Abstract
Background
Thus far, the incidence, mortality, and temporal trend data of oropharyngeal cancers (OPC) in China were few. We estimated the incidence, mortality, and temporal patterns of OPC in China during 2008–2012 according to the data from 135 population-based cancer registries to better understand the epidemiological pattern of OPC and to provide more precise information for OPC control in China.
Methods
According to the data of diagnosed OPC reported to 135 cancer registries during 2008–2012, we calculated age-standardized rate of incidence and mortality by 2000 Chinese standard population (ASRIC and ASRMC) and by 1985 Segi’s world standard population (ASRIW and ASRMW) by age, sex, and geographic regions; annual percentage changes of OPC incidence and mortality were calculated using Joinpoint trend analysis.
Results
ASRIW and ASRMW were 2.22/100,000 person-years and 0.94/100,000 person-years, respectively. The incidence and mortality in urban areas were higher than those in rural areas. ASRIC and ASRIW of males were higher than those of females. The overall ASRIC of OPC was significantly increased by 6.2% annually between 2003 and 2006 (P = 0.038), but remained stable between 2007 and 2012 (P = 0.392). ASRIC and ASRMC of males and in rural areas were significantly increased in the last decade (P < 0.05), but the rates of females remained stable during the same period (P > 0.05).
Conclusions
Across multiple cancer registries in China, there was an obvious increase in OPC in the recent decade, especially for incidence and mortality of males and in rural areas, whereas the rates of females remained stable. A healthy lifestyle should be advocated and early diagnosis and early treatment of OPC should be enhanced.
Similar content being viewed by others
Background
Cancer caused over 8 million deaths and became the second leading cause of death behind cardiovascular disease worldwide [1]. It has become a major public health issue in many countries [2]. The incidence of oral cavity cancers has decreased in recent years in most parts of the world. However, the incidence of oropharyngeal cancers (OPC) has increased over the past decades, predominantly in developed regions of Europe, North America, and parts of East Asia and among males younger than age 60 years [3, 4]. This type of cancer was traditionally considered to be a disease of middle-aged to elderly adults and was mainly observed in males. Nevertheless, increasing numbers of cases have been reported under 45 years of age over the past decades [5, 6]. These observed differences might be related to differences in the etiology of oral cancers at different anatomical sites [3]. Lip, oral cavity, and pharyngeal cancers account for approximately 3.8% of all cancer cases and 3.6% of cancer deaths worldwide; additionally, there was an estimated global age-standardized rate (ASR) of incidence of 1.4/100,000 for OPC (2.3/100,000 for males and 0.5/100,000 for females), and South-central Asia had the highest proportion of new global cases (35.1%) [7]. Literature on the incidence, mortality, and temporal patterns of OPC in China is scarce. Although previous estimates of the national OPC incidence have been reported, they either covered a small proportion of the Chinese population or were focused on specific years [8,9,10]. Thus far, large-scale epidemiological studies of OPC and information on temporal trends of OPC in China are limited. We estimated the incidence and mortality of OPC in China in 2008–2012 as well as temporal trends for OPC incidence and mortality during 2003–2012 according to the data of 135 population-based cancer registries to better understand OPC epidemiological patterns and to provide more precise scientific information for its control and prevention in China.
Materials and methods
Data sources
We quantified 20,618 new diagnoses of OPC and 9635 death cases that were reported to the 135 population-based cancer registries between 1 January 2008 and 31 December 2012 in China. These registries covered a population of 318,623,600 person-years in males and 310,710,310 person-years in females. The 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes included in this study were C00–C14 (except for C11) [11]. All 135 cancer registries collected data on new cancer cases/deaths from local hospitals, community health centers, vital statistics, or the Civil Administration Bureau [12]. According to the regional division method of the China National Bureau of Statistics, 31 provinces across the country are divided into the eastern, central, and western regions. The 135 cancer registries included 56 in cities (urban areas), covering approximately 382,669,450 person-years, and 79 in counties (rural areas), covering approximately 246,664,460 person-years (Additional file 1: Table S1). Nationwide population data by 5-year sex group and age were provided by statistics or public security census [11]. Individual registries submitted corresponding population databases to the National Central Cancer Registry (NCCR) of China. This information was obtained from the local Public Security/Statistical Bureaus or from calculations on the basis of census data. Thirty-two of these 135 cancer registries, which provided full datasets for new cancer patients diagnosed during 2003–2012 and covered a population of 537,368,837 person-years, were included in incidence/mortality trend analyses (Additional file 1: Table S1). The same standards were used for data collection and report in the two datasets.
Statistical indices and methods
The quality of the dataset from each cancer registry was reviewed and evaluated by NCCR based on the Guidelines for Chinese Cancer Registration and data quality criteria of the International Agency for Research on Cancer/International Association of Cancer Registries (IARC/IACR) [11, 13]. The mortality to incidence (M/I) ratio, the percentage of cancer cases identified with death certification only (DCO%), the proportion of morphologic verification (MV%), and the percentage of uncertified cancer (UB%) were adopted to evaluate the reliability, validity, and completeness of the cancer database [11]. Data from each cancer registry, which was consistently classified as category A or B, were included in our study. Incidence and mortality of OPC were estimated with stratification by gender, area, and age groups. Statistical analysis was conducted using SAS 9.3 version (SAS Institute Inc, Cary, NC, US) and MS EXCEL 2007 (Microsoft, Redmond, WA, USA). IARCcrgTools 2.05, which was issued by IARC/IACR, was adopted for data check and evaluation. Age-standardized rates by standard population of China in 2000 (ASRC) and that of the Segi’s world standard population in 1985 (ASRW) were calculated [8]. Temporal trends of incidence and mortality from 2003 to 2012 (32 registries) were analyzed using Joinpoint Software 4.5 (https://surveillance.cancer.gov/joinpoint/), fitting joinpoint models to the log-transformed data, and the rates were standardized by the world standard population. To reduce the possibility of reporting spurious changes in trends over the period, all models were restricted to a maximum of 2 joinpoints. Trends analysis was expressed as an annual percentage change (APC), and the Z test was used to evaluate whether the APC was significantly different from zero. The terms “increase” and “decrease” were adopted when the slope (APC of the trend) was statistically significant (P < 0.05).
Results
OPC incidence in China
According to the pool of datasets from the 135 cancer registries, 20,618 new cases were diagnosed as OPC between 2008 and 2012 in China: 13,582 in males and 7036 in females. The crude incidence was 3.28/100,000 person-years (4.26/100,000 in males and 2.26/100,000 in females), accounting for 1.16% of overall new diagnosed cancers and ranking the 20th among all cancer sites. Age-standardized rates of incidence by 2000 Chinese standard population (ASRIC) and by 1985 Segi’s world standard population (ASRIW) were 2.27/100,000 person-years and 2.22/100,000 person-years, respectively. The cumulative incidence rate (0–74 years old) of OPC was 0.25%. The incidence in urban areas was higher than that in rural areas. The incidence in males was also higher than that in females. The incidence in the Eastern region was higher than those in the Western region and the Central region (Table 1).
OPC mortality in China
There were 9335 death cases of OPC in China between 2008 and 2012, which included 6575 males and 2760 females. The crude mortality was 1.48/100,000 person-years, accounting for 0.83% of overall cancer deaths, and was ranked the 18th among all cancer sites. Age-standardized rates of mortality by 2000 Chinese standard population (ASRMC) and by 1985 Segi’s world standard population (ASRMW) were 0.95/100,000 and 0.94/100,000, respectively. The cumulative death rate (0–74 years old) was 0.10%. The mortality of OPC was higher in males than in females. The mortality in urban areas was higher than that of rural areas. The mortality in females was lower than that in males. The ASRMC and ASRMW in the Western region were higher than those in the Eastern and Central regions (Table 2).
Age-specific incidence and mortality of OPC
The incidence of OPC was relatively low before age 30, and then began to rise slowly; it increased dramatically after age 40, peaked at age 80, and then slightly decreased after age 85 (Fig. 1a). The age-specific incidence was higher in urban areas than in rural areas in almost all the age-groups (except for the 0–10 years age group). The incidence in males was higher in urban areas than in rural areas after age 15, whereas the incidence in females was higher in urban areas than in rural areas in every age group after age 35.
Age-specific incidence and mortality of oropharyngeal cancer (OPC) in China, 2008–2012. a The age-specific incidence increased remarkably from age 40–45, peaked at age 80–84 for both males and females, and decreased thereafter. The incidence in males was higher than that in females; the incidence in urban areas was higher than that in rural areas. b The age-specific mortality increased remarkably from age 40–45, peaked at age 80–84 for males and at age 85 and above for females. The mortality in males was higher than that in females; the mortality in urban areas was higher than that in rural areas
The mortality of OPC was relatively low before age 40, then began to increase, and reached a peak after age 85 (Fig. 1b). The mortality of OPC in rural areas was the highest at age 80–84. The age-specific mortality in males was higher in urban areas than in rural areas after age 25, whereas the mortality in females was higher in urban areas than in rural areas after age 70. The mortality in urban areas was higher than that in rural areas after age 35.
Time trend analysis
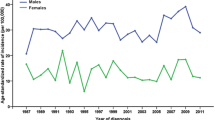
The ASRIC of OPC increased during 2003–2006 (annual percentage change [APC] = 6.2%, 95% confidence interval [CI] = 0.5% to 12.3%) and remained stable during 2007–2012 (APC = 0.7%, 95% CI = − 1.2% to 2.6%). Overall, the OPC incidence increased for males (APC = 2.5%, 95% CI = 1.5% to 3.5%) but not for females (APC = 5.5%, 95% CI = − 0.9% to 12.3%). A marginally significant temporal trend of ASRIC was observed in urban areas (APC = 8.5%, 95% CI = 0.0 to 17.6%, P = 0.049). These data reveal a significant increase of OPC in China in the recent decade, especially for males in rural areas (Tables 3, 4, Fig. 2a).
Trends of ASRIC and ASRMC of OPC in China, 2003–2012. a The ASRIC increased during 2003–2006, remained stable during 2007–2012. The OPC incidence increased in males, especially in rural areas. b The ASRMC was stable over the study period (2003–2012) for females, whereas significant upward trends were observed for males. There was an increase in mortality over the time period both in urban and rural areas
The ASRMC was stable over the study period (2003–2012) for females (APC = 0.8%, 95% CI = − 1.1% to 2.7%), whereas significant upward trends were observed for males (APC = 2.9%, 95% CI = 2.0% to 3.9%). There was an increase in ASRMC over the time period in both urban areas (APC = 2.3%, 95% CI = 1.4% to 3.1%) and rural areas (APC = 3.2%, 95% CI = 0.9% to 5.6%) (Tables 3, 5, Fig. 2).
Discussion
The current analysis included 20,618 new diagnoses of OPC and 9335 death cases. Our data showed that the ASRIW of OPC was 2.22/100,000 and the ASRMW was 0.94/100,000 in China. Additionally, the overall ASRIC of OPC significantly increased by 6.2% annually during 2003–2006 (P < 0.05), but remained stable during 2007–2012. The ASRIC and ASRMC for males and in rural areas significantly increased in the recent decade (P < 0.05); the incidence significantly increased by 30.7% in males (P < 0.01) and by 61.1% in rural areas (P < 0.01). Nevertheless, ASRIC and ASRMC remained stable in females during those periods. The ASRIW of OPC in China was slightly above the average level of Colombia, Costa Rica, and Ecuador [3], but lower than the United States of America (15.6/100,000 for males and 6.1/100,000 for females) [14]. In contrast, the incidence of OPC in males is high in North France with a rate of 42.3/100,000 in males in the Somme and Bas Rhin regions [14].
Tobacco smoking, alcohol consumption, and human papillomavirus types 16 and 18 (HPV16/18) infections were identified as the major risk factors for OPC, with tobacco smoking and alcohol consumption having synergistic effects [15,16,17]. Tobacco use is a mutagenic factor that increases the risk of oral cavity and pharyngeal cancers [18]. China constitutes approximately 40% of the world’s total tobacco use, predominantly in males, making China the largest tobacco consumer worldwide [19]. Additionally, a large increase in consumption was observed in urban rather than rural areas over the past three decades [19]. It might partly explain the reason why the incidence of OPC was higher in urban areas than in rural areas. In addition, although the mechanisms for alcohol-related carcinogenesis were not completely clarified, the IARC listed both alcoholic beverages and acetaldehyde, including its major metabolites, as human carcinogens. Alcohol drinking is an established risk factor for head and neck cancer, and this relationship was even stronger among cancers of the oropharynx and hypopharynx [20]. Li et al. [21] reported that the proportions of current drinkers among Chinese males, females, and the whole population were 55.6%, 15.0%, and 35.7%, respectively. Additionally, among current drinkers, the proportions of excessive, frequent, and binge drinkers were 59.1%, 26.0%, 53.7% in urban areas and 64.7%, 26.4%, 59.3% in rural areas, respectively. Furthermore, a significant association between heavy alcohol drinking and malnutrition was observed, which may have led to reduced intake of vegetables, fruits, and some other foods with cancer-preventive effects [22]. Additionally, the local cytotoxic activity of ethanol might explain the synergistic effect of tobacco and alcohol consumption on the risk of OPC. The cytotoxic effect of ethanol on the cells lining these tissues activated the division of stem cells located in deeper layers to replace dead cells. By activating their division, alcohol leaves the DNA of stem cells highly exposed to the DNA-damaging activity of tobacco carcinogens [23].
In head and neck squamous cell carcinoma, HPV has been regarded as an important pathogen, in addition to alcohol and tobacco consumption [24]. An increase in the incidence of oropharyngeal squamous cell carcinoma (OPSCC) was observed in some countries [3, 4], and this has been attributed to HPV infection [25]. Currently, the natural history of oral HPV infection has not been fully studied [26]. Nevertheless, some progress has been made in recent decades. It was reported that HPV DNA was detected in approximately 19%–75% of OPSCCs worldwide, 85%–95% of which belong to the HPV-16 type [27, 28]. A recent study performed in China also showed that HPV infection was detected in 16.7% of OPSCC specimens [27], which was similar to the detection in 18%–36% of OPSCCs reported in studies from developed countries [28]. The development of HPV-associated OPSCC was found to be associated with high-risk sexual behaviours, including an increased number of sexual partners/oral sex partners, and homosexual intercourse [3]. In addition, a significantly higher prevalence of oral HPV infection was found in subjects who had performed oral sex than in subjects who had not, consistent with previous studies that showed that oral sex might be a risk factor for oral HPV infection [29]. Using data from cancer registries and data of HPV prevalence, recent studies in Australia [30], the United States [31], and Sweden [32] have indicated that changes in sexual behaviours among recent birth cohorts have led to increased oral HPV exposure and to an increased incidence of OPC. Nevertheless, the natural history of oral HPV infection is still largely unknown, and ongoing research is underway to explore why males are more susceptible to acquisition and persistent HPV infection than females [33].
The present study demonstrated temporal trends of OPC incidence and mortality according to the ICD-10 classification. Joinpoint analysis provided a clear picture of temporal patterns in different segments of time. As such, we were able to show the significant changes in trends that have occurred during the study period. Using the APC model, we detected significant differences in incidence and mortality trends of OPC between males and females. Furthermore, OPC incidence significantly increased in rural and urban areas among males. The present study confirmed the increases in incidence and mortality of OPC in the last decade, especially for males in rural areas. Nevertheless, the ASRIC and ASRMC were stable over the study period for females, which may be because of an increased awareness of the need of sunscreen or low smoking rates in females [19]. Our results were consistent with previous studies on trends of OPC in other countries [16, 34, 35].
Our observations of increasing OPC incidence in males and in rural areas help us better understand its epidemiological situation in China and provide more precise scientific information for its control and prevention. The main strength of our study is the population-based design with a large sample size of OPC patients in China. Furthermore, we included 32 cancer registries that had data of sufficient quality over the 10-year period (2003–2012) for inclusion in incidence and mortality trend analyses. Additionally, our epidemiologic study was nationwide and covered a period of 5 years.
Nevertheless, there were limitations that should be addressed. First, the information submitted by the cancer registries was incomplete because the private behaviours such as oral/vaginal sexual behaviours were not known. Second, it did not contain information of other risk factors, such as individual tobacco and alcohol consumption. Therefore, the effect of these factors on temporal trends could only be assessed. Finally, the small number of cases in some subgroups (i.e., small number of death cases in the Western region) may preclude further analysis by age in females.
Conclusions
We analyzed temporal trends of incidence and mortality of OPC by sex and geographic regions using a large sample from population-based cancer registries in China. The results confirmed an increase in OPC incidence and mortality in the recent decade, especially for males in rural areas. However, the age-standardized rates were stable over the study period for females. The implementation of public health measures such as screening programms and risk factor control should be adopted for reducing the incidence in China. Studies with larger sample sizes are needed to further explore the roles of HPV infection, tobacco and alcohol consumption, and sexual behaviours in the development of OPC.
Change history
26 February 2019
In the original publication of this article [1], the number of death cases in ‘Materials and methods’ section is incorrect.
Abbreviations
- ASR:
-
age-standardized rate
- OPC:
-
oropharyngeal cancer
- ASRIC:
-
age-standardized rate of incidence by 2000 Chinese standard population
- ASRIW:
-
age-standardized rate of incidence by 1985 Segi’s world standard population
- ASRMC:
-
age-standardized rate of mortality by 2000 Chinese standard population
- ASRMW:
-
age-standardized rate of mortality by 1985 Segi’s world standard population
- ICD-10:
-
the 10th revision of the International Statistical Classification of Diseases and Related Health Problems
- NCCR:
-
National Central Cancer Registry of China
- IARC/IACR:
-
International Agency for Research on Cancer/International Association of Cancer Registries
- M/I:
-
mortality to incidence
- DCO%:
-
the percentage of cancer cases identified with death certification only
- MV%:
-
the proportion of morphologic verification
- UB%:
-
the percentage of uncertified cancer
- APC:
-
annual percentage change
- HPV:
-
human papillomavirus
- OPSCC:
-
oropharyngeal squamous cell carcinoma
References
Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–27. https://doi.org/10.1001/jamaoncol.2015.0735.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. https://doi.org/10.3322/caac.20138.
Chaturvedi AK, Anderson WF, Lortet-Tieulent J. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–9. https://doi.org/10.1200/JCO.2013.50.3870.
Auluck A, Walker BB, Hislop G, Lear SA, Schuurman N, Rosin M. Population-based incidence trends of oropharyngeal and oral cavity cancers by sex among the poorest and underprivileged populations. BMC Cancer. 2014;14:316. https://doi.org/10.1186/1471-2407-14-316.
Abram MH, van Heerden WF, Rheeder P, Girdler-Brown BV, van Zyl AW. Epidemiology of oral squamous cell carcinoma. SADJ. 2012;67(10):550–3.
Majchrzak E, Szybiak B, Wegner A, Pienkowski P, Pazdrowski J, Luczewski L, et al. Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol Oncol. 2014;48(1):1–10. https://doi.org/10.2478/raon-2013-0057.
Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67(1):51–64. https://doi.org/10.3322/caac.21384.
Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26(1):48–58. https://doi.org/10.3978/j.issn.1000-9604.2014.01.08.
Li GL, Chen WQ. Representativeness of population-based cancer registration in China–comparison of urban and rural areas. Asian Pac J Cancer Prev. 2009;10(4):559–64.
Zheng CM, Ge MH, Zhang SS, Tan Z, Wang P, Zheng RS, et al. Oral cavity cancer incidence and mortality in China, 2010. J Cancer Res Ther. 2015;11(Suppl 2):C149–54. https://doi.org/10.4103/0973-1482.168176.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. https://doi.org/10.3322/caac.21338.
Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27(1):2–12. https://doi.org/10.3978/j.issn.1000-9604.2015.01.06.
Matsuda T, Matsuda A. Burden of cancer incidence below the age of 40 in Asia 2002 extrapolated from the Cancer Incidence in Five Continents Vol. IX. Jpn J Clin Oncol. 2013;43(4):449–50. https://doi.org/10.1093/jjco/hyt045.
Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–16. https://doi.org/10.1016/j.oraloncology.2008.06.002.
Wang F, Zhang H, Xue Y, Wen J, Zhou J, Yang X, et al. A systematic investigation of the association between HPV and the clinicopathological parameters and prognosis of oral and oropharyngeal squamous cell carcinomas. Cancer Med. 2017;6(5):910–7. https://doi.org/10.1002/cam4.1045.
Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. https://doi.org/10.1200/JCO.2011.36.4596.
Farsi NJ, Rousseau MC, Schlecht N, Castonguay G, Allison P, Nguyen-Tan PF, et al. Aetiological heterogeneity of head and neck squamous cell carcinomas: the role of human papillomavirus infections, smoking and alcohol. Carcinogenesis. 2017;38(12):1188–95. https://doi.org/10.1093/carcin/bgx106.
Ide R, Mizoue T, Fujino Y, Hoshiyama Y, Sakata K, Tamakoshi A, et al. Cigarette smoking, alcohol drinking, and oral and pharyngeal cancer mortality in Japan. Oral Dis. 2008;14(4):314–9.
Liu S, Zhang M, Yang L, Li Y, Wang L, Huang Z, et al. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health. 2017;71(2):154–61. https://doi.org/10.1136/jech-2016-207805.
Kawakita D, Matsuo K. Alcohol and head and neck cancer. Cancer Metastasis Rev. 2017;36(3):425–34. https://doi.org/10.1007/s10555-017-9690-0.
Li Y, Jiang Y, Zhang M, Yin P, Wu F, Zhao W. Drinking behaviour among men and women in China: the 2007 China Chronic Disease and Risk Factor Surveillance. Addiction. 2011;106(11):1946–56. https://doi.org/10.1111/j.1360-0443.2011.03514.x.
Wang PL, Xiao FT, Gong BC, Liu FN. Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget. 2017;8(58):99013–23. https://doi.org/10.18632/oncotarget.20918.
Lopez-Lazaro M. A local mechanism by which alcohol consumption causes cancer. Oral Oncol. 2016;62:149–52. https://doi.org/10.1016/j.oraloncology.2016.10.001.
Genden EM, Sambur IM, de Almeida JR, Posner M, Rinaldo A, Rodrigo JP, et al. Human papillomavirus and oropharyngeal squamous cell carcinoma: what the clinician should know. Eur Arch Otorhinolaryngol. 2013;270(2):405–16. https://doi.org/10.1007/s00405-012-2086-4.
Strojan P, Zadnik V, Sifrer R, Lanisnik B, Didanovic V, Jereb S, et al. Incidence trends in head and neck squamous cell carcinoma in Slovenia, 1983–2009: role of human papillomavirus infection. Eur Arch Otorhinolaryngol. 2015;272(12):3805–14. https://doi.org/10.1007/s00405-014-3459-7.
Zhang C, Liu F, Pan Y, Deng Q, Li X, He Z, et al. Incidence and clearance of oral human papillomavirus infection: a population-based cohort study in rural China. Oncotarget. 2017;8(35):59831–44. https://doi.org/10.18632/oncotarget.16306.
Huang H, Zhang B, Chen W, Zhou SM, Zhang YX, Gao L, et al. Human papillomavirus infection and prognostic predictors in patients with oropharyngeal squamous cell carcinoma. Asian Pac J Cancer Prev. 2012;13(3):891–6.
Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–75. https://doi.org/10.1158/1055-9965.EPI-04-0551.
Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–42. https://doi.org/10.1200/JCO.2015.61.6995.
Hong AM, Grulich AE, Jones D, Lee CS, Garland SM, Dobbins TA, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine. 2010;28(19):3269–72. https://doi.org/10.1016/j.vaccine.2010.02.098.
Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–9. https://doi.org/10.1200/JCO.2007.14.1713.
Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119(11):2620–3. https://doi.org/10.1002/ijc.22177.
Beachler DC, Sugar EA, Margolick JB, Weber KM, Strickler HD, Wiley DJ, et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am J Epidemiol. 2015;181(1):40–53. https://doi.org/10.1093/aje/kwu247.
Carlander AF, Gronhoj Larsen C, Jensen DH, Garnaes E, Kiss K, Andersen L, et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: a Danish population-based study from 2011 to 2014. Eur J Cancer. 2017;70:75–82. https://doi.org/10.1016/j.ejca.2016.10.015.
Osazuwa-Peters N, Simpson MC, Massa ST, Adjei Boakye E, Antisdel JL, Varvares MA. 40-year incidence trends for oropharyngeal squamous cell carcinoma in the United States. Oral Oncol. 2017;74:90–7. https://doi.org/10.1016/j.oraloncology.2017.09.015.
Authors’ contributions
JL analyzed the data and wrote the paper. SWZ prepared and calculated the data. XLY data check and analysis, information retrieval. LPZ and WQC conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge the cooperation of all the population-based cancer registries in providing cancer statistics, data collection, sorting, and verification, and database establishment.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors consent for publication this study in Cancer Communications.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by a Program Grant in Fundamental Research from the Ministry of Science and Technology of China (No. 2014FY121100), by the National Natural Science Foundation of China (No. 81602931), supported partly by Natural Science Foundation of Jiangxi, China (No. 20171BAB215052), and supported by Jiangxi Provincial Health Planning Committee science and technology project (Nos. 20172004 and 20186008).
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
List of 135 cancer registries which provided full datasets for new cancer patients diagnosed during 2008–2012.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, J., Yang, Xl., Zhang, SW. et al. Incidence, mortality, and temporal patterns of oropharyngeal cancer in China: a population-based study. Cancer Commun 38, 75 (2018). https://doi.org/10.1186/s40880-018-0345-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40880-018-0345-5