript. KT and KO made significant comments on statistical analysis. All authors commented on the previous versions of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This single-center retrospective study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Review Committee of Hokkaido University Hospital (IRB No. 021-0077; Nov 26, 2021).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atsumi, C., Aikawa, K., Takahashi, K. et al. The comparison of postoperative analgesic requirements between modified thoracoabdominal nerve block through perichondrial approach versus wound infiltration analgesia in patients undergoing gynecological laparoscopic surgery: a retrospective, exploratory study. JA Clin Rep 9, 39 (2023). https://doi.org/10.1186/s40981-023-00632-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40981-023-00632-w