Abstract
Aims
Chimeric Antigen Receptor-T (CAR-T) cell infusion is a rapidly evolving antitumor therapy; however, cardiovascular (CV) complications, likely associated with cytokine release syndrome (CRS) and systemic inflammation, have been reported to occur. The CARdio-Tox study aimed at elucidating incidence and determinants of cardiotoxicity related to CAR-T cell therapy.
Methods
Patients with blood malignancies candidate to CAR-T cells were prospectively evaluated by echocardiography at baseline and 7 and 30 days after infusion. The study endpoints were i) incidence of cancer therapy-related cardiac dysfunction (CTRCD), CTRCD were also balanced for any grade CRS, but CTRCD occurred of Cardiology Guidelines on Cardio-Oncology (decrements of left ventricular ejection fraction (LVEF) or global longitudinal strain (GLS) and/or elevations of cardiac biomarkers (high sensitivity troponin I, natriuretic peptides) and ii), correlations of echocardiographic metrics with inflammatory biomarkers.
Results
Incidence of CTRCD was high at 7 days (59,3%), particularly in subjects with CRS. The integrated definition of CTRCD allowed the identification of the majority of cases (50%). Moreover, early LVEF and GLS decrements were inversely correlated with fibrinogen and interleukin-2 receptor levels (p always ≤ 0.01).
Conclusions
There is a high incidence of early CTRCD in patients treated with CAR-T cells, and a link between CTRCD and inflammation can be demonstrated. Dedicated patient monitoring protocols are advised.
Graphical Abstract

Similar content being viewed by others
Introduction
Chimeric antigen receptor (CAR)-T cell therapy represents an effective therapeutic opportunity for patients with advanced hematological malignancies, delivering a significant improvement in response rates [1,2,3]; however, data from clinical trials and real-word reports show that numerous adverse events may occur [4, 5]. The main CAR-T cell toxicity is represented by the cytokine release syndrome (CRS), a subtype of systemic inflammatory response syndrome [6] which is characterized by an excessive systemic inflammatory response secondary to the interaction between the engineered T-cells, immune effectors and the tumor microenvironment [7]. Clinical presentation of CRS ranges from mild flu-like symptoms to life-threatening multiorgan dysfunction [7]. These manifestations do not spare the cardiovascular (CV) system, as denoted by cases of severe cardiac dysfunction, arrhythmias, and cardiovascular death [8,9,10].
Cause-and-effect relations between CRS severity and CV toxicity have been hypothesized [8,9,10], but prospective studies that probed optimal modalities for detecting and monitoring inflammation and CV toxicity in this unique patient population are scarce [11]. The CARdio-TOX study is a single center, prospective, proof-of-concept study of adult patients affected by refractory or relapsed (R/R) hematologic malignancies and treated with CAR-T cells. We recently reported that patients recruited in CARdio-TOX exhibited impaired left ventricle ejection fraction (LVEF) and global longitudinal strain (GLS) as early as 7 days after CAR-T cells infusion, with concomitant changes of several exploratory diastolic metrics occurring at the same time point [12]. Patient re-evaluation at 30 days showed an incomplete recovery of systolic and diastolic parameters, possibly denoting that acute myocardial damage may or may not resolve over time and pave the road to chronic toxicity and late clinical outcomes.
In the present study, we aimed at elucidating both the incidence of Cancer therapy-related cardiac dysfunction (CTRCD), defined as an aggregate of echocardiographic and biomarker abnormalities, and correlations between imaging alterations and bio-humoral indexes of inflammation.
Patients and methods
Study design and patient characteristics
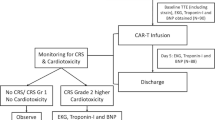
CARdio-TOX is a non-profit, investigator-initiated, prospective, single center, real-life study conducted at the Department of Cardiovascular Medicine of Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, between April 2022 and April 2023. Adult patients eligible to CAR-T cell therapy underwent clinical, electrocardiographic and echocardiographic evaluations at baseline and then at 7 and 30 days after CAR-T cell administration. Three anti CD19 CAR-T cell preparations were used according to clinicians’ indications: Axicabtagene Ciloleucel (Yescarta, Kite Pharmaceuticals, Santa Monica, California), Tisagenlecleucel (Kymriah, Novartis Pharmaceuticals, East Hanover, New Jersey), Brexucabtagene Autoleucel (Tecartus, Kite Pharma EU B.V.). Inclusion criteria were age > 18, years, LVEF ≥ 50% and a confirmed diagnosis of R/R CD19+ B-cell malignancy (lymphoma or acute lymphoblastic leukemia) with two or more prior systemic therapies. Exclusion criteria were age < 18 years and left ventricular ejection fraction (LVEF) < 50% or poor acoustic window at baseline echocardiography. The following information was extracted from patient medical records: prior CV events, CV risk factors (arterial hypertension, smoking, diabetes mellitus, dyslipidemia), history of potentially cardiotoxic therapies (chemotherapy, immunotherapy, left chest radiation therapy, autologous hematopoietic stem cell transplantation [HSCT].
The primary objective was the incidence of CTRCD at 7 days after CAR-T cell infusion. CTRCD was defined according to 2022 European Society of Cardiology (ESC) Cardio-Oncology guidelines (LVEF reduction by ≥ 10% points to an LVEF of 40–49%, or LVEF reduction by < 10% points to an LVEF of 40– 49%, or a decline of global longitudinal strain (GLS) by ≥ 15% from baseline, or increases in cardiac biomarkers such as troponin or B-type natriuretic peptide) [13]. Indexes of LV dysfunction (LVEF, GLS) were then correlated with serum inflammatory biomarkers such as C-reactive protein (CRP), fibrinogen, ferritin, soluble interleukin 2 receptor (sIL-2r), interleukin 6 (IL-6).
The study was approved by the Institutional Ethic Committee. No extramural funding supported this work. The authors are solely responsible for study design and conduct, study analyses, drafting and editing of the paper, as well as its final content.
Echocardiographic evaluation
Transthoracic 2D echocardiography (TTE) was performed using Philips EPIQ7C (Philips Medical Systems, Andover, Massachusetts, USA). Colour, pulsed-wave and continuous wave Doppler images were acquired from the parasternal, apical and subcostal views [14, 15]. All images were digitally stored for offline analyses by an experienced operator (L.M.). 2D-Strain (2D-ST) analysis was determined from views acquired during three consecutive cardiac cycles, using a TomTec-Arena TM software (TomTec Imaging Systems, Unterschleissheim, Germany). LV-GLS was calculated from the average values of four-chamber, two-chamber, and three-chamber curves. LV dimension, LA volume with strain analysis and right ventricle (RV) longitudinal function were measured according to recommendations by the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) [14,15,16]. LV diastolic function, non-invasive estimation of LV filling pressures, and valvular heart diseases were evaluated according to current recommendations [17, 18]. Intra-observer and inter-observer variability assessment and values for our echocardiographic laboratory have been previously described [19].
Cardiac and inflammatory biomarkers
Blood samples for cardiac and inflammatory biomarkers were drawn before and 7 days after CAR-T cell infusion. High-sensitivity troponin I (hsTnI), soluble protein ST2 (sST2) and the aminoterminal fragment of prohormone BNP (Nt-proBNP) were used as cardiac biomarkers; IL6, sIL2r, ferritin and fibrinogen were used as inflammatory biomarkers as per institutional clinical practice. All biomarkers were measured according to validated protocols of the institutional Medicinal Chemistry department. CRS grade and any required treatment for CRS management were in accordance to the American Society for Transplantation and Cellular Therapy (ASTCT) consensus [20].
Statistical analyses
Dichotomous variables were expressed as counts (percentage). The distribution of continuous variables was tested using Kolmogorov–Smirnov test. Mean ± standard deviation was used to express continuous variables with normal distribution, while median (interquartile range) was used for variables with non-normal distribution. Continuous variables were compared using an unpaired Student’s t test or Mann–Whitney U test. Categorical data were evaluated using the χ2 test or Fisher exact test, as appropriate.
Differences in each continuous echocardiographic parameter and inflammatory biomarker between day 7 and baseline were expressed as ∆:100*(day 7 minus baseline)/baseline.
All tests were two-sided, and statistical significance was set at P < 0.05. All analyses were performed using SPSS (SPSS version 23, Inc., Chicago, IL, USA) statistical software.
Results
Patient characteristics and CTRCD incidence
Forty-seven patients candidate for CAR-T cell therapy were screened, of whom 27 were eventually enrolled (Fig. 1).Sixteen patients (59,3% of the study population) were diagnosed CTRCD as per guidelines definition [13]. Patients with or without CTRCD were balanced for age, oncologic characteristics, common comorbidities and risk factors (hypertension, diabetes, smoking), as well as baseline laboratory and imaging findings; however, cardiotoxicity occurred more frequently in females (p = 0.042). CRS occurred in a total of 24 patients (88.8%) and the anti IL6 receptor antibody, tocilizumab, was used in 19 (70.4%) patients. Patients with or without CTRCD were also balanced for any grade CRS, but CTRCD occurred more often in patients with grade > 2 CRS [20]. Fever and usage of tocilizumab were therefore more frequent in patients with CTRCD (see also Table 1). As for CV events, we recorded only one case of non-fatal cardiac arrest in the context of severe CRS, a case of acute heart failure and a case of paroxysmal atrial fibrillation.
Patterns of CTRCD
Out of 16 patients diagnosed with CTRCD, 2 (12,5%) were characterized by only elevations of hsTnI or NT-proBNP, 6 (37,5%) were characterized by GLS decline ≥ 15% from baseline, and 8 (50%) were characterized by a composite of biomarkers and GLS with or without LVEF decrements (Fig. 2). Overall, GLS decrements were observed in 14 of 16 cardiotoxicity cases, followed by serum biomarkers elevations and LVEF decrements (9 and 5 of 16 cases, respectively). Patterns of LVEF and GLS changes are shown in Fig. 3, while a complete description of echocardiographic findings is reported in Table 1, Supplementary Materials.
Temporal trends of LVEF and GLS in patients treated with CAR-T cells. Each panel shows individual data and means ± SD (at baseline and 7 and 30 days after CAR-T cells). Single or triple asterisks indicate P < 0.05 or P < 0.001 for LVEF and GLS at day 7 or 30 versus baseline. Abbreviations GLS = global longitudinal strain; LVEF = left ventricular ejection fraction
Changes in serum cardiac biomarkers at day 7 after CAR-T cell therapy are reported in Table 2; Fig. 4. In addition to significant elevations of hsTnI and NT-proBNP, there was a significant increase of sST2, marker of myocardial fibrosis (see also Table 2).
Correlations between left ventricular systolic function and inflammatory markers
We characterized whether day 7 changes of LVEF and GLS, parameters of systolic function reported in ESC guidelines definition of CTRCD [13], correlated with changes of inflammatory biomarkers at the same time point. A statistically significant inverse correlation occurred between changes of LVEF or GLS and sIL2r or fibrinogen (Fig. 5). No correlation was observed with CRP, IL6, ferritin (not shown).
Significant Inverse Correlations between Early Changes in Echocardiographic Parameters and Inflammatory Indexes. Data were linear regression analyses with 95% confidence intervals of percentage differences of echocardiographic parameters versus inflammatory indexes, all expressed as 100*(day 7 minus baseline)/baseline. Panel A, LVEF versus fibrinogen; Panel B, LVEF versus sIL2r; panel C, GLS versus fibrinogen; panel D, GLS versus sIL2r. Similar results were obtained by non-parametric two-tailed correlation (p = 0.052, 0.005, 0.062 and 0.0001 in panels A, B, C and D, respectively). Abbreviations GLS = left ventricular global longitudinal strain; sIL2r = soluble interleukin 2 receptor; LVEF = left ventricular ejection fraction; d7 = day 7; BL, baseline
Discussion
Uncertainties remain around the actual incidence of CTRCD in patients treated with CAR-T cells [21,22,23,24,25,26,27]. A study of 137 patients found that 5.8% of them developed a significant drop in LVEF (defined as a decrease of at least 10% points to a value below 50%), mainly associated with the occurrence of grade ≥ 2 CRS [22]. Other studies showed that a reduction of LVEF below 50% or > 10% from baseline during index hospitalization occurred in 10.3% of 116 patients, with a decline in median LVEF from 58 to 37% at ∼ 12 days from CAR T-cell infusion [23]. Again, most of patients diagnosed with CTRCD had grade ≥ 2 CRS, further highlighting possible cause-and-effect relations between systemic inflammation and cardiotoxicity. On the other hand, the recently released ESC Cardio-Oncology guidelines recommend a definition of CTRCD that integrates abnormalities of imaging parameters and serum biomarkers, such as LVEF, GLS, troponin and natriuretic peptide [13]. In accordance with this definition, we were able to diagnose CTRCD in as many as 16 patients treated with CAR-T cells. Had we defined CTRCD only on the basis of LVEF decrements, its incidence would have been 18,5% (5 cases out of 27 patients).
The high incidence of CTRCD observed in our study warrants further considerations. Our patients received serial echocardiographic and biomarkers evaluations, which were done prospectively at pre-specified time points. This approach likely avoided the risk of underestimating CTRCD, that otherwise would bias studies in which imaging and laboratory evaluations were event-driven. In this context, it was not until recently when Lefebvre et al. prospectively evaluated 44 patients prior to and 2 days, 1 week, 1 month, and 6 months after CAR-T cell infusion [11]. No significant changes in LVEF were noticed across study visits and only a modest decrease in GLS was shown to occur at the early time points. Interestingly, only 52% of subjects developed CRS as opposed to at least 70% in previous reports and 89% in our present study; moreover, CRS was of a low grade in 95% of cases [11]. Lower incidence and severity of CRS may help to explain the milder cardiovascular manifestations observed by Lefebvre et al. compared to our study.
Having said that the high incidence of CTRCD observed in our study originates from the updated definition of CTRCD released by ESC [13], largely based on subclinical indexes like GLS and serum biomarkers, we believe that detecting such early manifestations might be important for improving CV outcomes in the cancer patient. A multicenter registry of 202 CART-cell patients receiving anti-CD19 therapies aimed at assessing a composite endpoint of heart failure, cardiogenic shock, or myocardial infarction [25]. Sixteen percent of subjects experienced severe cardiac events, which were independently associated with overall mortality (hazard ratio 2.8). In analyzing determinants of event occurrence the authors identified a role for CV risk factors, in particular hypertension and history of atrial fibrillation or heart failure; interestingly, however, there was no information on clinical usage of anti-IL6 medications to mitigate CRS in these patients [25]. Post-marketing analyses similarly showed a fatality rate of CV and pulmonary adverse events, including late-occurring cardiomyopathy, tachyarrhythmias, pleural and pericardial effusions, as high as 30.9% [26]. None of our patients presented at treatment with a history of heart failure, ischemic heart disease, or arrhythmias, which probably explains why we did not record a significant incidence of fatal CV events after CAR-T cell therapy. However, the high incidence of CTRCD that we characterized as early as 7 days after CAR-T cell infusion, serves a rationale to intensify CV surveillance in high-risk patients with a potentially worse CV outcome. The ESC cardio-oncology guidelines advocate intensive surveillance in cases of signs and symptoms of congestion or high-grade CRS, but they do not clarify how often and how long patients should be surveilled [13].
There are further differences, as well as similarities, between our study and previously published reports. Older age, dyslipidemia and coronary artery disease were reported to increase the risk of cardiomyopathy after CAR-T cell therapy [22]. As already mentioned, our study did not recruit patients with a history of ischemic heart disease but hypertension, diabetes and smoking were equally represented among patients with or without CTRCD. On a different note, but in agreement with others [22, 23], we also found that neither the number of prior lines of therapy nor the cumulative anthracycline dose were significantly different among patients with or without CTRCD. This latter finding denotes the distinct nature of CAR-T cells cardiotoxicity, as previous treatment with anthracyclines usually aggravates the risk of cardiotoxicity upon patient’s exposure to subsequent cancer therapies.
Limitations
We acknowledge this was a single institution study, with a small sample size. The lack of a control arm in which patients with the same oncologic diagnosis received treatments with other drugs, possibly including newly developed bispecific antibodies that also cause some degree of CRS, preclude further considerations on the actual risk:benefit of CAR-T cells in terms of CV liability. Furthermore, the very low incidence of major CV events in our study population, likely reflecting the extensive use of anti-IL6 medication and short-follow-up, does not allow us to approximate how well such events would have been predicted by the imaging and bio-humoral markers we used to define CTRCD. Finally, the majority of subjects enrolled were males, preventing generalization of findings to females.
Strengths
In addition to denoting the value of integrating echocardiographic parameters with serum biomarkers, this study provides novel information on the relations between inflammation and CTRCD. We in fact confirmed a higher incidence of CTRCD in patients with grade ≥ 2 CRS, but we also investigated, for the first time in adult patients [27], on the association between changes in LVEF or GLS and inflammatory biomarkers. We did not find correlations with CRP, IL6 and ferritin, as tocilizumab interferes with IL6 assay [28] and reduces both CRP [29] and ferritin levels [30]; however, we found significant correlations with sIL2R, whose levels are relatively stable after tocilizumab initiation [31], and with fibrinogen, which shows longer half-life than ferritin [32, 33] and thus attains circulating levels more suitable for correlation analyses once tocilizumab has been started. These findings strengthen a causative link between inflammation and CAR-T cells cardiotoxicity, paving the road to further studies in these settings [34].
In addition, besides conventional cardiac biomarkers such as hsTnI and NT-proBNP, we characterized early changes of sST2, currently considered as an index of myocardial remodeling and fibrosis [35]. sST2 significantly increased at day 7, similar to hsTnI and Nt-proBNP (Table 2). As the ST2 gene is upregulated in the setting of myocardial stretch, these findings raise one more research issue in the settings of CAR-T cells cardiotoxicity [35].
Conclusions
This prospective study shows, for the first time, that a large proportion of patients treated with anti CD19 CAR-T cells may experience acute CTRCD, as defined by recent Cardio-Oncology guidelines. Moreover, a remarkable association of CTRCD with an inflammatory primum movens is confirmed by more direct correlations than in previous studies [36]. We therefore propose a systematic approach of clinical surveillance and comprehensive evaluation of patients undergoing CAR-T cell therapy, including both imaging and laboratory indexes as suggested by cardio-oncology guidelines. Early monitoring would remarkably assist the identification of patients at risk of developing severe cardiomyopathy. An extended follow-up, which was beyond the aims of this proof-of-concept study, would in turn elucidate the size effect of acute cardiotoxicity on late clinical outcomes.
Data availability
The datasets used and analysed in this study are available from the corresponding author on reasonable request.
Abbreviations
- CAR-T:
-
chimeric antigen receptor-T
- CRP:
-
c-reactive protein
- CRS:
-
cytokine release syndrome
- CTRCD:
-
cancer therapy-related cardiac dysfunction
- CV:
-
cardiovascular
- IL:
-
interleukin
- LA:
-
left atrium
- LVEF:
-
left ventricular ejection fraction
- LVGLS:
-
left ventricular global longitudinal strain
References
Jain T, Bar M, Kansagra AJ, et al. Use of Chimeric Antigen Receptor T Cell Therapy in clinical practice for Relapsed/Refractory aggressive B cell Non-hodgkin Lymphoma: an Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transpl. 2019;25:2305–21. https://doi.org/10.1016/j.bbmt.2019.08.015.
Kansagra AJ, Frey NV, Bar M, et al. Clinical Utilization of Chimeric Antigen Receptor T Cells in B Cell Acute Lymphoblastic Leukemia: An Expert Opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and marrow transplantation. Biol Blood Marrow Transpl. 2019;25:e76–85. https://doi.org/10.1016/j.bbmt.2018.12.068.
Tang HKC, Wang B, Tan HX, et al. CAR T-Cell therapy for Cancer: latest updates and challenges, with a focus on B-Lymphoid malignancies and selected solid tumours. Cells. 2023;12:1586. https://doi.org/10.3390/cells12121586.
Mucha SR, Rajendram P. Management and Prevention of Cellular-Therapy-related toxicity: early and late complications. Curr Oncol. 2023;30:5003–23. https://doi.org/10.3390/curroncol30050378.
Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. https://doi.org/10.1016/j.blre.2018.11.002.
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–95. https://doi.org/10.1182/blood-2014-05-552729.
Shah D, Soper B, Shopland L. Cytokine release syndrome and cancer immunotherapies - historical challenges and promising futures. Front Immunol. 2023;14:1190379. https://doi.org/10.3389/fimmu.2023.1190379.
Strati P, Gregory T, Majhail NS, Jain N. Chimeric Antigen receptor T-Cell therapy for hematologic malignancies: a practical review. JCO Oncol Pract. 2023;OP2200819. https://doi.org/10.1200/OP.22.00819.
Marar RI, Abbasi MA, Prathivadhi-Bhayankaram S, et al. Cardiotoxicities of Novel therapies in Hematologic malignancies: chimeric Antigen receptor T-Cell therapy and bispecific T-Cell Engager Therapy. JCO Oncol Pract. 2023;19:331–42. https://doi.org/10.1200/OP.22.00713.
Ganatra S, Dani SS, Yang EH, Zaha VG, Nohria A. Cardiotoxicity of T-Cell antineoplastic therapies: JACC: CardioOncology primer. JACC CardioOncol. 2022;4:616–23. https://doi.org/10.1016/j.jaccao.2022.07.014.
Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M. Cardiovascular effects of CAR T Cell Therapy: a retrospective study. JACC CardioOncol. 2020;2(2):193–203. https://doi.org/10.1016/j.jaccao.2020.04.012.
Camilli M, Lombardo A, Crea F, Minotti G. Temporal Patterns of Left Ventricular Systolic and Diastolic Metrics Changes in Adult Patients with Hematological Malignancies Treated With Chimeric Antigen Receptor (CAR)-T Cells: Results from the CARdio-Tox Prospective Study. Eur Heart J Cardiovasc Imaging. In presshttps://doi.org/10.1093/ehjci/jead317.
Lyon AR, López-Fernández T, Couch LS, ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–4361. https://doi.org/10.1093/eurheartj/ehac244.
Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. https://doi.org/10.1016/j.echo.2014.10.003. PMID: 25559473.
Galderisi M, Cosyns B, Edvardsen T et al. 2016–2018 EACVI Scientific Documents Committee; 2016–2018 EACVI Scientific Documents Committee. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18:1301–1310. https://doi.org/10.1093/ehjci/jex244. PMID: 29045589.
Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. https://doi.org/10.1093/ehjci/jey042.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by Echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. https://doi.org/10.1016/j.echo.2016.01.011.
Vahanian A, Beyersdorf F, Praz F, ESC/EACTS Scientific Document Group, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. https://doi.org/10.1093/eurheartj/ehab395.
Camilli M, Iannaccone G, Russo M, et al. Early improvement of strain imaging parameters predicts long-term response to sacubitril/valsartan in patients with heart failure with reduced ejection fraction: an observational prospective study. Int J Cardiol. 2023;131110. https://doi.org/10.1016/j.ijcard.2023.06.001.
Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and neurologic toxicity Associated with Immune Effector cells. Biol Blood Marrow Transpl. 2019;25:625–38. https://doi.org/10.1016/j.bbmt.2018.12.758.
Totzeck M, Anker MS, Rassaf T. CAR T-cell cancer therapies: do not forget the heart. Eur Heart J. 2023;44:2043–5. https://doi.org/10.1093/eurheartj/ehad175.
Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular events among adults treated with chimeric Antigen receptor T-Cells (CAR-T). J Am Coll Cardiol. 2019;74:3099–108. https://doi.org/10.1016/j.jacc.2019.10.038.
Ganatra S, Redd R, Hayek SS, et al. Chimeric Antigen receptor T-Cell therapy-Associated Cardiomyopathy in patients with refractory or relapsed Non-hodgkin Lymphoma. Circulation. 2020;142:1687–90. https://doi.org/10.1161/CIRCULATIONAHA.120.048100.
Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M. Cardiovascular effects of CAR T Cell Therapy: a retrospective study. JACC CardioOncol. 2020;2:193–203. https://doi.org/10.1016/j.jaccao.2020.04.012.
Mahmood SS, Riedell PA, Feldman S, et al. Biomarkers and cardiovascular outcomes in chimeric antigen receptor T-cell therapy recipients. Eur Heart J. 2023;44:2029–42. https://doi.org/10.1093/eurheartj/ehad117.
Goldman A, Maor E, Bomze D, et al. Adverse Cardiovascular and pulmonary events Associated with chimeric Antigen receptor T-Cell therapy. J Am Coll Cardiol. 2021;78:1800–13. https://doi.org/10.1016/j.jacc.2021.08.044.
Shalabi H, Sachdev V, Kulshreshtha A, et al. Impact of cytokine release syndrome on cardiac function following CD19 CAR-T cell therapy in children and young adults with hematological malignancies. J Immunother Cancer. 2020;8:e001159. https://doi.org/10.1136/jitc-2020-001159.
Chen F, Teachey DT, Pequignot E, et al. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. 2016;434:1–8. https://doi.org/10.1016/j.jim.2016.03.005.
Berman M, Berliner S, Bashouti N, Elkayam O, Ziv-Baran T. Reduced C-reactive protein level at hospital admission in patients treated with Tocilizumab - An attention may be required. Heliyon. 2023;9(6):e16665. https://doi.org/10.1016/j.heliyon.2023.e16665.
Guz D, Gafter-Gvili A, Lev N, Sahaf Levin G, Lev S. Tocilizumab Treatment Effect on Iron Homeostasis in severe COVID-19 patients. Acta Haematol. 2022;145(4):440–7. https://doi.org/10.1159/000522307. Epub 2022 Jan 31.
Azmy V, Kaman K, Tang D, et al. Cytokine profiles before and after Immune Modulation in Hospitalized patients with COVID-19. J Clin Immunol. 2021;41(4):738–47. https://doi.org/10.1007/s10875-020-00949-6.
Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23(3):95–104. https://doi.org/10.1016/j.blre.2008.08.001.
Vilar R, Fish RJ, Casini A, Neerman-Arbez M. Fibrin(ogen) in human disease: both friend and foe. Haematologica. 2020;105(2):284–96. https://doi.org/10.3324/haematol.2019.236901.
Camilli M, Maggio L, Tinti L, et al. Chimeric antigen receptor-T cell therapy-related cardiotoxicity in adults and children cancer patients: a clinical appraisal. Front Cardiovasc Med. 2023;10:1090103. https://doi.org/10.3389/fcvm.2023.1090103.
Coglianese EE, Larson MG, Vasan RS, et al. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem. 2012;58:1673–81. https://doi.org/10.1373/clinchem.2012.192153.
Lee DH, Chandrasekhar S, Jain MD, et al. Cardiac and inflammatory biomarker differences in adverse cardiac events after chimeric antigen receptor T-Cell therapy: an exploratory study. Cardiooncology. 2023;9:18. https://doi.org/10.1186/s40959-023-00170-5.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MC, MV, TF, LM, FB, GC, AB, PL, LT, ADR, GC, EG: idealization, statistical analysis and writing. GL, FB, RAM, FS, SS, SH, GAL, FC: supervision. AL, GM: idealization, writing and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained for the present study by Policlinico Universitario Agostino Gemelli IRCSS, Rome (ID6000).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Camilli, M., Viscovo, M., Felici, T. et al. Inflammation and acute cardiotoxicity in adult hematological patients treated with CAR-T cells: results from a pilot proof-of-concept study. Cardio-Oncology 10, 18 (2024). https://doi.org/10.1186/s40959-024-00218-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40959-024-00218-0