Abstract
Background
Programmed cell death ligand 1 (PD-L1) is an immune checkpoint molecule that attenuates the immune response. PD-L1 contributes to failed antitumor immunity; thereby, blockade of PD-L1 with monoclonal antibody enhances the immune response. Recently, it was reported that PD-L1 was regulated by protein 53 (p53). Besides, cytokeratin 17 (CK17) is thought to be a diagnostic marker of oral squamous cell carcinoma (OSCC). Our aim was to evaluate the correlation between the immunohistochemical expression of PD-L1, p53 and CK17 with clinicopathological characteristics and disease-specific survival in patients with OSCC.
Methods
A total of 48 patients with OSCC were included in this study. Immunohistochemical staining was performed to evaluate the correlation among the expressions of PD-L1, p53 and CK17, and furthermore the correlation among various clinicopathological factors, PD-L1, p53 and CK17.
Results
The positive rate of p53, CK17, PD-L1 (tumor cells) and PD-L1 (tumor-infiltrating lymphocytes) was 63.2%, 91.7%, 48.9% and 57.1%. A statistically significant correlation between p53 expression and T stage and TNM stage (p = 0.049, p = 0.03, respectively) was observed. Also, a statistically significant correlation between p53 and PD-L1 (TCs) expression (p = 0.0009) was observed. Five-year disease-specific survival rate was not significantly correlated with gender, TNM stage, p53 expression, PD-L1 expression and CK17 expression.
Conclusion
The expression of p53 and PD-L1 shows significantly positive correlation in oral squamous cell carcinoma in tumor cells. Also, a significant correlation between p53 expression and T stage and TNM stage was observed. No other significant correlation between PD-L1 staining or CK17 and clinical or pathologic characteristics was identified.
Similar content being viewed by others
Background
Programmed death ligand 1 (PD-L1) is a cell-surface protein that has been proved to be overexpressed on various cells including tumor cells, lymphocytes and other tissues in many human cancers [1]. PD-L1 promotes T-cell tolerance and escape host immunity. It is reported that increased PD-L1 and PD-1 expression is predictive of nodal metastasis and poor prognosis in oral squamous cell carcinoma [2, 3].
Protein 53 (p53) is one of the most commonly mutated genes in cancer [4]. It is critical in regulating cell division, apoptosis, senescence and DNA damage and repair. p53 is also important for modulating the immune response. When DNA is damaged, p53 gene transcription is increased and wild-type p53 protein is concentrated, which result in the arrest of cell cycle at the G1/S phase and apoptosis of cancer cells. When the p53 gene is mutated, the mutant p53 protein loses its cancer inhibition function and promotes the transformation of normal cells to malignant cells. Mutant p53 is present in almost all types of human malignant tumor. Also, mutant p53 is closely correlated with oral squamous cell carcinoma [5,6,7].
Cytokeratins (CKs), intermediate filament of the cytoskeletons, are candidates for diagnostic markers of OSCC, as they are overexpressed in OSCC compared to normal mucosa. The significant up-regulation and the strong overexpression of CK17 could exhibit its utility as a diagnostic marker of OSCC [8,9,10].
In this study, we aimed to evaluate the correlation between the immunohistochemical expression of PD-L1, p53 and CK17 with clinicopathological characteristics and disease-specific survival in patients with OSCC.
Methods
The study comprised a total of 49 selected patients (29 males and 20 females) with oral squamous cell carcinomas (OSCCs) at the Department of Oral and Maxillofacial Surgery, Wakayama Medical University Hospital, between 1990 and 2010. Patients were randomly selected. This study followed the Declaration of Helsinki on medical protocol and ethics, and the regional ethical review board of Wakayama Medical University approved the study (Protocol Identification Number 2512).
The patients ranged in age from 49 to 91 years, with a mean age of 65.7 years. The primary malignant tumors were located on the lower gingiva in 18 cases, the tongue in 11 cases, the upper gingiva in 5 cases, the oral floor in 7 cases, the maxillary sinus in 3 cases, the hard palate in 2 cases, and the soft palate in 3 cases. Tumor staging was performed according to the specifications of the TNM classification of malignant tumors (UICC 1997). The mode of tumor invasion was assessed according to the classification by Yamamoto et al as follows: grade 1 = well-defined borderline; grade 2 = cords, less marked borderline; grade 3 = groups of cells, no distinct borderline; and grade 4 = diffuse invasion (4C = cordlike type; 4D = widespread type) [11].
Immunohistochemical staining
The PD-L1, P53 and CK17 expression OSCC tissues were evaluated from serial deparaffinized sections. The OSCC biopsy specimens had been fixed with formalin from 24 to 48 h at room temperature and treated with routine processing as in a previous study [12, 13]. Four micrometer-thick sections of paraffin-embedded tissues were mounted on precoated slides and air-dried overnight at 58°C. The serial sections were prepared for staining and were incubated with primary antibodies for 12 h. Immunohistochemistry (IHC) was performed using a Discovery Auto-Stainer with automated protocols (Ventana Medical Systems, Inc.; Roche) as previously described [13, 14].
The following commercial antibodies were purchased: PD-L1 (1:1, rabbit monoclonal, 790-4905; Ventana Medical Systems, Inc.; Roche, USA), p53 (1:100, mouse monoclonal, M7001; Dako Denmark, Glostrup, Denmark) and CK17 (1:50, mouse monoclonal, M7046; Dako Denmark, Glostrup, Denmark).
Evaluation of staining results
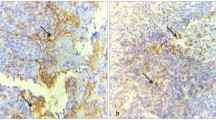
The tumor biopsy sample was considered positive for PD-L1 expression in tumor cells if moderate-to-strong membrane staining was observed in ≥5% of tumor cells (TCs) based on previous larger studies (Fig.1a, b). Tumor-infiltrating lymphocytes (TILs) were considered to have positive PD-L1 expression if ≥1% of TILs exhibited moderate-to-strong membrane staining, as previously described (Fig. 1a, c) [7].
Representative images of PD-L1, p53 and CK17 expression by IHC. a PD-L1 expression negative. b PD-L1 expression of TCs moderate positive. c PD-L1 expression of TILs moderate positive. d p53 expression negative. e p53 expression moderate positive. f p53 expression strong positive. g CK17 expression negative. h CK17 expression weak positive. i CK17 expression strong positive. Scale bars: 100μm
p53 expression was considered positive when ≥10% of the TC nuclei (Fig. 1d, e, f) [7].
The CK17-stained sections with <5 % reactive cells were considered to be negative, and those with more than 5 % reactive cells were defined as positive (Fig. 1g, h, i) The sections were divided into two groups as follows: over 60 % positive cells were defined as “strong” cases; less than 60 % positive cells were defined as “weak” cases [8].
The Chi-square (χ2) test and Fisher’s exact test were used to analyze differences in categorical variables (including gender, age, TNM stage, differentiation, mode of invasion) between positive PD-L1/P53/CK17 expression groups and negative PD-L1/P53/CK17 expression groups. In addition, univariate survival analysis and survival curves were conducted by Kaplan–Meier method and the log-rank test was routinely used to test for significant differences.
Results
The differentiation degree was well differentiated in 35 cases, moderately differentiated in 11 and poorly differentiated in three cases. The mode of invasion was grade 1 in 3 cases, grade 2 in 9, grade 3 in 17, grade 4C in 16 and grade 4D in 4 cases. Positive CK17, p53, PD-L1 (TCs) and PD-L1 (TILs) staining was present in 91.7%, 63.2%, 48.9% and 57.1% of all cases, respectively.
Table 1 shows the clinical and pathologic characteristics associated with PD-L1, p53 and CK17 expression. With respect to tumor stage, a statistically significant correlation between p53 expression and T stage and TNM stage (p = 0.049, p = 0.03, respectively) was observed. Also, a statistically significant correlation between p53 and PD-L1 (TCs) expression (p = 0.0009) was observed. No other significant correlation between PD-L1 staining or CK17 and clinical or pathologic characteristics was identified (Table 1).
However, the 5-year disease-specific survival rate by Kaplan–Meier method of the cases with PD-L1 (no expression) or CK17 (strong expression) tended to be slightly low, but not significant (Fig. 2a, b, d). Five-year disease-specific survival rate was not significantly correlated with p53 expression (Fig. 2c). The survival rate was not significantly correlated even with PD-L1 (+) and p53 (+).
Hazard ratio indicated that the 5-year disease-specific survival rate was not significantly correlated with gender, TNM stage, p53 expression, PD-L1 expression, and CK17 expression (Table 2).
Discussion
In this study, it is shown that the expression of PD-L1 is correlated with the expression of p53 in oral squamous cell carcinoma.
PD-L1 overexpression is recognized in many human cancers, promoting T-cell tolerance and escape host immunity. Early clinical trials using monoclonal antibodies that block the PD1/PDL1 interaction have shown promise in some patients with advanced cancer. OSCC patients with high PD-L1 expression had poor clinical outcome and might require PD-L1-targeted immunotherapy to improve their prognosis. Mutant p53 is present in almost all types of human tumor and is closely correlated with the development of OSCC. Mutated p53 loses its ability to suppress the function of oncogenes. Furthermore, mutant p53 may function as an oncogene to stimulate cell division and promote the growth of tumor cells [6].. Although whether p53 is involved in tumor immune evasion has been poorly understood, Cortez reported that PD-L1 is regulated by p53 via micro RNA (miR-34a) using a series of experiments involving lung cancer cell lines [15].
Regarding tumor cells, the expression of PD-L1 and p53 is positively correlated, because wild-type p53 is rapidly degraded (~0.5h); however, as the resolution time of variant p53 protein is delayed (›2h) and the protein is accumulated in the nucleus, the variant p53 protein is identified as overexpression [16, 17]. Although wild-type p53 inhibits the expression of PD-L1 directly, when variant p53 which has lost a function is accumulated, PD-L1 is overexpressed. Thus, it is thought that the expressions of p53 and PD-L1 show positive correlation in oral squamous cell carcinoma in this study.
Furthermore, based on the results of Cancer Genome Atlas exome data analysis, there is a link between P53 status and mutation burden in tumors [18]. That is to say that the evaluation of P53 status could be used as a surrogate biomarker for mutation burden [19]. At the same time, although many different factors modulate the clinical response to an immune checkpoint inhibitor, the strong relationship between the tumor mutational burden and the activity of anti-PD-1 therapies across multiple cancers has been highlighted and the association of p53 and PD-L1 also suggested.
Conclusion
In this study, the expression of p53 and PD-L1 shows a positive correlation in oral squamous cell carcinoma in tumor cells for the first time. No other significant correlation between PD-L1 staining or CK17 and clinical or pathologic characteristics was identified.
Availability of data and materials
Please contact the author for data requests.
Abbreviations
- CK17:
-
Cytokeratin 17
- IHC:
-
Immunohistochemistry
- OSCC:
-
Oral squamous cell carcinoma
- p53:
-
Protein 53
- PD-L1:
-
Programmed cell death ligand 1
- TCs:
-
Tumor cells
- TILs:
-
Tumor-infiltrating lymphocytes
References
Sharmal P, Allison JP (2015) The future of immune checkpoint therapy. Science 348(6230):56–61
Maruse Y, Kawano S, Jinno T, Matsubara R, Goto Y, Kaneko N et al (2018) Significant association of increased PD-L1 and PD-1 expression with nodal metastasis and a poor prognosis in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg 47:836–845
Lin YM, Sung WW, Hsieh MJ, Tsai SC, Lai HW, Yang SM et al (2015) High PD-L1 Expression Correlates with Metastasis and Poor Prognosis in Oral Squamous Cell Carcinoma. PLoS ONE 10(11):e0142656. https://doi.org/10.1371/journal.pone.0142656
Pfeifer GP, Holmquist GP (1997) Mutagenesis in the P53 gene. Biochim Biophys Acta 1333(1):M1–M8
Elias J, Dimitrio L, Clairambault J, Natalini R (2014) The p53 protein and its molecular network: modelling a missing link between DNA damage and cell fate. Biochim Biophys Acta 1844(1 Pt B):232–247
Li Y, Zhang J (2015) Expression of mutant p53 in oral squamous cell carcinoma is correlated with the effectiveness of intra-arterial chemotherapy. Oncol Lett 10:2883–2887
Yu X, Zhang X, Wang F, Lin Y, Wang W, Chen Y et al (2018) Correlation and prognostic significance of PD-L1 and P53 expression in resected primary pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis 10(3):1891–1902
Kitamura R, Toyoshima T, Tanaka H, Kawano S, Kiyosue T, Matsubara R et al (2012) Association of cytokeratin 17 expression with differentiation in oral squamous cell carcinoma. J Cancer Res Clin Oncol 138:1299–1310
Xu XC, Lee JS, Lippman SM, Ro JY, Hong WK, Lotan R (1995) Increased expression of cytokeratins CK8 and CK19 is associated with head and neck carcinogenesis. Cancer Epidemiol Biomarkers Prev 4(8):871–876
Toyoshima T, Vairaktaris E, Nkenke E, Schlegel KA, Neukam FW, Ries J (2008) Cytokeratin 17 mRNA expression has potential for diagnostic marker of oral squamous cell carcinoma. J Cancer Res Clin Oncol 134(4):515–521
Yamamoto E, Kohama G, Iwai M, Hiratsuka H (1983) Mode of invasion, Bleomycin sensitivity and clinical course in squamous cell carcinoma of the oral cavity. Cancer 51:2175–2180
Hiraishi Y, Wada T, Nakatani K, Negoro K, Fujita S (2006) Immunohistochemical Expression of EGFR and p-EGFR in Oral Squamous Cell Carcinomas. Pathol Oncol Res 12(2):87–91
Sato F, Bhawal U, Tojyo I, Fujita S, Murata S, Muragaki Y (2019) Differential expression of claudin-4, occluding, SOX2 and proliferating cell nuclear antigen between basaloid squamous cell carcinoma and squamous cell carcinoma. Mol Med Rep 20:1977–1985
Sato F, Kohsaka A, Takahashi K, Otao S, Kitada Y, Iwasaki Y et al (2017) Smad3 and Bmal1 regulate p21 and S100A4 expression in myocardial stromal fibroblasts via TNF-α. Histochem Cell Biol 148:617–624
Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y et al (2015) PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst 108(1): djv303
Gronostajski RM, Goldberg AL, Pardee AB (1984) Energy requirement for degradation of tumor-associated protein p53. Mol Cell Biol 4:442–448
Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, Levine AJ (1988) Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol 8:531–539
Shim HS, Kenudson M, Zheng Z, Liebers M, Cha YJ, Ho QH et al (2015) Unique Genetic and Survival Characteristics of Invasive Mucinous Adenocarcinoma of the Lung. J Thorac Oncol 10:1156–1162
Cha YJ, Kim HR, Lee CY, Cho BC, Shim HS (2016) Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer 97:73–80
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research (16K11697) from the Japan Society for the Promotion of Science.
Funding
There is no funding related to this article.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. IT read and wrote the manuscript. IT, YS, TN, ME and FS performed most of the experiments. IT, KO and YH prepared retrospective data. YM revised and corrected the manuscript. IT and SF designed and wrote the entire article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study followed the Declaration of Helsinki on medical protocol and ethics, approved by the regional ethical review board of Wakayama Medical University (Protocol Identification Number 2512). General consent was given by the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tojyo, I., Shintani, Y., Nakanishi, T. et al. PD-L1 expression correlated with p53 expression in oral squamous cell carcinoma. Maxillofac Plast Reconstr Surg 41, 56 (2019). https://doi.org/10.1186/s40902-019-0239-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40902-019-0239-8