Abstract
Obstructive sleep apnea (OSA) and hypertension are two important modifiable risk factors for cardiovascular disease and mortality. Numerous studies have highlighted the interplay between these two conditions. We provide a critical review of the current literature on the role of the OSA as a risk factor for hypertension and its effect on blood pressure (BP). We discuss several key topics: the effect of OSA on nocturnal BP, BP response to continuous positive airway pressure (CPAP) treatment, CPAP effect on BP in refractory hypertension, the role of OSA in BP variability (BPV), and maladaptive cardiac remodeling mediated by OSA’s effect on BP. Finally, we discuss the unique aspects of ethnicity and social determinants of health on OSA with a focus on Asian populations and the disparity in BP control and cardiovascular outcomes.
Similar content being viewed by others
Background
Hypertension is one of the most important risk factors for cardiovascular disease and mortality. Numerous studies have suggested the crucial role of sleep in blood pressure (BP) regulation. Recently, sleep has also been included by the American Heart Association as one of the 8 elements essential for cardiovascular health [1]. Obstructive sleep apnea (OSA) is a common sleep disorder characterized by repetitive upper airway obstruction during sleep and is a well-established cause of poor sleep. The goal of this paper is to provide a critical overview of the interplay between OSA and hypertension by summarizing the existing evidence regarding pathophysiological link and implications of OSA treatment with a special focus on the areas that have not been sufficiently discussed in the literature. We further discuss gaps and future directions on this topic.
OSA and hypertension: epidemiology
Hypertension is one of the most well-recognized modifiable risk factors for cardiovascular morbidity and mortality [2]. Although the etiology of hypertension is largely idiopathic (“essential”), genetic predisposition, lifestyle factors including diet, exercise, smoking, and stress, and comorbidities such as metabolic dysfunction and renal dysfunction have an important pathophysiological link to hypertension [3, 4].
Sleep is another important lifestyle factor that is increasingly recognized to have an impact on BP regulation [5]. In particular, numerous studies have suggested that a pathological sleep disorder such as OSA is an independent risk factor for hypertension. OSA is a common sleep disorder affecting nearly 10–15% of women and 15–30% of men [5, 6]. It is a spectrum of conditions that includes a wide range of severity of upper airway obstructions during sleep. OSA impairs sleep quality due to frequent episodes of OSA-related arousal and compromises sleep architecture. Patients with OSA exhibit a high prevalence of hypertension and those with hypertension have a high prevalence of OSA. Furthermore, several prospective studies hint that OSA may increase the risk of incident hypertension. A landmark study from the Wisconsin Sleep Cohort study (WSCS) showed that OSA is associated with an increased future risk of hypertension independent of age, sex, body mass index (BMI), and other potential confounders at baseline in a dose-dependent manner; the more severe the OSA, the higher risk of incident hypertension [7]. On the contrary, in some studies the association between OSA and incident hypertension attenuates and becomes insignificant after adjusting for potential confounders such as age, sex, and BMI. For example, the association between OSA and incident hypertension in the Sleep Heart Health Study (SHHS) was no longer significant after controlling for BMI when followed over 5 years [8]. This discrepancy can be explained in part by the age difference between the cohorts in either study; SHHS patients were much older than those in WSCS (60 years versus 47 years) [9]. The strength of the association between OSA severity and incident hypertension appears to decline with age. In SHHS, a reduced correlation between OSA severity and incident hypertension above an age cut-off of 60 years was demonstrated. Similarly, in the Vitoria Sleep Cohort (VSC), there was a dose-dependent association between OSA severity and incident hypertension in 1,180 middle- and old-aged patients followed for 7.5 years, but the association was reduced and no longer significant after controlling for age [10]. Other reasons for the disparate results between WSCS, SHHS, and VSC may be due to population differences and differences in assessments for OSA. For example, the reference in WSCS used an AHI of 0/hr, whereas in SHHS, the reference used a range of AHI: 0–4.9/hr. In contrast, the reference in VSC used 0–2.9/hr (1st quartile for Respiratory Disturbance Index (RDI)).
In Asian adults, daytime BP was associated with age and OSA severity in a Taiwanese cross-sectional study [11]. In this study, the BP and age were correlated in patients with mild to moderate OSA, but not in those with severe OSA. When compared to the Westerners, Asians were found to have a greater prevalence of increased nighttime BP or morning BP surge, as assessed by ambulatory BP monitoring (ABPM). In a Japanese study on 38 adult patients with severe OSA, both daytime and nighttime BPs were significantly reduced and 15 of 22 non-dippers became dippers after continuous positive airway pressure (CPAP) treatment for 3 days [12]. However, a prospective longitudinal study on the association between OSA and incident hypertension in Asian populations is still lacking.
OSA and hypertension: pathophysiology
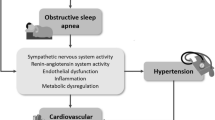
The pathophysiology of hypertension in OSA is complex and involves both direct and indirect mechanisms. The acute impact of OSA is well established. Each OSA event leads to varying degrees of cascades of cardiovascular and central nervous system responses including sympathetic surge, hypoxemia, hypercapnia, intrathoracic pressure swings and arousal [13,14,15]. Sympathetic responses can be manifested as an abrupt BP surge and peripheral vasoconstriction [16]. The mechanism of sympathetic surge is unclear but may be related to hypoxia-induced stimulation of the carotid body chemoreceptors, causing reflex sympathetic stimulation of the medullary cardiorespiratory centers [17]. Moreover, the intrathoracic pressure swing generated when massive efforts to breath against an obstructed airway are made can elicit sympathetic surge [18]. Intriguingly, increased sympathetic activity has been observed in patients with OSA during daytime [19, 20].
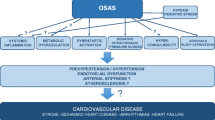
In the long term, OSA is associated with reduced endothelial nitric oxide availability, whereas oxidative stress and inflammation are enhanced [13,14,15, 21]. Other mechanisms that may be responsible for hypertension in OSA are increased arterial stiffness, increased renin–angiotensin–aldosterone activity, and altered baroreceptor reflexes (Fig. 1). Not only does OSA mediate hypertension through these pathways, but it may also regulate BP through these same mechanisms [22, 23]. These effects in combination with other common risk factors that accompany patients with OSA such as obesity and metabolic dysfunction can exert negative effects on BP regulation.
OSA and BP variability
BP is a biological signal characterized by marked fluctuations occurring within a 24-h period. This BP variability (BPV) is regulated by several mechanisms that balance adequate organ perfusion with possible damage from excessively high BP [24]. OSA may disrupt these regular fluctuations through its complex pathophysiologic relationship with hypertension as previously highlighted; excessive BPV over time may reflect an alteration in regulatory mechanisms such as poor autonomic control or vascular damage [25]. Furthermore, more studies are supporting sustained BPV as a strong and independent risk factor for cardiovascular disease and target organ damage beyond mean BP [26,27,28].
BPV is studied in the very short-term (beat-to-beat), short-term (within 24 h), mid-term (day-to-day), and long-term (week-to-week or month-to-month, also called visit-to-visit BPV) [22, 29]. Beat-to-beat BP measurements have been commonly used to measure cardiac baroreflex sensitivity [30]. Previous studies have demonstrated that beat-to-beat BPV is higher in OSA compared with non-OSA subjects [25, 31] and have suggested that this may reflect poor baroreflex control in patients with OSA [25]. Beat-to-beat BPV in patients with OSA may also provide unique information on BP behavior in relation to sleep apneic events. Multiple factors including patient-level underlying factors and sleep apnea event characteristics are expected to determine the severity of BP surge following sleep apneic events. However, some studies have shown a stronger relationship between apnea length [32] and degree of desaturation with the highest BPs typically observed during REM sleep [16, 33, 34]. Short-term BPV has been evaluated mainly as changes in mean BP from day to night in the form of dipping/non-dipping or morning BP surge. Yet, a few studies have shown that nocturnal systolic BPV and 24-h systolic BPV are also significantly increased in hypertensive OSA patients when compared with hypertensive non-OSA patients [35,36,37]. As for mid-term and long-term BPV, limited studies are available. Studies have thus far demonstrated that patients with OSA had increased visit-to-visit BPV and that patients with good adherence to CPAP therapy had the significantly reduced visit-to-visit BPV [38]. Further evidence is needed to better characterize the impact of OSA on BP regulation, particularly the mid and long-term. Assessment and treatment of BPV in OSA patients may add to the risk stratification and the evaluation of CPAP treatment [39].
OSA and nocturnal dipping
OSA may also contribute to impaired normal BP dipping. Previous studies have shown that a non-dipping pattern is found in 48% to 84% of patients with OSA. Its frequency and related nighttime BP also increases with OSA severity [40,41,42]. Non-dipping BP patterns also have utility in predicting the presence of OSA, despite their nonspecific nature. Crinion et al. found that moderate to severe OSA was associated with a 5.5-fold increased chance of having non-dipping BP after adjusting for potential confounders in patients with hypertension. [43] Interestingly, Genta-Pereira et al. found that the type of BP dipping may modify the prediction of having OSA. [44] In this study, reverse systolic BP dippers were independently associated with OSA, whereas both reduced and reverse diastolic dippers increased the likelihood of OSA. Surprisingly, the presence of snoring and two largely used sleep questionnaires only modestly improved the accuracy for predicting OSA [44].
Evaluating BP in OSA through ambulatory BP monitoring
The close association between OSA and hypertension highlights how BP assessment is crucial in patients with OSA whether they have an established diagnosis of hypertension or not. Capturing the myriad hemodynamic changes promoted by respiratory events, however, is not practical even in a sophisticated clinical sleep laboratory. 24-h ABPM in OSA can provide important insights and help identify patients with masked hypertension, isolated nocturnal hypertension, and daytime hypertension with pronounced nocturnal hypertension [29]. It has been observed that patients with newly diagnosed OSA have significantly higher 24-h systolic BPV when compared to those without OSA [35]. Such increased BPV may cause office BP to be less reliable, highlighting the benefit of the ABPM in patients with OSA [45].
Continuous beat to beat BP monitoring, though not readily available, also provides more insights into the pathophysiology of the OSA in relation to nocturnal hypertension. More specifically, if it is coupled with sleep studies, OSA related BP surge information can be derived [46]. This in turn, will help us understand the severity of OSA and better predict cardiovascular outcomes.
Impact of OSA treatment on BP
Over the last three decades, several research groups worldwide have tried to investigate the effect of OSA treatment on BP with conflicting results. While many studies have confirmed a modest but significant BP reduction, others have failed to show BP improvements in patients treated either with CPAP or mandibular advancement device [47]. In general, the impact of OSA treatment on in-office BP is modest – around 2 to 3 mmHg – and most studies focus on the effects of CPAP [48, 49]. This reduction is more pronounced for nocturnal BP [49,50,51]. However, these same meta-analyses also highlight important heterogeneity between studies. These differences were confirmed when a risk of bias analysis was performed, making it difficult to compare the results of the clinical trials. This suggests that there are subgroups or phenotypes of OSA patients who exhibit a more robust BP reduction when treated for OSA. For example, Castro Gratinoni et al. showed that the presence of a non-dipping pattern in patients with OSA was a predictor of better BP reduction (Nighttime mean BP change: non-dipper/low heart rate = − 6.2 ± 8.32 mmHg vs. dipper/low heart rate = 6 ± 6.97 mmHg) in response to CPAP. [45] Another intriguing finding that is still poorly understood is that there was a significant correlation between the degree of daytime sleepiness and the reduction in daytime SBP/DBP in patients with OSA [52]. Table 1 depicts the current characteristics that predict a higher impact on reducing BP after OSA treatment. These predictors need to be validated in longitudinal studies to determine if BP improvements in specific subgroups can be translated into a reduction of cardiovascular events.
Some additional insights could come from the ANDANTE project [53] (a Worldwide Individual Data Meta-Analysis of the Effect of Sleep Apnea Treatment on Blood Pressure) and the MORPHEOS trial [54] (a multicenter randomized controlled trial designed to evaluate the BP lowering effects of CPAP in OSA patients with uncontrolled hypertension under pill counting). Future studies will need to consider the pitfalls and limitations of previous studies such as suboptimal CPAP compliance.
Additional subgroups that may respond well to CPAP treatment include resistant hypertension (RH) and refractory hypertension (RFH). RH is defined as BP that remains uncontrolled despite the use of at least three antihypertensive drugs or BP that requires at least 4 antihypertensive drugs to control [55]. RFH is defined as BP that remains uncontrolled despite the use of at least 5 antihypertensive drugs [56]. The prevalence of RH and RFH in patients with OSA is greater than 70% and 90% respectively [57, 58]. A recent meta-analysis on the effect of CPAP in RH concluded that good CPAP adherence significantly reduces both 24-h systolic and diastolic BP values by 4–5 mmHg, especially at night [59]. Other randomized studies showed up to 10 mmHg of the BP reduction [60,61,62]. These decreases are seen more prominently (8–10 mmHg) in patients with RFH [63]. Of course, CPAP adherence was a crucial factor associated with more significant reductions in nocturnal BP [63]. Therefore, patients with RH not explained by other causes should be referred for sleep study to rule out OSA.
OSA, hypertension, and its impact on cardiac remodeling
Hypertension is an important contributor to cardiac remodeling. OSA’s adverse cardiovascular effect is thought to be mainly mediated through hypertension although a direct effect on the myocardium likely exists. Studies have shown that OSA is associated with left ventricular (LV) hypertrophy, LV diastolic dysfunction, and impairment in LV mechanics [64,65,66]. The first studies on this topic using conventional echocardiography reported baseline OSA was associated with reduced LV systolic function by LV ejection fraction (EF) by echocardiography obtained over an average of 18-year follow up [64]. Since then, a prospective randomized sham-controlled trial showed that treatment with CPAP for 3 months in patients with severe OSA resulted in significantly improved LV diastolic function compared to sham treatment [67]. The development of new imaging techniques such as speckle tracking and 3D echocardiography provided more insightful information on LV and right ventricle (RV) systolic function in OSA patients [67,68,69,70]. It also provided information on the efficacy of treatment of OSA with CPAP on reverse cardiac remodeling [70]. These studies suggested that LV mechanics, particularly global longitudinal strain (GLS), might be the most sensitive marker of initial subtle changes and LV remodeling in OSA patients. They also suggest that there are beneficial LV changes after starting CPAP therapy. However, separating out the effect of common comorbidities – hypertension, obesity, and diabetes – on cardiac remodeling is difficult. Most studies tried to adjust the resultant cardiac changes in parameters such as LV hypertrophy, but it is difficult to avoid the influence of these comorbidities as confounding factors. A recent meta-analysis showed that LV GLS is not only significantly decreased in OSA patients, but there appears to be a dose–response with OSA severity even among normotensive OSA patients [71]. Another meta-analysis that investigated the influence of CPAP reported that CPAP treatment was related to positive effects on LV and RV function in patients with OSA, particularly in terms of LV and RV GLS [72]. These results suggest that the assessment of cardiac mechanics by speckle tracking echocardiography should be included in the routine echocardiographic examination of patients with OSA.
There are several possible mechanisms that could clarify the relationship between OSA and cardiac remodeling. The overactivation of the sympathetic nervous system during repeated episodes of apnea results in episodic BP surge, regardless of the presence of daytime hypertension. Such nocturnal hypertension is a potent mediator of cardiac remodeling in both the LV and RV. Moreover, elevated BP associated with OSA may act as negative feedback, resulting in worsened OSA, which may accelerate LV and RV remodeling. Intermittent hypoxia stimulates an inflammatory reaction involving proinflammatory cytokines, growth factors, and oxidative stress. The activation of both humoral systems induces proliferation of extracellular matrix, interstitial myocardial fibrosis, and an increase in myocardial stiffness, which contributes to the development of LV and RV diastolic dysfunction and impairment of GLS. Interventricular dependence could be an additional mechanism responsible for LV and RV changes due to increased pulmonary arterial pressure that retrogradely transfers from the RV through the pulmonary circulation to the LA causing increased LV filling pressure and LV diastolic dysfunction. Cardiac remodeling can be clinically manifested as heart failure or arrythmia. Several studies suggested that OSA is an independent risk factor for heart failure and atrial fibrillation in patients without other underlying cardiac disorders [73,74,75].
OSA, arterial stiffness, and LV hemodynamics
Previous evidence has shown that sympathetic activation, systemic inflammation, and endothelial dysfunction are significantly increased in patients with OSA compared to those without OSA. These adverse consequences are closely related to BP, ventricular hemodynamics, and arterial stiffness. Moreover, elevated BP, hemodynamic changes, and arterial stiffness also mutually affect each other. Arterial stiffening causes the early return of reflected waves to elevate systolic BP and pulse pressure. Further increases in BP promote matrix synthesis, causing subsequent increases in vascular thickness and stiffening and increasing the load of stiff components within the arterial wall [76]. Ventricular-vascular uncoupling is also associated with increased systolic BP and subclinical LV remodeling [77, 78].
Recent studies have shown that CPAP treatment significantly improves stroke volume, ventricular-vascular coupling, and arterial stiffness measured by pulse wave doppler in patients with OSA [67]. Although the exact mechanism of these improvements is still unclear, some evidence suggests that CPAP therapy improves endothelial dysfunction, decreases oxidative stress markers, and restores ventricular hemodynamics and sympathetic nervous system activity [79]. OSA promotes the clinical combination of hypertension, ventricular-vascular uncoupling, and arterial stiffness, which is a major step toward the development of cardiovascular disease. Therefore, measuring the LV hemodynamics and arterial stiffness, as well as BP monitoring, can be an important clinical tool for predicting cardiovascular complications and treatment efficacy in patients with OSA.
OSA-hypertension and the role of social and racial-ethnicity
A burgeoning body of literature suggests that the relationship between OSA and hypertension is substantially affected by social determinants of health [80, 81]. Racial difference is a significant factor contributing to the disparities in BP outcomes among adults with OSA. One such study suggests that untreated moderate or severe OSA may be related to a two-fold higher odds of resistant hypertension in Black adults [82]. In a nationally representative sample, probable OSA was associated with an over fourfold increased odds of predicting hypertension among overweight Black participants (95% confidence interval [CI], 1.86–12.03) and a twofold increased odds among obese Hispanic participants (95% CI, 1.16–3.49) [81]. Although Asians have comparable rates of OSA compared to Whites, Asians have a higher prevalence of increased nighttime BP or morning BP surge [83]. This implies that Asians may be more susceptible to poor BP outcomes in the setting of OSA than their white counterparts [83, 84]. Indeed, studies have observed a link between OSA and abnormal dipping patterns of BP in Asian adults [83]. However, these findings were primarily established based on Japanese populations [12, 85] and more studies are needed to examine the relationship between OSA and hypertension in other Asian sub-groups given the substantial heterogeneity across Asian backgrounds. Other socioeconomic factors that increase the burden of OSA and hypertension may include living in a disadvantaged neighborhood [86, 87], lacking health insurance [88], and not being married [89, 90].
Addressing health disparities in OSA to improve hypertension care
Considering the high prevalence of OSA and hypertension, as well as the health consequences associated with these two conditions, future investigations should focus on identifying the social determinants that underlie the OSA-hypertension relationship. These important inquiries may assist with developing tailored interventions to reduce the health disparities among high-risk populations such as racial and ethnic minority groups. Improving identification and treatment of OSA in socioeconomically disadvantaged ethnicities will require novel and innovative care delivery models. One method may be simplifying the screening process. With technological advances, OSA evaluation can be typically performed with a home sleep apnea test. Telemonitoring can be an effective tool to follow up on patients requiring therapy. Integration of sleep services into primary care or other specialties may increase access for these populations. For example, incorporating OSA screening into cardiology practice may simplify the referral and treatment process.
In the US, the American Academy of Sleep Medicine has signaled national interest in this area through development of the Specialty Practice Accreditation program for cardiology clinics. This simplified referral and therapy approach may benefit blacks, who are most vulnerable to hypertension and its untoward complications [91]. However, whether such efforts will improve OSA care in the socioeconomically disadvantaged population as a whole remains to be seen. Not only must changes be implemented at the site of practice, but there must also be reform at the level of manufacture and distribution of the treatment modalities such as CPAP equipment to make therapy more readily accessible. Efforts to continue to streamline and simplify the diagnosis, treatment, and reimbursement for CPAP equipment will improve outcomes in vulnerable populations most susceptible to social and economic hardships who also have the most disparity in cardiovascular outcomes.
Gaps and future prospects
While prospective longitudinal studies have linked OSA to increased risk of hypertension [7, 92], such a finding has not been consistent [8, 10]. In patients with OSA, OSA treatment with CPAP has been shown to improve BP but the effect has been modest. Symptomatic phenotype may play a role in determining the benefit of CPAP on BP wherein the BP reduction is less pronounced in non-sleepy OSA patients [93,94,95,96,97]. The impact of OSA on nocturnal BP requires more in-depth investigation. This is because of the inherent limitation of using conventional ABPM device, which only yields intermittent BP readings in sleep. BP response to OSA itself may be an important indicator for adverse cardiovascular prognosis. Beat to beat BP measurement or hypoxia triggered BP monitoring will provide deeper insights into this [98]. This can be possible through direct BP measurement [16], estimation using other available physiological measurements such as pulse arrival time [99, 100], or potentially photoplethysmography signals derived from wearable devices [101,102,103]. More studies are essential to examine the clinical value of continuous BP monitoring in sleep.
There is a shift from a single in-lab polysomnography to multi-night portable home sleep studies and beyond (wearables). Such a transition will allow us to examine the role of sleep on BP control at a much higher dimension. Finally, studies investigating the benefit of alternative OSA therapies other than CPAP in BP and other cardiovascular health outcomes are necessary. Several studies have suggested that the beneficial effect of mandibular advancement device may not be inferior [50]. The effect of positional therapy, a highly underappreciated therapy, on BP requires further scrutiny [104]. The investigation of the effect of non-CPAP therapy on BP is important given the poor CPAP compliance and the emergence of novel therapies [105,106,107].
Conclusion
OSA is an important contributor to the pathophysiology of hypertension. The role of OSA has been most well recognized in nocturnal as well as resistant hypertension. The fact that OSA is so common in otherwise healthy individuals raises a question about to what extent OSA needs to be treated. There is some evidence that those who exhibit symptoms related to OSA may be most vulnerable to the risk of OSA and may benefit the most from OSA therapy. Patients with OSA with hypertension are at risk of cardiac remodeling, through which secondary cardiovascular consequence (e.g., heart failure, atrial fibrillation) many ensue. The relationship between OSA and hypertension is substantially affected by social determinants of health. Future investigations should focus on identifying the social determinants that underly the OSA-hypertension relationship. Advance in BP monitoring technology will likely bring a new level of understanding of the relationship between OSA and BP.
Availability of data and materials
Not applicable.
Abbreviations
- APBM:
-
Ambulatory BP monitoring
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BPV:
-
Blood pressure variability
- CPAP:
-
Continuous positive airway pressure
- EF:
-
Ejection fraction
- GLS:
-
Global longitudinal strain
- LV:
-
Left ventricle
- OSA:
-
Obstructive sleep apnea
- RDI:
-
Respiratory Disturbance Index
- RH:
-
Resistant hypertension (RH)
- RFH:
-
Refractory hypertension (RFH)
- RV:
-
Right ventricle
- SHHS:
-
Sleep Heart Health Study
- VSC:
-
Vitoria Sleep Cohort
- WSCS:
-
Wisconsin Sleep Cohort study
References
American Heart Association adds sleep to cardiovascular health checklist. American Heart Association https://newsroom.heart.org/news/american-heart-association-adds-sleep-to-cardiovascular-health-checklist.
Mills KT, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50.
Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785–802.
Beevers G, Lip GYH, O’Brien E. The pathophysiology of hypertension. BMJ. 2001;322:912–6.
Peppard PE, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14.
Young T, et al. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108:246–9.
Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84.
O’Connor GT, et al. Prospective study of sleep-disordered breathing and hypertension: the sleep heart health study. Am J Respir Crit Care Med. 2009;179:1159–64.
Haas DC, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the sleep heart health study. Circulation. 2005;111:614–21.
Cano-Pumarega I, et al. Obstructive sleep apnea and systemic hypertension. Am J Respir Crit Care Med. 2011;184:1299–304.
Chao C-C, Wu J-L, Chang Y-T, Lin C-Y. Combined effect of obstructive sleep apnea and age on daytime blood pressure. Eur Arch Otorhinolaryngol. 2012;269:1527–32.
Akashiba T, et al. Nasal continuous positive airway pressure changes blood pressure “non-dippers” to “dippers” in patients with obstructive sleep apnea. Sleep. 1999;22:849–53.
Bangash A, Wajid F, Poolacherla R, Mim FK, Rutkofsky IH. Obstructive sleep apnea and hypertension: a review of the relationship and pathogenic association. Cureus. 2020;12:8241.
Ahmad M, Makati D, Akbar S. Review of and updates on hypertension in obstructive sleep apnea. Int J Hypertens. 2017;2017:1848375.
Konecny T, Kara T, Somers VK. Obstructive Sleep Apnea and Hypertension. Hypertension. 2014;63:203–9.
Kwon Y, et al. Elucidation of obstructive sleep apnoea related blood pressure surge using a novel continuous beat-to-beat blood pressure monitoring system. J Hypertens. 2022;40:520–7.
Prabhakar NR. Carotid body chemo-reflex: a driver of autonomic abnormalities in sleep apnea. Exp Physiol. 2016;101:975–85.
Somers VK, Dyken ME, Skinner JL. Autonomic and hemodynamic responses and interactions during the Mueller maneuver in humans. J Auton Nerv Syst. 1993;44:253–9.
Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904.
Carlson JT, et al. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–8.
Atkeson A, Yeh SY, Malhotra A, Jelic S. Endothelial function in obstructive sleep apnea. Prog Cardiovasc Dis. 2009;51:351–62.
Parati G, et al. European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–66.
Drager LF, et al. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2015;309:H1101-1111.
Shahoud JS, Sanvictores T, Aeddula NR. Physiology, Arterial Pressure Regulation. Treasure Island (FL): StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK538509/.
Pal A, et al. Beat-to-beat blood pressure variability in patients with obstructive sleep apnea. J Clin Sleep Med. 2021;17:381–92.
Mehlum MH, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39:2243–51.
Palatini P, et al. Short-term blood pressure variability outweighs average 24-h blood pressure in the prediction of cardiovascular events in hypertension of the young. J Hypertens. 2019;37:1419–26.
Yinon L, et al. A prospective study of variability in systolic blood pressure and mortality in a rural Bangladeshi population cohort. Prev Med. 2013;57:807–12.
Pio-Abreu A, Moreno H, Drager LF. Obstructive sleep apnea and ambulatory blood pressure monitoring: current evidence and research gaps. J Hum Hypertens. 2021;35:315–24.
Wessel N, et al. Instantaneous cardiac Baroreflex sensitivity: xBRS method quantifies heart rate blood pressure variability ratio at rest and during slow breathing. Front Neurosci. 2020;14:547433.
Narkiewicz K, et al. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–7.
Ali NJ, Davies RJ, Fleetham JA, Stradling JR. The acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apnea. Chest. 1992;101:1526–32.
Sforza E, Capecchi V, Lugaresi E. Haemodynamic effects of short-term nasal continuous positive airway pressure therapy in sleep apnoea syndrome: monitoring by a finger arterial pressure device. Eur Respir J. 1992;5:858–63.
Kumagai H, et al. Nocturnal blood pressure fluctuations in patients with rapid eye movement-related obstructive sleep apnea. J Clin Med. 2021;10:5023.
Ke X, et al. Association of 24 h–systolic blood pressure variability and cardiovascular disease in patients with obstructive sleep apnea. BMC Cardiovasc Disord. 2017;17:287.
Shi J, et al. Obstructive sleep apnea increases systolic and diastolic blood pressure variability in hypertensive patients. Blood Press Monit. 2017;22:208–12.
Kang K-T, Chiu S-N, Weng W-C, Lee P-L, Hsu W-C. 24-hour ambulatory blood pressure variability in children with obstructive sleep apnea. Laryngoscope. 2021;131:2126–32.
Shiina K, et al. Obstructive sleep apnea as possible causal factor for visit-to-visit blood pressure variability. Circ J. 2016;80:1787–94.
Marrone O, Bonsignore MR. Blood-pressure variability in patients with obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:229–42.
Suzuki M, Guilleminault C, Otsuka K, Shiomi T. Blood pressure ‘dipping’ and ‘non-dipping’ in obstructive sleep apnea syndrome patients. Sleep. 1996;19:382–7.
Nabe B, Lies A, Pankow W, Kohl FV, Lohmann FW. Determinants of circadian blood pressure rhythm and blood pressure variability in obstructive sleep apnoea. J Sleep Res. 1995;4:97–101.
Seif F, et al. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J Hypertens. 2014;32:267–75.
Crinion SJ, et al. Nondipping Nocturnal blood pressure predicts sleep apnea in patients with hypertension. J Clin Sleep Med. 2019;15:957–63.
Genta-Pereira DC, et al. Nondipping blood pressure patterns predict obstructive sleep apnea in patients undergoing ambulatory blood pressure monitoring. Hypertension. 2018;72:979–85.
Castro-Grattoni AL, et al. Blood pressure response to CPAP treatment in subjects with obstructive sleep apnoea: the predictive value of 24-h ambulatory blood pressure monitoring. Eur Respir J. 2017;50:1700651.
Kwon Y, et al. Blood pressure monitoring in sleep: time to wake up. Blood Press Monit. 2020;25:61–8.
Parati G, Pengo MF, Lombardi C. Obstructive sleep apnea and hypertension: why treatment does not consistently improve blood pressure. Curr Hypertens Rep. 2019;21:30.
Fatureto-Borges F, Lorenzi-Filho G, Drager LF. Effectiveness of continuous positive airway pressure in lowering blood pressure in patients with obstructive sleep apnea: a critical review of the literature. Integr Blood Press Contr. 2016;9:43–7.
Fava C, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest. 2014;145:762–71.
Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280–93.
Pengo MF, et al. Obstructive sleep apnoea treatment and blood pressure: which phenotypes predict a response? A systematic review and meta-analysis. Eur Respir J. 2020;55:1901945.
Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;08:587–96.
Pengo, M. F., Steier, J., Parati, G., ANDANTE collaborators, & Researchers Collaborating in the ANDANTE PROJECT. The ANDANTE project: a worldwide individual data meta-analysis of the effect of sleep apnea treatment on blood pressure. Arch Bronconeumol (Engl Ed) S0300–2896(21)00149–6 (2021) https://doi.org/10.1016/j.arbres.2021.05.002.
Cruz FCSG, et al. The effect of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea and uncontrolled hypertension - Study design and challenges during the COVID-19 pandemic. Clinics (Sao Paulo). 2021;76:e2926.
Carey RM, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–90.
Acelajado MC, Hughes ZH, Oparil S, Calhoun DA. Treatment of resistant and refractory hypertension. Circ Res. 2019;124:1061–70.
Oscullo G, et al. Resistant/refractory hypertension and sleep apnoea: current knowledge and future challenges. J Clin Med. 2019;8:1872.
Logan AG, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7.
Labarca G, et al. Efficacy of Continuous Positive Airway Pressure (CPAP) in patients with Obstructive Sleep Apnea (OSA) and Resistant Hypertension (RH): Systematic review and meta-analysis. Sleep Med Rev. 2021;58: 101446.
de Oliveira AC, et al. The antihypertensive effect of positive airway pressure on resistant hypertension of patients with obstructive sleep apnea: a randomized, double-blind, clinical trial. Am J Respir Crit Care Med. 2014;190:345–7.
Pedrosa RP, et al. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144:1487–94.
Martínez-García M-A, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15.
Navarro-Soriano C, et al. Effect of continuous positive airway pressure in patients with true refractory hypertension and sleep apnea: a post-hoc intention-to-treat analysis of the HIPARCO randomized clinical trial. J Hypertens. 2019;37:1269–75.
Korcarz CE, et al. Effects of obstructive sleep apnea and obesity on cardiac remodeling: the Wisconsin sleep cohort study. Sleep. 2016;39:1187–95.
Cuspidi C, et al. Obstructive sleep apnoea syndrome and left ventricular hypertrophy: a meta-analysis of echocardiographic studies. J Hypertens. 2020;38:1640–9.
Lisi E, et al. Diastolic dysfunction in controlled hypertensive patients with mild-moderate obstructive sleep apnea. Int J Cardiol. 2015;187:686–92.
Shim CY, et al. Effects of continuous positive airway pressure therapy on left ventricular diastolic function: a randomised, sham-controlled clinical trial. Eur Respir J. 2018;51:1701774.
Kucher N, et al. Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J. 2003;24:1651–6.
Wang D, et al. Left ventricular subclinical dysfunction associated with myocardial deformation changes in obstructive sleep apnea patients estimated by real-time 3D speckle-tracking echocardiography. Sleep Breath. 2016;20:135–44.
Kim D, et al. Continuous positive airway pressure therapy restores cardiac mechanical function in patients with severe obstructive sleep apnea: a randomized Sham-controlled study. J Am Soc Echocardiogr. 2019;32:826–35.
Tadic M, et al. Is myocardial strain an early marker of systolic dysfunction in obstructive sleep apnoea? Findings from a meta-analysis of echocardiographic studies. J Hypertens. 2022;40:1461–8.
Tadic M, et al. The impact of continuous positive airway pressure on cardiac mechanics: Findings from a meta-analysis of echocardiographic studies. J Clin Hypertens. 2022;24:795–803.
Oldenburg O, et al. Sleep-disordered breathing in patients with symptomatic heart failure a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Failure. 2007;9:251–7.
Holt A, et al. Sleep apnea, the risk of developing heart failure, and potential benefits of Continuous Positive Airway Pressure (CPAP) Therapy. J Am Heart Assoc. 2018;7: e008684.
Mehra R, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the sleep heart health study. Am J Respir Crit Care Med. 2006;173:910–6.
Safar ME, et al. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72:796–805.
Tso JV, et al. Hypertension and ventricular-arterial uncoupling in collegiate American Football Athletes. J Am Heart Assoc. 2022;11: e023430.
Shim CY, et al. The relationship between ventricular-vascular uncoupling during exercise and impaired left ventricular longitudinal functional reserve in hypertensive patients. J Am Soc Hypertens. 2013;7:198–205.
Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569–76.
Imayama I, et al. Socioeconomic status impacts blood pressure response to positive airway pressure treatment. J Clin Sleep Med. 2022;18:1287. https://doi.org/10.5664/jcsm.9844.
Sands-Lincoln M, et al. The association between obstructive sleep apnea and hypertension by race/ethnicity in a nationally representative sample. J Clin Hypertens (Greenwich). 2013;15:593–9.
Johnson DA, et al. Association between sleep apnea and blood pressure control among blacks. Circulation. 2019;139:1275–84.
Hoshide S, et al. Characteristics of hypertension in obstructive sleep apnea: an Asian experience. J Clin Hypertens (Greenwich). 2021;23:489–95.
Dudley KA, Patel SR. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med. 2016;18:96–102.
Sekizuka H, Osada N, Akashi YJ. The factors affecting the non-dipper pattern in Japanese patients with severe obstructive sleep apnea. Intern Med. 2018;57:1553–9.
Johnson DA, Ohanele C, Alcántara C, Jackson CL. The need for social and environmental determinants of health research to understand and intervene on racial/ethnic disparities in obstructive sleep apnea. Clin Chest Med. 2022;43:199–216.
Usher T, Gaskin DJ, Bower K, Rohde C, Thorpe RJ. Residential segregation and hypertension prevalence in black and white older adults. J Appl Gerontol. 2018;37:177–202.
Patel J, Topf MC, Huntley C, Boon M. Does insurance status impact delivery of care with upper airway stimulation for OSA? Ann Otol Rhinol Laryngol. 2020;129:128–34.
Goosmann, M., Williams, A. M., Springer, K. & Yaremchuk, K. L. The Impact of Marital Status and Race in Obstructive Sleep Apnea. Ear Nose Throat J 2022;1455613221120068. https://doi.org/10.1177/01455613221120068.
Ramezankhani A, Azizi F, Hadaegh F. Associations of marital status with diabetes, hypertension, cardiovascular disease and all-cause mortality: a long term follow-up study. PLoS ONE. 2019;14:e0215593.
Healy WJ, et al. Reducing disparities in cardiovascular health in African Americans through integrated cardiovascular sleep care in outpatient setting. SLEEP Adv. 2022;3:zpac016.
Marin JM, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–76.
Barbé F, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8.
Robinson GV, Smith DM, Langford BA, Davies RJO, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27:1229–35.
Barbé F, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. A randomized, controlled trial. Ann Intern Med. 2001;134:1015–23.
Craig SE, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax. 2012;67:1090–6.
Parati G, Lombardi C. Control of hypertension in nonsleepy patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;181:650–2.
Kuwabara M, Hamasaki H, Tomitani N, Shiga T, Kario K. Novel triggered nocturnal blood pressure monitoring for sleep apnea syndrome: distribution and reproducibility of hypoxia-triggered nocturnal blood pressure measurements. J Clin Hypertens (Greenwich). 2017;19:30–7.
Kwon Y, et al. Pulse arrival time, a novel sleep cardiovascular marker: the multi-ethnic study of atherosclerosis. Thorax. 2021;76:1124–30.
Gesche H, Grosskurth D, Küchler G, Patzak A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol. 2012;112:309–15.
Xing X, et al. An unobtrusive and calibration-free blood pressure estimation method using photoplethysmography and biometrics. Sci Rep. 2019;9:8611.
Martínez G, et al. Can photoplethysmography replace arterial blood pressure in the assessment of blood pressure? J Clin Med. 2018;7:316.
Takazawa K, et al. Assessment of vasoactive agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension. 1998;32:365–70.
Berger M, et al. Avoiding the supine position during sleep lowers 24 h blood pressure in obstructive sleep apnea (OSA) patients. J Hum Hypertens. 1997;11:657–64.
Strollo PJ, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–49.
Randerath W, et al. Current and novel treatment options for obstructive sleep apnoea. ERJ Open Res. 2022;8:00126–2022.
Perger E, et al. Reboxetine plus oxybutynin for OSA treatment: a 1-week, randomized, placebo-controlled double-blind crossover trial. Chest. 2022;161:237–47.
Acknowledgements
This study was funded by a grant (KSH-2010) from the Korean Society of Hypertension.
Funding
YK was supported by NIH R01HL158765, R21HL167126, AG070576 and HL150502 grants.
Author information
Authors and Affiliations
Contributions
All authors participated in the conception of the study. WST, JW, JGL, MT, GML, MAMG, MP, XL, YC, LD, WH, GRH, and YK: drafting of manuscript, and critical revision. acquisition of data, drafting of manuscript, and critical revision. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors have nothing to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kwon, Y., Tzeng, W.S., Seo, J. et al. Obstructive sleep apnea and hypertension; critical overview. Clin Hypertens 30, 19 (2024). https://doi.org/10.1186/s40885-024-00276-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40885-024-00276-7